Abstract
The yolk syncytial layer (YSL) in the zebrafish embryo is a multinucleated syncytium essential for embryo development, but the molecular mechanisms underlying YSL formation remain largely unknown. Here we show that zebrafish solute carrier family 3 member 2 (Slc3a2) is expressed specifically in the YSL and that slc3a2 knockdown causes severe YSL defects including clustering of the yolk syncytial nuclei and enhanced cell fusion, accompanied by disruption of microtubule networks. Expression of a constitutively active RhoA mimics the YSL phenotypes caused by slc3a2 knockdown, whereas attenuation of RhoA or ROCK activity rescues the slc3a2-knockdown phenotypes. Furthermore, slc3a2 knockdown significantly reduces tyrosine phosphorylation of c-Src, and overexpression of a constitutively active Src restores the slc3a2-knockdown phenotypes. Our data demonstrate a signaling pathway regulating YSL formation in which Slc3a2 inhibits the RhoA/ROCK pathway via phosphorylation of c-Src to modulate YSL microtubule dynamics. This work illuminates processes at a very early stage of zebrafish embryogenesis and more generally informs the mechanism of cell dynamics during syncytium formation.
Keywords: CD98, epiboly, morphogenesis
The yolk syncytial layer (YSL) in the zebrafish embryo is an extraembryonic structure composed of a multinucleated syncytium located at the margin between the yolk and the blastoderm (1). Formation of the YSL is one of the earliest differentiation events in the embryo and plays a vital role in embryo development: The YSL is crucial in regulating dorso-anterior axis formation (2–6), epiboly movements involved in embryo patterning and morphogenesis (7–12), and cardiac progenitor cell movements (13).
The YSL forms between the 512-cell and 1k-cell stage just after the midblastula transition, the onset of zygotic transcription, when the marginal blastomeres undergo acute membrane collapse, thereby fusing to each other and to the adjoining yolk cell to form a thin syncytial ring around the blastodisc edge (14, 15). The YSL contains microtubules emanating from microtubule asters adjacent to the individual yolk syncytial nuclei (YSN) and extending vertically toward the vegetal pole (7–9). Disruption of the YSL microtubules by depolymerizing reagents such as nocodazole, UV irradiation, or cold temperature causes an altered and enlarged YSL and aggregation of the YSN, leading to a severe delay of epiboly (7–9). This effect suggests that the microtubule networks in the YSL act as towing guides to direct the dynamic movements of the YSL and blastoderm toward the vegetal pole during epiboly (7).
Loss of function of some genes highly expressed in the YSL, such as β4.1 and β4.2 (voltage-gated Ca2+ channel β subunits) and cyp11a1 (a steroidogenic enzyme), cause aberrant YSN localization (12) or altered YSL structure with unstable microtubule arrays (10), leading to a severe delay of epiboly. However, these genes do not have any known molecular link to downstream pathways that modulate microtubules, and thus the signaling pathways by which microtubule dynamics in the YSL are regulated remain undefined (10, 14, 12).
A zebrafish slc3a2 gene (here named “slc3a2-a”) has been reported based on genome sequence (16), and another slc3a2 gene, slc3a2-b, was found in our in situ-based gene-discovery screen (17). Zebrafish solute carrier family 3 member 2 (Slc3a2) proteins have about 60% sequence identity to the mammalian Slc3a2/4F2hc/CD98 heavy chain (CD98hc), an 85-kDa glycosylated type II membrane protein, with conservation in intracellular, transmembrane, and extracellular domains. Mammalian Slc3a2/4F2hc/CD98hc binds to integrin β1 and β3 subunits through its intracellular domain and hence is involved in various integrin-mediated functions independent of the CD98 light chain (18–22). Mammalian Slc3a2/4F2hc/CD98hc regulates integrin-mediated cell spreading, cell migration, and adhesion in mouse embryonic stem cells (19) and human placenta trophoblasts (21). Furthermore, anti–CD98hc/FRP-1 antibodies regulate virus-mediated cell fusion (23, 24) and formation of multinucleated giant monocytes (25), and knockdown of Slc3a2/4F2hc/CD98hc inhibits placental syncytial trophoblast formation (26).
Here we show that zebrafish Slc3a2 is indispensable for regulating microtubule dynamics in the YSL and that RhoA, ROCK, and Src act downstream of Slc3a2 in YSL formation.
Results
Zebrafish slc3a2 Is Expressed Specifically in the YSL.
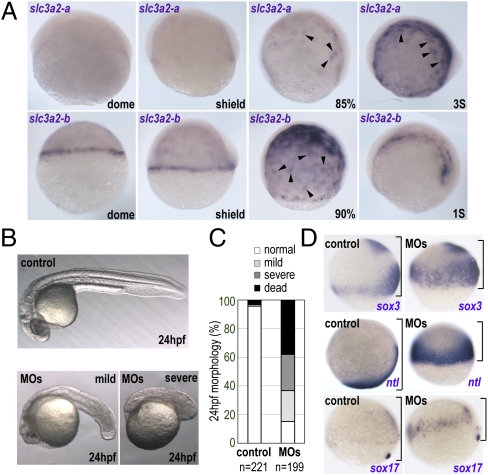
slc3a2-a and slc3a2-b transcripts were detected from the midblastula stage onward exclusively in the YSL. Expression of both genes increased through epiboly stages, when the mRNA was localized preferentially in the vicinity of the YSN (Fig. 1A), and continued through somitogenesis stages. The YSL-specific expression of slc3a2-b was confirmed by sectioning of in situ-stained embryos (Fig. S1). Specific expression of slc3a2 in the YSL suggests a role in the function and/or formation of this structure.
