Abstract
There is extensive evidence that glucocorticoid hormones impair the retrieval of memory of emotionally arousing experiences. Although it is known that glucocorticoid effects on memory retrieval impairment depend on rapid interactions with arousal-induced noradrenergic activity, the exact mechanism underlying this presumably nongenomically mediated glucocorticoid action remains to be elucidated. Here, we show that the hippocampal endocannabinoid system, a rapidly activated retrograde messenger system, is involved in mediating glucocorticoid effects on retrieval of contextual fear memory. Systemic administration of corticosterone (0.3–3 mg/kg) to male Sprague–Dawley rats 1 h before retention testing impaired the retrieval of contextual fear memory without impairing the retrieval of auditory fear memory or directly affecting the expression of freezing behavior. Importantly, a blockade of hippocampal CB1 receptors with AM251 prevented the impairing effect of corticosterone on retrieval of contextual fear memory, whereas the same impairing dose of corticosterone increased hippocampal levels of the endocannabinoid 2-arachidonoylglycerol. We also found that antagonism of hippocampal β-adrenoceptor activity with local infusions of propranolol blocked the memory retrieval impairment induced by the CB receptor agonist WIN55,212–2. Thus, these findings strongly suggest that the endocannabinoid system plays an intermediary role in regulating rapid glucocorticoid effects on noradrenergic activity in impairing memory retrieval of emotionally arousing experiences.
Keywords: cannabinoid receptor, norepinephrine, emotional arousal, fear conditioning, posttraumatic stress disorder
It is well-established that glucocorticoid (GC) hormones, released from the adrenal cortex during stressful episodes, can modulate different memory processes (1–4). Although most studies focused on GC effects on the acquisition and consolidation of memory, extensive evidence also indicates that acutely elevated GC levels at the time of retention testing impair the retrieval of memory of spatial and contextual training (5–9). Because a glucocorticoid receptor (GR) agonist infused into the hippocampus before retention induces comparable memory retrieval impairment (10, 11), such findings suggest that GC effects on memory retrieval depend, at least in part, on activation of GRs in the hippocampus. Findings of studies of human subjects are consistent with the findings of animal studies and indicate that exogenous GC administration or exposure to a psychosocial stressor shortly before retention testing impairs retrieval of declarative (mostly episodic) information (7, 12, 13) and reduces hippocampal activity (14). Moreover, previous findings indicate that emotionally arousing information is especially sensitive to the retrieval-impairing effects of GCs (8) and that emotional arousal during the test situation enables GC effects on memory retrieval (15). Findings of recent clinical studies suggest that the administration of stress doses of GCs may have therapeutic value by attenuating the reexperiencing of highly traumatic memories in patients who have posttraumatic stress disorder (PTSD) and other anxiety disorders (16–19).
Our previous finding that GCs interact with arousal-induced noradrenergic activity in impairing the retrieval of hippocampus-dependent memory (10, 11, 20, 21) might explain why GCs selectively impair memory retrieval of emotionally arousing or traumatic experiences (8). However, it is not understood how GCs interact with the noradrenergic system in influencing memory retrieval, because these effects seem to be too rapid to act through the classical genomic mode of action of GCs (6, 10, 11, 20–23). Findings of recent studies investigating the cellular mechanism underlying the rapid effects of GCs suggest a possible involvement of the endocannabinoid system (24–27). Endogenous ligands for cannabinoid CB1 receptors [i.e., anandamide (AEA) and 2-arachidonoylglycerol (2-AG)] are synthesized on demand through cleavage of membrane precursors and serve as retrograde messengers at central synapses (28). They bind to G protein-coupled CB1 receptors at presynaptic sites to regulate ion channel activity and neurotransmitter release (29). It is now well-established that stress and GCs can induce rapid changes in endocannabinoid signaling in stress-responsive brain regions (30, 31). Although these effects have been mostly studied with respect to nongenomically mediated effects of GCs on hypothalamic–pituitary–adrenocortical axis activity (25, 26, 30), CB1 receptors are also abundantly expressed in the hippocampus, basolateral amygdala, and other brain regions, where they modulate synaptic transmission, neuronal firing, and memory (29, 32–34).
We previously reported evidence that GCs interact with the endocannabinoid system within the basolateral amygdala in enhancing the consolidation of memory of emotionally arousing training experiences (32, 35). In the present study, we investigated whether the endocannabinoid system is involved in mediating GC-induced memory retrieval impairment. We focus here on retrieval of contextual fear memory, because we first found that a systemic injection of corticosterone (CORT) administered shortly before retention testing impairs the retrieval of contextual but not auditory fear memory. Furthermore, in view of the extensive evidence indicating that GC effects on memory retrieval depend on arousal-induced noradrenergic activity, we also examined whether endocannabinoids interact with the noradrenergic system within the hippocampus in impairing retrieval of contextual fear memory.
Results
Systemic CORT Administration Dose-Dependently Impairs Retrieval of Contextual but Not Auditory Fear Memory.
