Abstract
Trafficking of AMPA receptors (AMPARs) plays a key role in synaptic transmission. However, a general framework integrating the two major mechanisms regulating AMPAR delivery at postsynapses (i.e., surface diffusion and internal recycling) is lacking. To this aim, we built a model based on numerical trajectories of individual AMPARs, including free diffusion in the extrasynaptic space, confinement in the synapse, and trapping at the postsynaptic density (PSD) through reversible interactions with scaffold proteins. The AMPAR/scaffold kinetic rates were adjusted by comparing computer simulations to single-particle tracking and fluorescence recovery after photobleaching experiments in primary neurons, in different conditions of synapse density and maturation. The model predicts that the steady-state AMPAR number at synapses is bidirectionally controlled by AMPAR/scaffold binding affinity and PSD size. To reveal the impact of recycling processes in basal conditions and upon synaptic potentiation or depression, spatially and temporally defined exocytic and endocytic events were introduced. The model predicts that local recycling of AMPARs close to the PSD, coupled to short-range surface diffusion, provides rapid control of AMPAR number at synapses. In contrast, because of long-range diffusion limitations, extrasynaptic recycling is intrinsically slower and less synapse-specific. Thus, by discriminating the relative contributions of AMPAR diffusion, trapping, and recycling events on spatial and temporal bases, this model provides unique insights on the dynamic regulation of synaptic strength.
Keywords: glutamate receptor, scaffold molecules, synaptic plasticity, theoretical model, diffusion/trapping
Controlling the number of AMPA-type glutamate receptors (AMPARs) at excitatory synapses is of fundamental importance in synaptic transmission (1). AMPARs are anchored at the postsynaptic density (PSD) via specific interactions with scaffold molecules, but can dynamically exchange between intracellular and extrasynaptic membrane compartments. This turnover involves two major mechanisms: endo/exocytic recycling and surface diffusion (2).
A number of studies have demonstrated the importance of AMPAR recycling in synaptic plasticity. Synaptic potentiation induces AMPAR exocytosis, whereas disrupting basal exocytosis leads to a run-down of AMPAR-dependent synaptic transmission and reduces long-term potentiation (LTP) (3–8). Inversely, inhibition of basal endocytosis gradually increases AMPAR excitatory postsynaptic currents (EPSCs), and occludes long-term depression (LTD) (1, 9). Furthermore, an endocytic zone (EZ) located near the PSD and responsible for local AMPAR recycling is essential for regulating synaptic transmission (10–12). Despite these advances, the exact locations and kinetics of AMPAR exocytosis and endocytosis, both in basal conditions and in response to LTP and LTD stimuli, respectively, are still unclear (13).
The other mechanism controlling AMPAR trafficking at synapses is surface diffusion (14). Fluorescence recovery after photobleaching (FRAP) and single-particle tracking (SPT) experiments have shown that AMPARs diffuse freely in the extrasynaptic space and are confined at the synapse (5, 15–17). Surface-diffusing AMPARs are captured at PSDs via PDZ domain scaffold proteins, including postsynaptic density protein 95 (PSD-95), which interacts with AMPAR auxiliary subunits (i.e., transmembrane AMPAR regulatory proteins, or TARPs), or synapse-associated protein 97 (SAP-97) and protein interacting with protein kinase C (PICK)/glutamate receptor interacting protein (GRIP), which recognize GluA1 and GluA2 AMPAR subunits, respectively (1, 18–20). Importantly, AMPAR diffusion and trapping at the synapse are bidirectionally regulated by synaptic activity (19, 21). However, the relative importance of diffusion and binding in regulating AMPAR dynamics is difficult to assess, because the kinetic rates characterizing AMPAR/scaffold interactions are unknown.
Despite a crucial role of AMPAR trafficking in synaptic function, a general model describing the kinetic interplay between AMPAR diffusion and vesicular recycling is still lacking. Previous theoretical papers described AMPAR diffusion in synapses (22–25), but those contained many unknown parameters and remained far from actual experimental paradigms. We built here a unified quantitative framework integrating exo/endocytic events and diffusion/trapping at postsynaptic sites, with only two adjustable parameters: the AMPAR/scaffold binding and unbinding rates. Our model closely sticks to SPT, FRAP, and electrophysiology data, and allows predictions of AMPAR dynamics at synapses in various biological conditions.