Fig. 1.
Zebrafish slc3a2 is expressed specifically in the YSL and is essential for embryo development. (A) Expression of zebrafish slc3a2-a and slc3a3-b at different embryonic stages. Intensive slc3a2-a and slc3a2-b expression was observed around the YSN (arrowheads). (B) slc3a2 knockdown (MOs) causes a small head and short body axis at 24 hpf. Shown are examples of mild and severe morphological phenotypes. (C) Morphology at 24 hpf was categorized as normal (white), mild (light gray), or severe (dark gray) phenotypes presenting a small head and short body axis or as dead (black). Data shown are from three experiments; the total number of embryos is shown below each bar. (D) slc3a2 knockdown induces severe epiboly delay. Whole-mount in situ analyses for sox3, no-tail (ntl), and sox17 at the late gastrula stage are shown. The distance between the animal pole and the blastoderm margin is indicated by a black bracket.
Slc3a2 Is Required for Normal Embryonic Morphogenesis.
To examine the functions of Slc3a2 (hereafter, “slc3a2” refers to slc3a2-a and slc3a2-b) in embryogenesis, loss of function was achieved by injecting morpholino (MO) antisense oligonucleotides against slc3a2-a and slc3a2-b. Single knockdown of slc3a2-a or slc3a2-b induced relatively mild morphological defects (a shorter tail, smaller head) in embryos at 24 h postfertilization (hpf) (Fig. S2A, b and c). In contrast, double knockdown of both slc3a2-a and slc3a2-b (slc3a2-MOs) showed marked developmental defects at 24 hpf (Fig. 1B and Fig. S2A, d) including lethality (38.1 ± 6.0%), a highly reduced head and truncated body axis (25.3 ± 4.8%), and a milder phenotype of a small head and short tail (21.6 ± 9.0%) (Fig. 1C). To confirm the specificity of the phenotypes caused by the slc3a2-MOs, we used two different MOs for each slc3a2-a and slc3a2-b gene (Materials and Methods) and observed similar morphological phenotypes (Fig. S2A, e). Rescue experiments are presented below in the context of YSL phenotypes. These data indicate slc3a2 is indispensable for normal embryogenesis in zebrafish and that there is at least partial functional redundancy between the slc3a2-a and slc3a2-b paralogues.
Slc3a2 Regulates Epiboly Movements.
To examine how loss of function of Slc3a2 affects embryonic patterning, we analyzed the expression of different marker genes by whole-mount in situ hybridization. sox3 (a neural ectoderm marker), no-tail (ntl, a mesoderm marker), and sox17 (an endoderm marker) were expressed in their normal spatial relationships after slc3a2 knockdown, but the embryos showed a marked delay of epiboly (Fig. 1D). At 24 hpf, despite the severe morphological defects in slc3a2 morphants (slc3a2-MOs), the expression domains of tissue-specific markers, such as myoD in somitic muscle (Fig. S2C, a and b) and pax2 at the isthmus, optic stalk, otic vesicle, spinal cord, and pronephric duct (Fig. S2C, c and d), were comparable to those in control embryos. These data suggest that Slc3a2 is essential for gastrulation movements and for embryonic morphogenesis rather than for tissue specification.
Slc3a2 Knockdown Enhances Plasma Membrane Fusion in the YSL and Enveloping Layer.
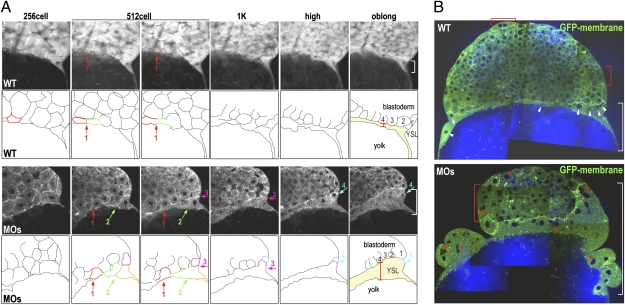
Human SLC3a2/4F2hc/CD98hc regulates cell fusion in a variety of cell types (23–26). We therefore speculated that abrogation of Slc3a2 in the zebrafish embryo might alter plasma membrane fusion during YSL formation. To examine this possibility, plasma membrane dynamics during YSL formation was analyzed using two-photon microscopy to image membrane-tagged GFP-labeled cells (27). Acquisition of both two-photon fluorescence (TPF) (for GFP) and coherent anti-Stokes Raman scattering (CARS) (for detecting lipids) enabled visualization of plasma membranes of the blastomeres and the yolk cell throughout cell-division cycles. Consistent with previous reports (1), metasynchronous membrane fusion in the marginal blastomeres was observed during transition between the 512- and 1k-cell stages in control embryos (Fig. 2A and Movie S1). For the subsequent YSL division cycles (from the 1k-cell to the oblong stage), the positions of the YSN in control embryos did not change; thus the YSN remained stationary underneath the blastoderm margin (Fig. 2A and Movie S1). In contrast, in the slc3a2-MOs, cell–cell fusion continued until the oblong stage, resulting in enlargement of the YSL (Fig. 2 A and B). Some of the YSN in slc3a2-MOs were clustered (Fig. 2B) and moved freely around, unlike in control embryos (Movie S2). Interestingly, some of the enveloping layer (EVL) cells that share a common cellular origin with the YSL (15) were able to fuse to become syncytial cells both in control and in slc3a2-MOs (Fig. 2B and Movies S2 and S3). However, the quantity of fused EVL cells and, consequently, the number of nuclei within the syncytial region were increased significantly in slc3a2-MOs (Fig. 2B). Collectively, these data indicate that the stage-specific cell-to-cell membrane-fusion events are disorganized in the absence of Slc3a2, suggesting that Slc3a2 regulates YSL formation through the spatiotemporal regulation of membrane collapse during syncytium formation.