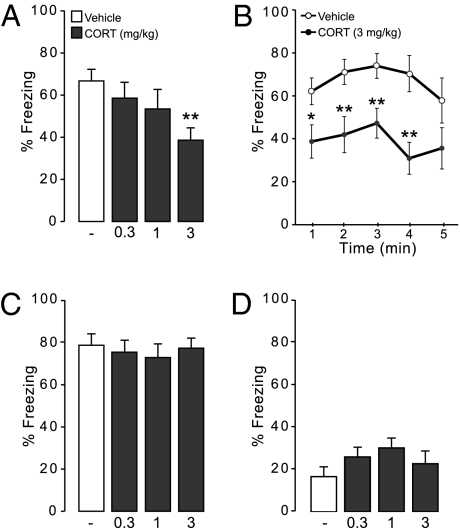
This experiment investigated whether CORT administered systemically 1 h before retention testing impaired retrieval of contextual and auditory fear memory. During training, different groups of animals acquired the contextual (F7,301 = 81.62, P < 0.0001) and auditory (F7,196 = 61.56, P < 0.0001) fear conditioning tasks as indicated by progressively increasing freezing scores during shock trials. Furthermore, the groups that were assigned to receive control or drug treatments subsequently did not differ in acquisition performance (contextual fear conditioning: F3,43 = 1.60, P = 0.20; auditory fear conditioning: F3,28 = 0.82, P = 0.96) (Table S1); 24 h later, rats received a systemic injection of either vehicle or different doses of CORT (0.3, 1, or 3 mg/kg) 1 h before retention testing on the contextual and auditory fear conditioning tasks. As is shown in Fig. 1A, one-way ANOVA indicated that CORT treatment induced a dose-dependent reduction in overall percent freezing during retention testing on the contextual fear conditioning task (F3,43 = 2.98, P = 0.04). Fisher posthoc analysis revealed that the 3-mg/kg dose of CORT, but not lower doses, significantly decreased freezing levels (P < 0.01 compared with vehicle). We also analyzed whether freezing levels of rats administered the 3-mg/kg dose of CORT were lower throughout the retention test or whether CORT facilitated the extinction of fear during the retention test session. Repeated-measures ANOVA for freezing levels in five consecutive 1-min time bins (CORT 3 mg/kg and vehicle groups only) showed a significant effect of CORT treatment (F1,23 = 12.22, P = 0.001) but not of time (F4,92 = 1.69, P = 0.15) or interaction between CORT treatment and time (F4,92 = 0.65, P = 0.62), suggesting that freezing levels did not change over the course of the retention test; thus, the freezing of the CORT 3 mg/kg group was lower than the freezing of the vehicle group throughout the test (Fig. 1B). In contrast to contextual fear memory, systemic CORT treatment did not alter freezing levels during retention on the auditory fear conditioning task (F3,28 = 0.20, P = 0.89) (Fig. 1C).
Fig. 1.
Effect of systemic CORT administration on retrieval of fear memory. (A) Systemic CORT (0.3, 1, or 3 mg/kg) treatment administered 1 h before retention testing dose-dependently impairs retrieval of contextual fear memory. Results represent mean ± SEM. **P < 0.01 vs. vehicle (n = 11–13 per group). (B) Effect of systemic CORT (3 mg/kg) treatment on freezing during retrieval of contextual fear memory analyzed in 1-min time bins. Results represent mean ± SEM. *P < 0.05, **P < 0.01 vs. vehicle (n = 11–13 per group). (C) Systemic CORT (0.3, 1, or 3 mg/kg) treatment given 1 h before retention testing does not impair retrieval of auditory fear memory. Results represent mean ± SEM (n = 8 per group). (D) Effect of systemic CORT (0.3, 1, or 3 mg/kg) administration on basal freezing levels in a nontraining context. Results represent mean ± SEM (n = 10–15 per group).
To further exclude the possibility that CORT treatment might directly influence the expression of freezing, separate groups of animals were trained on the contextual fear conditioning task, and 24 h later, they were administered different doses of CORT (0.3, 1, and 3 mg/kg) 1 h before placing them in a context that was distinctly different from the training context. CORT treatment did not affect basal freezing levels in this nontraining context (F3, 47 = 1.24, P = 0.31) (Fig. 1D). Thus, these findings indicate that CORT selectively impaired conditioned freezing during retention of contextual fear memory and did not affect freezing during retention of the auditory fear conditioning task or induce any direct deficits in the expression of freezing behavior.
Endocannabinoid Signaling in the Hippocampus Mediates the Impairing Effect of CORT on Retrieval of Contextual Fear Memory.