Fig. 2.
Loss of function of Slc3a2 enhances cell–cell fusion and YSN clustering. (A) Sequences of TPF time-lapse images showing GFP membrane (Upper) and outlines of marginal cells and the YSL (Lower). Fusing cells are indicated by colored outlines, and fusion locations are indicated by colored arrows. The width of the YSL at the outer edge is shown by a white bracket. Cell–cell fusion occurs mostly around the 512-cell stage (red arrow in control, red and green arrows in MOs) but persists through the following stages in slc3a2-MOs (MOs) (pink and cyan arrows in MOs). (B) Merged images of CARS (for lipid; blue) and TPF (for GFP membrane; green) of control and MOs. Uniformly distributed YSN in the control are indicated by white arrowheads, and clustered YSN in MOs are indicated by red asterisks. Multinucleated EVL cells are indicated by red brackets.
Slc3a2 Regulates the Distribution of the YSN.
To examine further the roles of Slc3a2 in YSL organization, the YSN were labeled fluorescently by injecting Sytox green into the embryos. Consistent with previous studies (9, 12, 28), the YSN in control embryos were distributed evenly underneath the blastoderm (Fig. 3A, a), whereas the YSN in slc3a2-MOs often coalesced and were distributed unevenly (Fig. 3A, b; see also Fig. 2). In most cases, the external YSL in slc3a2-MOs was expanded from the blastoderm margin, spreading toward the vegetal pole, as compared with control embryos (Fig. 3A, b). The proportion of embryos showing normal YSL organization therefore was greatly reduced in slc3a2-MOs (Fig. 3B and Table S1). We confirmed that the abnormalities in YSL organization were caused specifically by slc3a2 knockdown, because overexpression of slc3a2-b not targeted by the MOs substantially rescued these phenotypes (Figs. 3 A, e and B and Table S1). These data indicate that Slc3a2 regulates the structure of the YSL and distribution of the YSN.
Fig. 3.
Loss of function of Slc3a2 severely alters YSL organization, which is restored by inhibition of RhoA or ROCK. (A) The YSN are visualized by Sytox green. Shown are lateral views of dome-stage embryos from control (a); slc3a2-MOs (MOs) (b); HN-slc3a2-b (HNslc3a2b) (c); RhoA-G14V (G14V) (d); slc3a2-MOs+slc3a2-b (MOs+slc3a2b) (e); slc3a2-MOs+RhoA-MO (MOs+RhoAMO) (f); slc3a2-MOs + treatment with 50 μM Y-27632 (MOs+Y27632) (g); and control treated with 50 μM Y-27632 (Y27632) (h). Clustered YSN are indicated by white arrowheads. Note rescue of YSL phenotypes by coinjecting slc3a2-b-mRNA and RhoA-MO or by treatment with 50 μM Y-27632. (B) Loss of function of Slc3a2 severely alters YSL organization, which is restored by inhibition of RhoA or ROCK or by overexpression of v-Src. Phenotype in the dome-stage embryo is categorized as normal (white) showing uniform distribution of the YSN without YSL deformation/expansion, abnormal (gray) showing altered YSN localization including uneven distribution of the YSN and YSN clustering with YSL deformation/expansion, or dead (black). Data are from more than three different experiments. Total number of embryos is shown below each bar. (C) Inhibition of RhoA or ROCK restores microtubule networks in the YSL in slc3a2-MOs. (a–d) control; (e–h) HNslc3a2b; (i–l) MOs; (m–p) MOs+slc3a2b; (q–t) MOs + RhoA-MO; (u–x) MOs + Y27632. Microtubule networks were visualized by α-tubulin antibody staining (a, e, i, m, q, and u), and the YSN were visualized by Sytox green (b, f, j, n, r, and v). Shown are merged images of α-tubulin (red) and Sytox green (green) (c, g, k, o, s, and w) and reconstituted section views of the merged images rotated by 90° (d, h, l, p, t, and x). Absence or disruption of microtubule arrays in the YSL is indicated by red arrowheads in e and i, and the clustered YSN are indicated by green asterisks in f and j. Control shows overlap of the YSN and dense YSL microtubule networks (white square in d), which are missing in both HNslc3a2b and MOs (h and l). YSL microtubule networks, YSN distribution, and overlapping of the YSN and YSL microtubules are all restored in MOs+slc3a2b, MOs+RhoA-MO, and MOs+Y27632 (m–p, q–t, and u–x).
Intracellular Domain of Slc3a2 Is Required to Regulate YSN Localization.
To examine the contribution of functional domains of Slc3a2 to the establishment of YSL structure and YSN localization, we generated a putative dominant-negative form of Slc3a2-b (HN-Slc3a2-b) in which the intracellular domain was replaced by the cytosolic domain (24 amino acids) of human parainfluenza virus type-2 hemagglutinin-neuraminidase (29). Overexpression of HN-Slc3a2-b caused morphological changes in 24-hpf embryos comparable to those seen in slc3a2-MOs (Fig. S2A, f). Consistently, many of the YSN in the HN-slc3a2-b–overexpressing embryos were clustered, and the YSL expanded toward the vegetal pole, again similar to the phenotype of slc3a2-MOs (Fig. 3 A, c and B). These data support the conclusion that Slc3a2 acts through its intracellular domain to regulate YSL structure and YSN localization.
Slc3a2 Is Required to Maintain Microtubule Arrays in the YSL.
Thick arrays of microtubules extend from the YSL toward the yolk along the animal–vegetal axis; disrupting these arrays by microtubule depolymerizing reagents or UV irradiation causes premature YSL formation and altered YSL organization (Fig. S3) (7–9). We therefore examined the microtubules in the YSL by α-tubulin antibody staining. In control embryos, dense arrays of microtubules in the YSL were seen underneath the blastoderm edge, where the YSN were dispersed uniformly (Fig. 3C, a–d). In contrast, YSL microtubule arrays in embryos injected with HN-slc3a2-b or slc3a2-MOs were disrupted, with regions devoid of microtubules (Fig. 3C, e, g–i, k, and l), and the YSN were arranged irregularly (Fig. 3C, f, g, j, and k). We further confirmed that overexpression of slc3a2-b rescued microtubule networks in the YSL and YSN localization in slc3a2-MOs (Fig. 3 C, m–p). These data indicate that Slc3a2 is required to retain microtubule networks in the YSL.
Inhibition of RhoA/ROCK Restores YSN Localization and YSL Microtubule Arrays After slc3a2 Knockdown.
Microtubule dynamics both regulate and are regulated by intracellular signaling pathways involving RhoA and the downstream kinase ROCK (30, 31). Using mammalian cell lines, we previously demonstrated an interrelationship between microtubules and RhoA/ROCK in which depolymerized microtubules activate RhoA, which in turn promotes depolymerization of microtubules through ROCK (31). This negative regulation of microtubules by Rho/ROCK signaling also has been shown to be critical for directional migration of macrophages in live zebrafish embryos (32) as well as for contact inhibition of migrating chicken embryonic heart fibroblasts (33). We therefore hypothesized that disruption of microtubules in the YSL of slc3a2-MOs could be caused by increased RhoA/ROCK activity. Indeed, overexpression of a constitutively active form of RhoA (G14V) in zebrafish embryos induced severe YSL deformation and YSN aggregation, similar to the phenotypes in slc3a2-MOs (Fig. 3 A, d and B and Table S1). These observations led us to examine whether inhibiting RhoA or ROCK activity could restore YSL structure and YSN localization after slc3a2-MOs knockdown. Embryos microinjected with a mixture of MOs against slc3a2 and RhoA (MOs+RhoA MO) retained intact YSL structure in which the YSN were distributed uniformly underneath the blastoderm margin (Fig. 3 A, f and B and Table S1). Similarly, treating slc3a2-MOs–injected embryos with a ROCK inhibitor, Y-27632, rescued the morphology of the YSL with even distribution of the YSN (Fig. 3 A, g and B and Table S1). We further examined whether the attenuation of RhoA or ROCK activity can restore microtubule networks and found that microtubule arrays were restored in embryos injected with MOs+RhoA-MO (Fig. 3C, q–t) as well as in the slc3a2-MOs–injected embryos treated with Y-27632 (Fig. 3C, u–x). These data indicate that Slc3a2 regulates microtubule networks in the YSL and distribution of the YSN by inhibiting the RhoA/ROCK pathway.
Slc3a2 Inhibits RhoA/ROCK Pathway via c-Src Kinase.
Mammalian SLC3a2/4F2hc/CD98hc contributes to integrin outside–in signaling to regulate various integrin-mediated cell functions, including cell adhesion and cell–cell fusion (19, 21–24). A downstream effector of integrin outside–in signaling is c-Src, which is phosphorylated at tyrosine 416 upon integrin–ligand binding (34). Because activation of c-Src transiently inhibits RhoA activity via p190RhoGAP (35), we hypothesized that zebrafish Slc3a2 might inhibit RhoA via c-Src activation. To examine this possibility, we analyzed the phosphorylation level of c-Src tyrosine 416 in slc3a2-MOs by Western blotting using c-Src phosphotyrosine–specific antibody. We found that slc3a2 knockdown substantially reduced tyrosine phosphorylation of c-Src (pY416) (Fig. 4A). Similarly, co-knockdown of Slc3a2 and RhoA also reduced the level of phosphorylated c-Src, even though this treatment restored normal YSN distribution and YSL microtubule networks (Fig. 4A). These data indicate that Slc3a2 is required for c-Src activation and that RhoA acts downstream of these two molecules in the signaling cascade regulating YSL organization. Finally, we examined whether overexpression of a constitutively active c-Src (v-Src) could rescue YSL phenotypes in slc3a2-MOs–injected embryos. The level of v-Src mRNA, 1.25 pg per embryo, did not affect embryogenesis through 24 hpf. We found that overexpression of v-Src restored YSL deformation (YSN clustering and YSL expansion) (Figs. 3B and 4B) and YSL microtubules (Fig. 4C) in slc3a2-MOs–treated embryos. Together, these data indicate Slc3a2 regulates c-Src to inhibit the RhoA/ROCK signaling pathway, thereby contributing to the even distribution of the YSN and to the preservation of YSL microtubule networks.