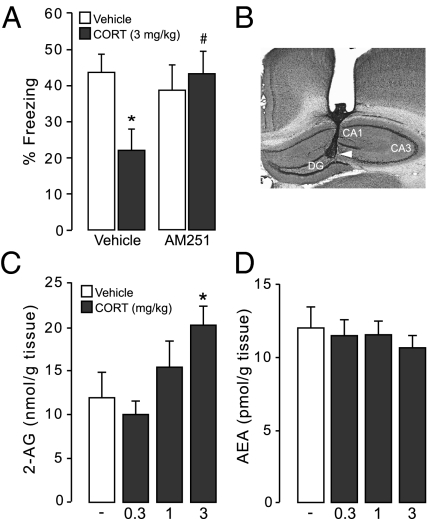
To investigate whether the endocannabinoid system of the hippocampus plays a role in mediating the impairing effect of CORT treatment on retrieval of contextual fear memory, bilateral infusions of the CB1 receptor antagonist AM251 (0.35 ng in 0.5 μL) were administered into the dorsal hippocampus 1 h before retention testing together with systemic injections of either vehicle or CORT (3 mg/kg). Repeated-measures ANOVA for freezing scores during training showed that all groups acquired the contextual fear conditioning task as indicated by progressively increasing freezing scores during shock trials (F7,203 = 63.66, P < 0.0001) without a difference in the acquisition rate between later drug groups (F3,29 = 0.79, P = 0.50) (Table S2). As is shown in Fig. 2A, two-way ANOVA for percent freezing during 24-h retention testing revealed no significant main effects of CORT (F1,29 = 1.93, P = 0.17) or AM251 (F1,29 = 1.76, P = 0.19) but a significant interaction effect between these two treatments (F1,29 = 4.61, P = 0.04). Fisher posthoc comparison tests showed that systemic CORT administration significantly reduced freezing in control rats administered vehicle into the hippocampus (P < 0.05). However, this effect of CORT on freezing behavior was blocked in animals administered AM251 into the hippocampus (P < 0.05 compared with CORT alone).
Fig. 2.
Role of the endocannabinoid system in regulating glucocorticoid effects on retrieval of contextual fear memory. (A) Hippocampal infusion of the CB1 receptor antagonist AM251 (0.35 ng in 0.5 μL) administered 1 h before retention testing blocks the impairment of retrieval of contextual fear memory induced by concurrent systemic CORT (3 mg/kg) treatment. Results represent mean ± SEM. *P < 0.05 vs. vehicle (n = 7–11 per group); #P < 0.05 vs. CORT alone. (B) Representative photomicrograph illustrating placement of cannula and needle tip in the dorsal hippocampus with subfields dentate gyrus (DG), CA1, and CA3. (C and D) Systemic CORT (0.3, 1, or 3 mg/kg) treatment dose-dependently increases hippocampal 2-AG but not AEA in the same time window of the retention test. All results represent mean ± SEM. *P < 0.05 vs. vehicle (n = 10–15 per group).
Next, we investigated whether CORT administration affected endocannabinoid tissue levels in the hippocampus. Rats were trained on the contextual fear conditioning task, and 24 h later, they were given a systemic injection of CORT (0.3, 1, or 3 mg/kg) 1 h before placing them in a nontraining but previously habituated context for 5 min. Immediately afterward, the hippocampus was dissected for endocannabinoid measurements. As is shown in Fig. 2 C and D, one-way ANOVA revealed that CORT treatment dose-dependently elevated hippocampal levels of the endocannabinoid 2-AG (F3,47 = 3.15, P = 0.03) without affecting levels of AEA (F3,47 = 0.23, P = 0.87) or other measured endocannabinoids such as oleoylethanolamide and palmitoylethanolamide (Table S3). Fisher posthoc analyses indicated that the highest dose of CORT (3 mg/kg), but not any of the lower and nonimpairing doses, increased 2-AG levels compared with vehicle (P < 0.05). Thus, our findings that CORT administration elevates 2-AG levels in the hippocampus, whereas a blockade of hippocampal CB1 receptors prevents CORT effects on memory retrieval impairment suggest that hippocampal endocannabinioid signaling is critically involved in mediating the impairing effects of CORT on retrieval of contextual fear memory.
Intrahippocampal Infusion of the CB Receptor Agonist WIN55,212–2 Impairs Retrieval of Contextual Fear Memory Through an Interaction with the Noradrenergic System.
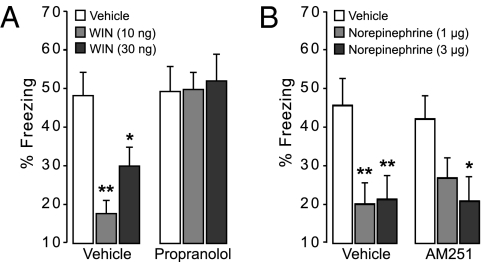
As described above, we previously reported that GC effects on memory retrieval of emotionally arousing experiences involve an essential interaction with arousal-induced noradrenergic activity (11, 36). Hence, in this experiment, we investigated whether cannabinoid effects on memory retrieval also depend on interactions with the noradrenergic system. To address this issue, we first examined whether bilateral microinfusions of the CB receptor agonist WIN55,212–2 (10 or 30 ng in 0.5 μL) administered into the dorsal hippocampus 1 h before the retention test impaired retrieval of contextual fear memory and whether concurrent administration of the β-adrenoceptor antagonist propranolol (1.25 μg) blocked the impairment. All animals acquired the contextual fear conditioning task as indicated by progressively increasing freezing scores during shock trials (F7,420 = 108.00, P < 0.0001) without a significant difference in freezing scores between later drug groups (F2,63 = 2.18, P = 0.12) (Table S4). As is shown in Fig. 3A, two-way ANOVA for percent freezing on the retention test showed a significant WIN55,212–2 effect (F2,63 = 3.26, P = 0.04) and a significant propranolol effect (F1,63 = 15.65, P = 0.0001) as well as a significant interaction effect between these two treatments (F2,63 = 3.63, P = 0.03). Posthoc analysis showed that both doses of WIN55,212–2 significantly impaired freezing levels (10 ng, P < 0.001 and 30 ng, P < 0.05 compared with vehicle). However, WIN55,212–2 did not reduce freezing levels in rats also administered propranolol. Thus, these findings indicate that, as with GCs, endocannabinoid effects on memory retrieval impairment depend on concurrent noradrenergic activity within the hippocampus.