Fig. 4.
Slc3a2 inhibits the RhoA/ROCK pathway via c-Src activation in regulating YSL organization. (A) slc3a2 knockdown reduces tyrosine phosphorylation of c-Src. Western blot shows pY416 c-Src and total c-Src in lysates from control, slc3a2-morphants (MOs), or slc3a2/RhoA morphants (MOs+RhoA-MO). Data shown are representative of three independent experiments. (B) Overexpression of v-Src restores YSN localization after slc3a2 knockdown. The YSN are visualized by Sytox green. Clustered YSN in MOs (white arrows) are restored by coinjecting v-Src mRNA (v-Src+MOs). (C) Overexpression of v-Src restores microtubule networks in the YSL in slc3a2-knockdown embryos. YSL microtubule networks (αTUB; red), YSN distribution (SytG; green), and overlap of YSN and YSL microtubules (white squares in merged images) are all restored by v-Src+MOs.
Discussion
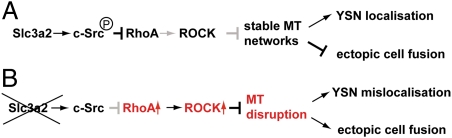
In this study, we demonstrate that Slc3a2 plays a critical role in YSL formation and YSL organization by regulating microtubule networks in the YSL. We find that knockdown of slc3a2 causes the disruption of microtubule networks in the YSL accompanied by clustering of the YSN and ectopic syncytium formation, leading to severe morphological defects in the zebrafish embryo. By rescue experiments, we found that all the slc3a2-knockdown phenotypes are rescued by attenuating RhoA or ROCK activity, suggesting that these phenotypes are caused by increased RhoA/ROCK activity. Furthermore, we showed that slc3a2 knockdown reduces tyrosine phosphorylation of c-Src, which links to integrin-mediated RhoA inhibition (35), suggesting that Slc3a2 normally activates c-Src to inhibit RhoA during YSL formation. Consistently, overexpression of constitutively active c-Src also rescues the YSL phenotypes caused by slc3a2 knockdown. Our data led us to construct a model in which Slc3a2 inhibits a RhoA/ROCK signaling pathway via c-Src activation, thereby regulating YSL microtubules and YSL organization (Fig. 5).
Fig. 5.
Model for the Slc3a2-mediated signaling pathway regulating YSN localization and cell–cell fusion in the YSL. (A) Slc3a2 is involved in integrin outside–in signaling, which activates c-Src, which in turn inhibits RhoA. Microtubule arrays in the YSL then are retained, contributing to regular YSN localization and normal cell–cell fusion. (B) slc3a2 knockdown abolishes c-Src activation; hence, microtubule networks in the YSL are disrupted by increased RhoA/ROCK activity in the YSL, thereby causing YSN clustering and enhanced ectopic cell–cell fusion.
Depolymerization of microtubules induces both premature marginal blastomere fusion and YSN clustering (7–9), suggesting that microtubule dynamics contribute to two events essential for YSL organization: cell–cell fusion and YSN localization (Fig. 5). Realignment of microtubules into parallel arrays along the axis of the fusing cells has been shown to be crucial for syncytial myotube formation (36–38). These data support the view that microtubules control the spatiotemporal aspects of plasma membrane fusion in YSL progenitors. How, then, can Slc3a2 modulate plasma membrane dynamics via microtubules? It is known that microtubule dynamics affect the regional distribution of adhesion molecules (38–40) and turnover of adherence junctions (41), thereby regulating adhesive strength and the integrity of cell–cell contacts. One possibility is that the local distribution of Slc3a2 regionally protects the microtubule networks, which in turn feed back on the localization of Slc3a2 or affect cellular distribution of other adhesion molecules (e.g., E-cadherin), thereby regulating the plasma membrane dynamics. Indeed, we found that slc3a2 knockdown appears to reduce the integrity of cell–cell contacts between the YSL and the marginal blastomeres; hence the marginal cells actively crawl or tumble within the looser extracellular space (Movie S2). In contrast, the marginal blastomeres in control embryos are tightly attached to each other and to the YSL, leaving less extracellular space at the marginal area (Movies S1 and S3).
We observed that not only cell–cell fusion in the YSL progenitors but also YSN localization correlates strongly with the integrity of microtubule networks in the YSL. The combined TPF and CARS time-lapse imaging demonstrates that the YSN move quickly in the enlarged YSL of slc3a2-MOs but are stationary in control embryos. The persistent and regularly distributed YSN in control embryos may ensure the direction of epiboly movements, because vertical microtubule arrays extending toward the vegetal pole emanate from microtubule asters close to the aligned individual YSN (8, 9). The YSN might be associated with the dense YSL microtubule mesh and therefore persist underneath the blastoderm margin. Indeed, α-tubulin staining shows that the YSN in control embryos locate with the dense microtubule networks in the YSL, whereas the YSN in the slc3a2-MOs–injected embryos are separated from the YSL microtubules.
Our data suggest that Slc3a2 normally inhibits RhoA in the YSL. This result might be similar to the previous report by Kabir-Salmani et al. (21) that stable expression of human CD98hc (Slc3a2, 4F2hc) in a CD98hc-null hepatocyte line facilitates integrin-mediated cell adhesion, which is accompanied by enhanced transient inhibition in RhoA activity. In contrast, Féral et al. (20) have reported that CD98hc-null mouse embryonic fibroblasts fail to induce the integrin-mediated delayed activation of RhoA that follows transient RhoA inhibition and contributes to stress fiber formation and fibronectin matrix assembly. The discrepancy in observed effects of mammalian SLC3a2/4F2hc/CD98hc on RhoA activity could be the result of cell-type specificity, so that SLC3a2/4F2hc/CD98hc can be influenced by and in turn affect different signaling molecules. Although mammalian SLC3a2/4F2hc/CD98hc is expressed in a wide variety of tissues (42), our in situ analyses revealed that zebrafish slc3a2 is expressed specifically in the YSL in the embryo. Considering the relatively low similarity of zebrafish Slc3a2 with mammalian SLC3a2/4F2hc/CD98hc (60%), it is possible that some functional diversifications may have occurred in different species.
Mammalian SLC3a2 is involved in the formation of multinucleated giant monocytes, placental syncytial trophoblast formation, and virus-mediated cell–cell fusion. Thus it is highly possible that Slc3a2 regulates microtubule dynamics via RhoA/ROCK pathway in cell–cell fusion and syncytium formation in these cells (23–26).
Materials and Methods
Zebrafish Strains and Maintenance.
Wild-type and Tg[β-actin:GFP] strains of zebrafish (27) were maintained according to standard procedures (1).
RNA Probe Synthesis and in Situ Hybridization.
The procedures for whole-mount in situ hybridization have been described previously (17, 43).
Constructs.
Details of plasmid constructions are described in SI Materials and Methods.
Synthesis and Microinjection of mRNA.
mRNAs were synthesized using the mMessange mMachine SP6 kit (Ambion). One nanoliter of 150 pg/nL slc3a2-b-mRNA or HN-slc3a2-b-mRNA, 15 pg/nL RhoA-b-G14V-mRNA, or 1.25 pg/nL GFP-FL-v-Src-mRNA was injected through the intact chorion into the blastomere at the one-cell stage.
MO Analysis and Injection.
The MOs against slc3a2-a (BC053256) (slc3a2-a-MO1: 5′-CCTTCATCTCGTCTTCTTTGTTCAT-3′ and slc3a2-a-MO2: 5′- TTTGTTTGATAGTAGTTTCAGCACT-3′) and slc3a2-b [BC044497 (17)] (slc3a2-b-MO1: 5′-CCTTCATCTCGTCTTCTTTGTTCAT-3′ and slc3a2-b-MO2: 5′-TATCCACTTCAGTGTCGTTGCTCAT-3′) were purchased from GeneTools. The MO against RhoA-b (RhoA-b-MO: 5′-TTCTTGCGAATTGCTGCCATATTTG-3′) was purchased from Open Biosystems. Each MO was used at 1:4 dilutions from a 1 mM stock. In all cases, 2 nL of solution was injected into the yolk proximal to the blastomeres of the embryo at the one- to four-cell stage. A combination of slc3a2-a-MO1 and slc3a2-b-MO1 (slc3a2-MOs) was used for all data shown except for the data shown in Fig. S2 A and B.
Sytox Green Injection, Drug Treatment, and Whole-Mount Microscopy.
To label the nuclei within the YSL, a total of ∼6 nL of 1 mM Sytox green (Invitrogen) was injected into the yolk at high stage (∼2 nL of Sytox green was injected into three or four different regions of the yolk proximal to the blastomeres). In some experiments, the embryos were treated with 50 μM of ROCK inhibitor Y-27632 (Calbiochem) in embryo medium from the 256-cell stage, before YSL formation. YSL organization was analyzed at dome stage by fluorescent dissection microscopy (Nikon SMZ1500).
TPF and CARS Microscopy.
Embryos from Tg[β-actin:GFP] fish were injected with slc3a2c-MOs as described above. Embryos then were dechorionated and mounted onto glass-bottomed 35-mm dishes (MatTek) with 0.7% low-melting agarose in embryo medium. Embryos held in this condition continued cleavage throughout the imaging period. TPF imaging and CARS microscopy were performed simultaneously; details have been described previously (44, 45) and are described in SI Materials and Methods. Time-lapse images of membrane-GFP dynamics were acquired using Flouroview v5 software (Olympus). Stacks of frame-averaged (four-frame) sections were collected and reconstructed by using Image J (http://rsbweb.nih.gov/ij/).
Immunostaining and Confocal Imaging.
Embryos were injected with MOs or mRNAs, and in some experiments Sytox green was injected subsequently as described above. Staining with anti–α-tubulin antibody (DM1A; Sigma) was performed as described by Gard (46). Stained samples were analyzed by a Zeiss LSM510 inverted confocal microscope. Z-stacks of line-averaged (eight-line) sections were obtained by scanning an area of 102.4 × 102.4 μm (0.1 μm pixel−1) with 6-μm steps over a total vertical distance of 180–240 μμ, and were reconstituted using LSM510 meta.