Fig. 3.
Endocannabinoid and norepinephrine interactions in the dorsal hippocampus on retrieval of contextual fear memory. (A) The CB receptor agonist WIN55,212–2 (WIN, 10 or 30 ng in 0.5 μL) infused into the hippocampus 1 h before the retention test impairs retrieval of contextual fear memory. Concurrent infusion of the β-adrenoceptor antagonist propranolol (1.25 μg) blocks this WIN55,212–2-induced memory retrieval impairment. Results represent mean ± SEM. *P < 0.05, **P < 0.001 vs. vehicle (n = 10–14 per group). (B) Intrahippocampal infusions of norepinephrine (1 or 3 μg in 0.5 μL) administered 1 h before the retention testing impair retrieval of contextual fear memory. Concurrent infusion of the CB1 receptor antagonist AM251 (0.35 ng) does not block this impairment. Results represent mean ± SEM. *P < 0.05, **P < 0.01 vs. vehicle (n = 11–15 per group).
The second part of this experiment investigated whether blockade of hippocampal CB1 receptors with AM251 (0.35 ng in 0.5 μL) would affect memory retrieval impairment induced by local infusions of norepinephrine (1 or 3 μg). During training, animals increased their freezing as shock trials progressed (F7,490 = 137.59, P < 0.0001), and there were no differences in the acquisition rate between later drug groups (F2,72 = 0.59, P = 0.56) (Table S5). As is shown in Fig. 3B, two-way ANOVA for percent freezing during 24-h retention testing revealed a significant norepinephrine effect (F2,72 = 8.28, P = 0.0005) but no significant AM251 effect (F1,72= 0.33, P = 0.86) or interaction between norepinephrine and AM251 (F2,72= 0.37, P = 0.70). Microinjection of either dose of norepinephrine into the dorsal hippocampus 1 h before retention testing significantly reduced conditioned freezing levels (1 μg, P < 0.01 and 3 μg, P < 0.01 compared with vehicle). As with norepinephrine administered alone, the 3-μg dose of norepinephrine infused together with the CB1 receptor antagonist induced a significant reduction in freezing (P < 0.05), whereas the 1-μg dose of norepinephrine just failed to reach significance (P = 0.08). Infusion of this low dose of AM251 alone did not alter freezing levels (P = 0.68). These findings indicate that the effect of noradrenergic activation is downstream of CB1 receptor activation.
To exclude the possibility that WIN55,212–2 or norepinephrine infusions into the hippocampus might have decreased freezing during the retention test by directly affecting the expression of freezing behavior, we investigated (in separate groups of animals) the effect of intrahippocampal infusions of the same doses of WIN55,212–2 (10 or 30 ng in 0.5 μL) or norepinephrine (1 or 3 μg in 0.5 μL) on freezing behavior during retention of auditory fear conditioning. Repeated-measures ANOVA comparing freezing levels in the WIN55,212–2-treated groups showed a significant effect of tone trial (F4,108 = 126.63, P < 0.0001) but no significant effect of WIN55,212–2 (F2,27 = 0.41, P = 0.66) or interaction between tone trial and WIN55,212–2 treatment (F8,108 = 0.84, P = 0.56) (Fig. S1A). Highly comparable, repeated-measures ANOVA comparing retention freezing scores of norepinephrine-treated groups showed a significant effect of tone trial (F4,108 = 90.82, P < 0.0001) but no significant effect of norepinephrine (F2,27 = 0.25, P = 0.77) or interaction between tone trial and norepinephrine treatment (F8,108= 0.86, P = 0.55) (Fig. S1B). Thus, these findings indicate that WIN55,212–2 or norepinephrine effects on contextual fear memory were not mediated by a general, nonspecific change in the expression of freezing behavior.
Discussion
The present study investigated a putative involvement of the hippocampal endocannabinoid system in regulating GC effects on the retrieval of fear memory. The interest of this question stems from previous work indicating that, in both rats and healthy human participants, GCs interact with arousal-induced noradrenergic mechanisms in impairing memory retrieval of emotionally arousing information (6–9). However, because these effects are too rapid to be mediated through genomic GC actions, the neurobiological processes underlying the GC influence on noradrenergic activity remained to be determined (37). The present findings indicate that a blockade of hippocampal CB1 receptors prevents the impairing effects of GCs on retrieval of contextual fear memory, whereas the administration of an impairing dose of CORT increases hippocampal levels of the endocannabinoid 2-AG. We also found that antagonism of hippocampal β-adrenoceptor activity blocks the memory retrieval impairment induced by the CB receptor agonist WIN55,212–2, whereas CB1 receptor blockade fails to alter memory retrieval impairment induced by concurrent hippocampal infusions of norepinephrine. These findings suggest that the endocannabinoid system is involved in mediating GC effects on the noradrenergic system in impairing memory retrieval.
Our finding that CORT administration shortly before retention testing impaired retrieval of contextual fear memory without affecting retrieval of auditory fear memory or baseline freezing is consistent with previous reports indicating that GCs impair memory retrieval of hippocampus-dependent contextual fear memory and that this stress hormone effect is not directly attributable to acute fear relief or deficits in the expression of freezing behavior (22, 38). Moreover, our finding that CORT did not facilitate the extinction of freezing within the course of the retention test is in line with other evidence indicating that CORT facilitates the consolidation, but not the acquisition, of fear extinction memory (38, 39). Findings of several other studies investigating the effects of stress, GCs, or specific GR agonists on memory retrieval of other training tasks in rats and requiring the expression of other behavioral responses as well as the expression of verbal reports in healthy human subjects support the view that GCs impair immediate and delayed recall of hippocampus-dependent memory (6, 7, 10, 11). There is extensive evidence that the hippocampus is involved in the retrieval of contextual, spatial, or declarative memory and is also a primary target for stress hormones (2, 40, 41). Moreover, prior findings indicate that direct infusions of GCs into the hippocampus impair the retrieval of spatial memory (10, 11) and that a single GC administration to human subjects decreases hippocampal activity during declarative memory retrieval (42). The findings of studies investigating whether GCs might also impair memory retrieval of hippocampus-independent learning tasks are consistent with our current observation that GCs seem to have little or no effect on retrieval of auditory fear memory or other hippocampus-independent memories (12, 22). However, we cannot exclude the possibility that CORT might have impaired the retrieval of memory of some specific features of the conditioning tone (e.g., frequency, intensity, duration, etc.) used in the present study.
Our finding that pretest blockade of hippocampal CB1 receptors with local infusions of AM251 prevented the GC-induced impairment of contextual fear memory retrieval indicates that endocannabinoid signaling plays an important role in regulating GC effects on memory retrieval. Moreover, comparable with the effect of systemic CORT administration, intrahippocampal infusions of the full CB agonist WIN55,212–2 impaired the retrieval of contextual but not auditory fear memory. This selective impairment of retrieval of contextual fear memory indicates that the WIN55,212–2 administration did not nonspecifically affect the expression of freezing behavior, a finding that is in accordance with other reported evidence that intrahippocampal administration of WIN55,212–2 or other cannabinoid agonists (δ-9-tetrahydrocannabinol or CP 55,940) impairs spatial memory without directly affecting the expression of behaviors that were assessed as an index of memory (43–45). Moreover, we found that CORT administration, in a dose that impairs memory retrieval, increased hippocampal levels of 2-AG but not AEA or other measured endocannabinoids in the same time course of the retention test. These findings are consistent with previous evidence that stress and GCs rapidly alter endocannabinoid signaling in a variety of stress-responsive brain regions, including the hippocampus (30, 46). Although some controversy exists in the literature, stress has been shown to mobilize 2-AG while concurrently decreasing AEA levels in the hippocampus (30, 31). Interestingly, GR antagonists block this stress-induced increase in hippocampal 2-AG levels (47). Although it is currently unknown how GCs might increase 2-AG levels (i.e., changes in synthesis, release, uptake, or degradation), the effect seems to depend on activation of a G protein-coupled receptor and intracellular cAMP-dependent protein kinase signaling (48).
Extensive evidence indicates that stress and GC effects on memory retrieval of emotionally arousing experiences depend crucially on an interaction with arousal-induced noradrenergic activity (10, 11, 22, 36). A β-adrenoceptor antagonist administered systemically or directly into the hippocampus or basolateral amygdala in rats blocks GC effects on memory retrieval. Moreover, GCs have been shown to rapidly increase the release of norepinephrine in the amygdala after an emotionally arousing experience (23) in a time frame that seems incompatible with the time frame of the classical genomic effects of GCs. The present findings indicate that GC-induced impairment of memory retrieval is mediated, at least in part, by rapid influences on the endocannabinoid system. Moreover, our finding that the β-adrenoceptor antagonist propranolol blocks the impairing effect of the CB receptor agonist WIN55,212–2, whereas a blockade of CB1 receptors with AM251 fails to prevent norepinephrine-induced memory retrieval impairment indicates that norepinephrine is functionally located downstream from the endocannabinoid system. Collectively, these findings strongly suggest that endocannabinoids play an intermediary role in regulating GC effects on the norepinephrine system in impairing memory retrieval. In support of this view, previous findings indicate that the administration of a synthetic cannabinoid agonist dose-dependently increased norepinephrine levels in limbic and cortical regions (49, 50).
A possible scenario is that endocannabinoids might influence noradrenergic function through an inhibition of GABAergic transmission (27, 32, 35). Although this possibility was originally proposed for GC-induced enhancement of memory consolidation involving the basolateral amygdala (32), CB1 receptors are also abundantly expressed on hippocampal GABAergic terminals and to a minor extent, glutamatergic terminals (51). An activation of CB1 receptors has consistently been shown to suppress the release of GABA in the hippocampus through a Ca2+-dependent depolarization-induced suppression of inhibition (28). In support of our finding that CORT might affect memory retrieval through increased 2-AG endocannabinoid signaling, recent findings suggest that, particularly, 2-AG is involved in the modulation of depolarization-induced suppression of inhibition and thus, the suppression of GABA release in the hippocampus (52–54). Additionally, substantial evidence from pharmacological studies on memory consolidation has indicated that a blockade of GABAergic transmission with specific antagonists increases norepinephrine release from presynaptic sites (55). Based on these findings, a similar working model for GC-induced impairment of memory retrieval can be proposed. GCs first boost the release of 2-AG in the hippocampus. This endocannabinoid then binds to CB1 receptors on GABAergic interneurons to suppress the release of GABA, resulting indirectly in elevated norepinephrine levels, which as we have shown in this study, impairs memory retrieval of salient information (Fig. S2).