Western Blot.
Embryos at the shield stage were lysed in cold lysis buffer [50 mM Tris (pH 7.5), 10 mM MgCl2, 0.5 M NaCl, and 2% (vol/vol) Igepal containing 1× protease inhibitor mixture and 50 mM NaVaO3] at 20 embryos per 200 μL lysis buffer. Lysates were clarified by centrifugation. Supernatants were mixed with lithium dodecyl sulfate (LDS) sample buffer (Invitrogen), heated, and analyzed by SDS/PAGE. pY416 Phospho-Src and total Src (Cell Signaling) blots were performed according to the manufacturer's instructions.
Supplementary Material
Acknowledgments
We thank Y. Ito for providing HN-cDNA, Y. Fujita for providing pCS2-GFP-FL-v-Src, M. Tada and S. J. Heasman for critical comments on the manuscript, and the fish facility in Biosciences, University of Exeter for fish maintenance. This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/F010222/1 (to T.K.) and in part by the intramural research program of the National Institute of Child Health and Human Development, National Institutes of Health (to I.B.D.) and Wellcome Trust funding (to S.W.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200642109/-/DCSupplemental.
References
- 1.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno T, Yamanaka M, Wakahara A, Kuroiwa A, Takeda H. Mesoderm induction in zebrafish. Nature. 1996;383(6596):131–132. [Google Scholar]
- 3.Hong SK, Jang MK, Brown JL, McBride AA, Feldman B. Embryonic mesoderm and endoderm induction requires the actions of non-embryonic Nodal-related ligands and Mxtx2. Development. 2011;138:787–795. doi: 10.1242/dev.058974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman B, et al. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- 5.Solnica-Krezel L, et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development. 1996;123:67–80. doi: 10.1242/dev.123.1.67. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka Y, et al. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev. 1998;12:2345–2353. doi: 10.1101/gad.12.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strähle U, Jesuthasan S. Ultraviolet irradiation impairs epiboly in zebrafish embryos: Evidence for a microtubule-dependent mechanism of epiboly. Development. 1993;119:909–919. doi: 10.1242/dev.119.3.909. [DOI] [PubMed] [Google Scholar]
- 8.Jesuthasan S, Stähle U. Dynamic microtubules and specification of the zebrafish embryonic axis. Curr Biol. 1997;7:31–42. doi: 10.1016/s0960-9822(06)00025-x. [DOI] [PubMed] [Google Scholar]
- 9.Solnica-Krezel L, Driever W. Microtubule arrays of the zebrafish yolk cell: Organization and function during epiboly. Development. 1994;120:2443–2455. doi: 10.1242/dev.120.9.2443. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HJ, Liang MR, Chen CT, Chung BC. Pregnenolone stabilizes microtubules and promotes zebrafish embryonic cell movement. Nature. 2006;439:480–483. doi: 10.1038/nature04436. [DOI] [PubMed] [Google Scholar]
- 11.Lachnit M, Kur E, Driever W. Alterations of the cytoskeleton in all three embryonic lineages contribute to the epiboly defect of Pou5f1/Oct4 deficient MZspg zebrafish embryos. Dev Biol. 2008;315:1–17. doi: 10.1016/j.ydbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Ebert AM, et al. Ca2+ channel-independent requirement for MAGUK family CACNB4 genes in initiation of zebrafish epiboly. Proc Natl Acad Sci USA. 2008;105:198–203. doi: 10.1073/pnas.0707948105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawahara A, et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho L, Heisenberg CP. The yolk syncytial layer in early zebrafish development. Trends Cell Biol. 2010;20:586–592. doi: 10.1016/j.tcb.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- 16.Yoder JA, Litman GW. The zebrafish fth1, slc3a2, men1, pc, fgf3 and cycd1 genes define two regions of conserved synteny between linkage group 7 and human chromosome 11q13. Gene. 2000;261:235–242. doi: 10.1016/s0378-1119(00)00503-5. [DOI] [PubMed] [Google Scholar]
- 17.Kudoh T, et al. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001;11:1979–1987. doi: 10.1101/gr.209601. [DOI] [PubMed] [Google Scholar]
- 18.Fenczik CA, et al. Distinct domains of CD98hc regulate integrins and amino acid transport. J Biol Chem. 2001;276:8746–8752. doi: 10.1074/jbc.M011239200. [DOI] [PubMed] [Google Scholar]
- 19.Feral CC, et al. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci USA. 2005;102:355–360. doi: 10.1073/pnas.0404852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Féral CC, et al. CD98hc (SLC3A2) participates in fibronectin matrix assembly by mediating integrin signaling. J Cell Biol. 2007;178:701–711. doi: 10.1083/jcb.200705090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabir-Salmani M, et al. The membrane-spanning domain of CD98 heavy chain promotes alpha(v)beta3 integrin signals in human extravillous trophoblasts. Mol Endocrinol. 2008;22:707–715. doi: 10.1210/me.2007-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prager GW, Féral CC, Kim C, Han J, Ginsberg MH. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem. 2007;282:24477–24484. doi: 10.1074/jbc.M702877200. [DOI] [PubMed] [Google Scholar]