As noted above, there is currently growing interest in GC influences on retrieval of memory of emotionally arousing experiences because of clinical findings indicating that GC administration to PTSD patients significantly reduces reexperiencing of highly traumatic memories and other chronic stress symptoms (16, 19). However, in a clinical setting, the sustained use of GCs is undesirable because of the pleiotropic nature of these hormones to affect a wide array of physiological functions (e.g., immune and metabolic functions). The present finding that the hippocampal endocannabinoid system is involved in mediating GC effects on memory retrieval impairment could aid in the development of non-GC–based therapies for PTSD. Although clinical studies have not yet investigated interactions between these two stress systems, recent findings indicate that administration of the synthetic cannabinoid nabilone to PTSD patients resulted in a highly comparable reduction of treatment-resistant daytime flashbacks and nightmares (56). Moreover, PTSD is often associated with high levels of cannabis consumption (57), which might be related, in part, to an inadequate activation of the endogenous GC and endocannabinoid systems in these patients (58). Furthermore, based on our finding that systemic CORT administration impaired the retrieval of hippocampus-dependent contextual fear memory without affecting the retrieval of hippocampus-independent auditory fear memory, it would seem important to also investigate whether GC or cannabinoid administration might selectively reduce the retrieval of hippocampus-dependent traumatic memories in PTSD patients.
Methods
Subjects.
Male adult Sprague–Dawley rats (280–330 g at time of surgery) from Charles River were kept individually in a temperature-controlled (22 °C) colony room and maintained on a standard 12-h light and 12-h dark cycle (07:00–19:00 h lights on) with ad libitum access to food and water. All behavioral procedures were performed during the light cycle between 10:00 and 15:00 h. All procedures were in compliance with the European Community's Council Directive on the use of laboratory animals of November 24, 1986 (86/609/EEC) and were approved by the Institutional Animal Care and Use Committee of the University of Groningen, Groningen, The Netherlands.
Surgery.
Animals, adapted to the vivarium for at least 1 wk, were anesthetized with a mixture of ketamine (37.5 mg/kg body weight; Alfasan) and dexmedetomidine (0.25 mg/kg; Orion), and surgery was performed according to a standardized protocol (59). Briefly, the skull was positioned in a stereotaxic frame (Kopf Instruments), and two stainless steel guide cannulae (11 mm, 23 gauge; Small Parts) were implanted bilaterally with the cannula tips 1.5 mm above the dorsal hippocampus (anteroposterior, −3.4 mm from Bregma; mediolateral, ±1.8 mm from the midline; dorsoventral, 2.7 mm below skull surface; incisor bar, −3.3 mm from interaural) (60). The cannulae were affixed to the skull with two anchoring screws and dental cement. Stylets (11-mm-long 00-insect dissection pins) inserted into each cannula to maintain patency were removed only for the infusion of drugs. After surgery, the rats were administered atipamezole hydrochloride (2.5 mg/kg; Orion) to reverse anesthesia and subsequently injected with 3 mL saline to facilitate clearance of drugs and prevent dehydration. The rats were allowed to recover for 10 d before initiation of training and were handled three times for 1 min each during this recovery period to accustom them to the infusion procedure.
Fear Conditioning.
After handling days were completed, all rats were habituated to the training context for 5 min without shock exposure. On the next day, animals were trained on either the contextual or auditory fear conditioning task. For contextual fear conditioning, each rat was placed in the fear conditioning apparatus (24 cm width × 25 cm depth × 34 cm height) and exposed to five foot shocks (1.4 mA, 1 s, 1-min intertrial interval) after 2 min of baseline; 24 h later, rats were reexposed to the fear conditioning context for 5 min. For auditory fear conditioning, animals were exposed to five tones (80 dB, 4 kHz, 10 s) coterminating with foot shock (1.4 mA, 1 s); 24 h later, animals were tested in a different context with tone trials only (five trials for 10 s each) after 3 min of baseline. Control groups were habituated to the training apparatus, and 24 h later, they were trained on the contextual fear conditioning task; however, on the retention test day, they were tested for 5 min in a different but previously habituated context. Freezing behavior was analyzed with Behafreeze software (http://www.pmbogusz.net/software/), and some of the groups were also analyzed manually blind to drug treatment as a quality control.
Endocannabinoid Quantification.
After rapid decapitation, the hippocampus was dissected, and lipid extraction was performed according to a standardized protocol as explained in SI Methods (61). For endocannabinoid measurements, automated online, solid-phase extraction using column switching with subsequent direct transfer to HPLC and a tandem MS system was applied. Pure solutions were used for calibration. The method is linear within the calibration ranges. All liquid chromatography MS analyses were carried out using an 1100 LC system (binary pump and autosampler; Agilent) coupled to an API 4000 mass spectrometer (Applied Biosystems) and equipped with a Turbo-Ion-Spray (ESI) source. Because in biological matrices, 2-AG (including its deuterated analog) rapidly isomerizes to 1-AG (62), we quantified 2-AG as the sum of both isomers.
Drug and Infusion Procedures.
All systemic and local drug manipulations were made 1 h before retention testing. For the first experiment, different doses of CORT (0.3, 1, and 3 mg/kg; Sigma-Aldrich) dissolved in 5% ethanol were administered s.c. CORT doses were based on previous findings (6, 38). For the second experiment, the selective CB1 receptor antagonist AM251 (0.35 ng in 0.5 μL per side; Sigma-Aldrich) was infused into the dorsal hippocampus together with an s.c. injection of either an impairing dose of CORT (3 mg/kg) or vehicle. AM251 was first dissolved in 100% DMSO and subsequently diluted in phosphate buffer to reach a final DMSO concentration of 2%. For the third experiment, the CB receptor agonist WIN55,212–2 (10 or 30 ng in 0.5 μL per side; Sigma-Aldrich) either alone or together with the β-adrenoceptor antagonist propranolol (1.25 μg; Sigma-Aldrich) was dissolved in a vehicle containing 2% DMSO and 0.2% Triton X-100 in phosphate buffer and infused into the dorsal hippocampus. For the last experiment, norepinephrine (1 or 3 μg in 0.5 μL per side; Sigma-Aldrich) either alone or together with AM251 (0.35 ng; Sigma-Aldrich) dissolved in 2% DMSO in phosphate buffer was infused into the dorsal hippocampus.
Bilateral infusions of drug or vehicle into the dorsal hippocampus were given by using 30-gauge injection needles connected to 10-μL Hamilton microsyringes by polyethylene (PE-20) tubing. The injection needles protruded 1.7 mm beyond the cannula tips, and a 0.5-μL injection volume per hemisphere was infused over a period of 50 s by an automated syringe pump (Stoelting). The injection needles were retained within the cannulae for an additional 20 s to prevent backflow of drug into the cannulae.
Histology.
Rats were anesthetized with an overdose of sodium pentobarbital (100 mg/kg, i.p.; Sigma-Aldrich) and perfused transcardially with a 0.9% saline (wt/vol) solution followed by 4% formaldehyde (wt/vol) dissolved in water. Brains were removed, and after cryoprotection in 25% sucrose, coronal sections of 50 μm were cut on a cryostat, mounted on gelatin-coated slides, and stained with cresyl violet. The location of the injection needle tips in the dorsal hippocampus was examined under a light microscope according to the standardized atlas plates in the work by Paxinos and Watson (60) by an observer blind to drug treatment condition. Rats with injection needle placements outside the hippocampus or with extensive tissue damage at the injection needle tips were excluded from analysis.
Statistics.
Data are expressed as mean ± SEM. Overall freezing scores on the retention test trials of the contextual and auditory fear conditioning tasks were analyzed with one- or two-way ANOVAs when appropriate. Endocannabinoid levels were analyzed with one-way ANOVA. To investigate the effect of time (for contextual fear conditioning) or tone trial (for auditory fear conditioning) on the freezing response, freezing retention scores were analyzed with repeated-measures ANOVA with time bin (1 min each) or tone trial as the within-subject factor. Freezing scores during the training session of the contextual and auditory fear conditioning tasks were always analyzed with repeated-measures ANOVA, with shock trial as the within-subject factor. The analyses were followed by Fisher LSD multiple comparison tests when appropriate. P values of less than 0.05 were considered statistically significant. The number of rats per group is indicated in Figs. 1–3 and Figs. S1 and S2.
Supplementary Material
Acknowledgments
We thank Jelle De Boer for assistance with data analysis and Petra Bakker, Angelika Jurdzinski, Hassiba Beldjoud, and Fany Messanvi for technical assistance with the collection of tissue for endocannabinoid measurements. This research was supported by Jan Kornelis De Cock Stichting Grant 6861063 (to P.A.) and National Science Foundation Grant IOB-0618211 (to B.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200742109/-/DCSupplemental.