- 23.Ohta H, et al. Molecular and biological characterization of fusion regulatory proteins (FRPs): Anti-FRP mAbs induced HIV-mediated cell fusion via an integrin system. EMBO J. 1994;13:2044–2055. doi: 10.1002/j.1460-2075.1994.tb06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto K, et al. An anti-fusion regulatory protein-1 monoclonal antibody suppresses human parainfluenza virus type 2-induced cell fusion. J Gen Virol. 1997;78:83–89. doi: 10.1099/0022-1317-78-1-83. [DOI] [PubMed] [Google Scholar]
- 25.Tajima M, et al. Suppression of FRP-1/CD98-mediated multinucleated giant cell and osteoclast formation by an anti-FRP-1/CD98 mAb, HBJ 127, that inhibits c-src expression. Cell Immunol. 1999;193:162–169. doi: 10.1006/cimm.1999.1467. [DOI] [PubMed] [Google Scholar]
- 26.Kudo Y, Boyd CA, Millo J, Sargent IL, Redman CW. Manipulation of CD98 expression affects both trophoblast cell fusion and amino acid transport activity during syncytialization of human placental BeWo cells. J Physiol. 2003;550:3–9. doi: 10.1113/jphysiol.2003.040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper MS, et al. Visualizing morphogenesis in transgenic zebrafish embryos using BODIPY TR methyl ester dye as a vital counterstain for GFP. Dev Dyn. 2005;232:359–368. doi: 10.1002/dvdy.20252. [DOI] [PubMed] [Google Scholar]
- 28.D'Amico LA, Cooper MS. Morphogenetic domains in the yolk syncytial layer of axiating zebrafish embryos. Dev Dyn. 2001;222:611–624. doi: 10.1002/dvdy.1216. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto K, et al. Paramyxovirus-induced syncytium cell formation is suppressed by a dominant negative fusion regulatory protein-1 (FRP-1)/CD98 mutated construct: An important role of FRP-1 in virus-induced cell fusion. J Gen Virol. 1997;78:775–783. doi: 10.1099/0022-1317-78-4-775. [DOI] [PubMed] [Google Scholar]
- 30.Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: Is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008;18:210–219. doi: 10.1016/j.tcb.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. Microtubules regulate migratory polarity through Rho/ROCK signaling in T cells. PLoS ONE. 2010;5:e8774. doi: 10.1371/journal.pone.0008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redd MJ, Kelly G, Dunn G, Way M, Martin P. Imaging macrophage chemotaxis in vivo: Studies of microtubule function in zebrafish wound inflammation. Cell Motil Cytoskeleton. 2006;63:415–422. doi: 10.1002/cm.20133. [DOI] [PubMed] [Google Scholar]
- 33.Kadir S, Astin JW, Tahtamouni L, Martin P, Nobes CD. Microtubule remodelling is required for the front-rear polarity switch during contact inhibition of locomotion. J Cell Sci. 2011;124:2642–2653. doi: 10.1242/jcs.087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong H, et al. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science. 2010;327:340–343. doi: 10.1126/science.1174779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arthur WT, Petch LA, Burridge K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr Biol. 2000;10:719–722. doi: 10.1016/s0960-9822(00)00537-6. [DOI] [PubMed] [Google Scholar]
- 36.Pizon V, Gerbal F, Diaz CC, Karsenti E. Microtubule-dependent transport and organization of sarcomeric myosin during skeletal muscle differentiation. EMBO J. 2005;24:3781–3792. doi: 10.1038/sj.emboj.7600842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Straube A, Merdes A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr Biol. 2007;17:1318–1325. doi: 10.1016/j.cub.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, et al. Microtubule plus-end binding protein EB1 is necessary for muscle cell differentiation, elongation and fusion. J Cell Sci. 2009;122:1401–1409. doi: 10.1242/jcs.039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stehbens SJ, et al. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci. 2006;119:1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- 40.Zaoui K, Honoré S, Isnardon D, Braguer D, Badache A. Memo-RhoA-mDia1 signaling controls microtubules, the actin network, and adhesion site formation in migrating cells. J Cell Biol. 2008;183:401–408. doi: 10.1083/jcb.200805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mary S, et al. Biogenesis of N-cadherin-dependent cell-cell contacts in living fibroblasts is a microtubule-dependent kinesin-driven mechanism. Mol Biol Cell. 2002;13:285–301. doi: 10.1091/mbc.01-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devés R, Boyd CA. Surface antigen CD98(4F2): Not a single membrane protein, but a family of proteins with multiple functions. J Membr Biol. 2000;173:165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- 43.Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–3592. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moger J, Johnston BD, Tyler CR. Imaging metal oxide nanoparticles in biological structures with CARS microscopy. Opt Express. 2008;16:3408–3419. doi: 10.1364/oe.16.003408. [DOI] [PubMed] [Google Scholar]
- 45.Mansfield J, et al. The elastin network: Its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy. J Anat. 2009;215:682–691. doi: 10.1111/j.1469-7580.2009.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gard DL. Organization, nucleation, and acetylation of microtubules in Xenopus laevis oocytes: A study by confocal immunofluorescence microscopy. Dev Biol. 1991;143:346–362. doi: 10.1016/0012-1606(91)90085-h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.