References
- 1.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 2.de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 3.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 4.Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:1–20. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS. Stress effects on memory: An update and integration. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2011.07.002. in press. [DOI] [PubMed] [Google Scholar]
- 6.de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 7.de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 8.Kuhlmann S, Kirschbaum C, Wolf OT. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol Learn Mem. 2005;83:158–162. doi: 10.1016/j.nlm.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Het S, Ramlow G, Wolf OT. A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology. 2005;30:771–784. doi: 10.1016/j.psyneuen.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Roozendaal B, Griffith QK, Buranday J, de Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proc Natl Acad Sci USA. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- 13.Kuhlmann S, Piel M, Wolf OT. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci. 2005;25:2977–2982. doi: 10.1523/JNEUROSCI.5139-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Quervain DJ, et al. Glucocorticoid-induced impairment of declarative memory retrieval is associated with reduced blood flow in the medial temporal lobe. Eur J Neurosci. 2003;17:1296–1302. doi: 10.1046/j.1460-9568.2003.02542.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlmann S, Wolf OT. Arousal and cortisol interact in modulating memory consolidation in healthy young men. Behav Neurosci. 2006;120:217–223. doi: 10.1037/0735-7044.120.1.217. [DOI] [PubMed] [Google Scholar]
- 16.Aerni A, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–1490. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 17.Schelling G, et al. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann N Y Acad Sci. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- 18.Schelling G, Roozendaal B, de Quervain DJ. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann N Y Acad Sci. 2004;1032:158–166. doi: 10.1196/annals.1314.013. [DOI] [PubMed] [Google Scholar]
- 19.de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004;81:150–154. doi: 10.1016/j.nlm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 22.Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. Stress and glucocorticoids impair memory retrieval via β2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci. 2011;31:14172–14181. doi: 10.1523/JNEUROSCI.2122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McReynolds JR, et al. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem. 2010;93:312–321. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill MN, et al. Functional interactions between stress and the endocannabinoid system: From synaptic signaling to behavioral output. J Neurosci. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasker JG, Di S, Malcher-Lopes R. Minireview: Rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–5556. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill MN, McEwen BS. Endocannabinoids: The silent partner of glucocorticoids in the synapse. Proc Natl Acad Sci USA. 2009;106:4579–4580. doi: 10.1073/pnas.0901519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 29.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 30.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: A novel mechanism for stress habituation. Eur J Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campolongo P, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsicano G, Lafenêtre P. Roles of the endocannabinoid system in learning and memory. Curr Top Behav Neurosci. 2009;1:201–230. doi: 10.1007/978-3-540-88955-7_8. [DOI] [PubMed] [Google Scholar]
- 34.Akirav I. The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front Behav Neurosci. 2011 doi: 10.3389/fnbeh.2011.00034. 10.3389/fnbeh.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atsak P, Roozendaal B, Campolongo P. Role of the endocannabinoid system in regulating glucocorticoid effects on memory for emotional experiences. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2011.08.047. 10.1016/j.neuroscience.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 36.de Quervain DJ, Aerni A, Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am J Psychiatry. 2007;164:967–969. doi: 10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- 37.Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: A matter of timing. Trends Cogn Sci. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Cai WH, Blundell J, Han J, Greene RW, Powell CM. Postreactivation glucocorticoids impair recall of established fear memory. J Neurosci. 2006;26:9560–9566. doi: 10.1523/JNEUROSCI.2397-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 40.Riedel G, et al. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 41.Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oei NY, et al. Glucocorticoids decrease hippocampal and prefrontal activation during declarative memory retrieval in young men. Brain Imaging Behav. 2007;1:31–41. doi: 10.1007/s11682-007-9003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119:282–290. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- 44.Wegener N, Kuhnert S, Thüns A, Roese R, Koch M. Effects of acute systemic and intra-cerebral stimulation of cannabinoid receptors on sensorimotor gating, locomotion and spatial memory in rats. Psychopharmacology (Berl) 2008;198:375–385. doi: 10.1007/s00213-008-1148-1. [DOI] [PubMed] [Google Scholar]
- 45.Egashira N, Mishima K, Iwasaki K, Fujiwara M. Intracerebral microinjections of delta 9-tetrahydrocannabinol: Search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Res. 2002;952:239–245. doi: 10.1016/s0006-8993(02)03247-x. [DOI] [PubMed] [Google Scholar]
- 46.Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010;35:1333–1338. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang M, et al. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol. 2012;26(1):56–70. doi: 10.1177/0269881111409606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 50.Page ME, et al. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–168. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. Neuropharmacology. 2008;54:58–67. doi: 10.1016/j.neuropharm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Tanimura A, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–327. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 54.Wang SH, Teixeira CM, Wheeler AL, Frankland PW. The precision of remote context memories does not require the hippocampus. Nat Neurosci. 2009;12:253–255. doi: 10.1038/nn.2263. [DOI] [PubMed] [Google Scholar]
- 55.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 56.Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD) CNS Neurosci Ther. 2009;15:84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calhoun PS, et al. Drug use and validity of substance use self-reports in veterans seeking help for posttraumatic stress disorder. J Consult Clin Psychol. 2000;68:923–927. [PubMed] [Google Scholar]
- 58.Yehuda R. Status of glucocorticoid alterations in post-traumatic stress disorder. Ann N Y Acad Sci. 2009;1179:56–69. doi: 10.1111/j.1749-6632.2009.04979.x. [DOI] [PubMed] [Google Scholar]
- 59.Fornari RV, et al. Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience. J Vis Exp. 2012 doi: 10.3791/3528. 10.3791/3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Ed. New York: Elsevier; 2005. [Google Scholar]
- 61.Vogeser M, et al. Release of anandamide from blood cells. Clin Chem Lab Med. 2006;44:488–491. doi: 10.1515/CCLM.2006.065. [DOI] [PubMed] [Google Scholar]
- 62.Vogeser M, Schelling G. Pitfalls in measuring the endocannabinoid 2-arachidonoyl glycerol in biological samples. Clin Chem Lab Med. 2007;45:1023–1025. doi: 10.1515/CCLM.2007.197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.