Results
The model integrates the three major components of AMPAR trafficking: surface diffusion, trapping at postsynapses, and recycling (Fig. 1A). The outputs of the computer program (Fig. S1) were simulated 2D trajectories of membrane-diffusing AMPARs, transiting between three distinct compartments: extrasynaptic space, synapse, and PSD (Fig. 1B and Movie S1), and directly comparable to SPT data (Fig. 1C). Model hypotheses and parameter values are given in SI Materials and Methods and Table S1.
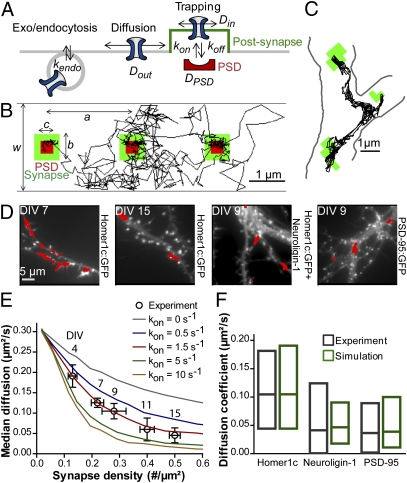
Fig. 1.
Model definition and comparison with SPT experiments. (A) Schematic diagram of the model. Kinetic parameters include Dout (extrasynaptic diffusion), Din (synaptic diffusion), DPSD (diffusion coefficient of the PSD), kon (AMPAR/scaffold binding rate), koff (AMPAR/scaffold dissociation rate), and kendo (endocytosis rate). (B) Simulated trajectory (50 s). The synapse is in green, the PSD in red, and dendrite borders in gray. Geometric parameters are: a (synapse spacing), b (synapse width), c (PSD width), w (dendrite width). (C) High-magnification 30-s trajectory of an AMPAR-bound Qdot on the surface of a DIV 9 neuron, Homer1c:GFP puncta being outlined in green. (D) AMPAR trajectories (red traces) are shown for DIV 7 and DIV 15 neurons expressing Homer1c:GFP (white), and for DIV 9 neurons coexpressing Homer1c:GFP (white) and neuroligin-1, or expressing PSD-95:GFP (white). (E) Experimental (○) and simulated (plain curves) AMPAR median diffusion coefficients obtained at different neuronal ages (DIV 4–15) or varying synaptic spacing (0.75–30 μm), respectively, were plotted against synapse density. The simulated curves were computed for different kon values, keeping koff = 0.1 s−1. (F) Interquartile distributions of AMPAR diffusion coefficients from experiments (black) and simulations (green). Neuroligin-1 expression, which doubled the number of Homer1c-positive puncta, was mimicked by a decrease in synapse spacing (a = 1 μm). Overexpressing PSD-95 was modeled by enhancing kon (2.5 s−1) to mimic an increase in PSD binding sites, plus an increase in PSD size (c = 0.4 μm).
Fitting the Model to SPT Experiments.
We first compared model predictions to SPT experiments performed in primary hippocampal neurons at different ages [days in vitro (DIV) 4–15] and transfected with Homer1c:GFP to identify postsynapses (Fig. 1 C–E). Endogenous AMPARs were labeled with anti-GluA2 conjugated Quantum dots (Qdots), and individual Qdot trajectories were recorded by fluorescence imaging. Simulations mimicked qualitatively well extrasynaptic diffusion and synaptic confinement of AMPARs observed in SPT experiments, with slight discrepancies attributed to limitations of the Qdot technique (Fig. 1 B and C and Fig. S2). The mean square displacement (MSD) was fitted by linear regression to yield a global diffusion coefficient (Fig. S3 A and B). The experimental distribution of diffusion coefficients was shifted to the left as neurons grew older (Fig. S3C), and matched by increasing obstacle density in the model (Fig. S3D). Overall, the median AMPAR diffusion coefficient decreased as synapse density was increased, in agreement with the model (Fig. 1E). Increasing kon to enhance AMPAR trapping at postsynapses reduced global AMPAR mobility (Fig. 1E), whereas increasing koff had the opposite effect (Fig. S4C), thus leading to several combinations of kon and koff that could equally fit SPT data (Fig. S4D). To experimentally alter AMPAR diffusion without affecting developmental age, we overexpressed either the adhesion protein neuroligin-1 to increase synapse density (26), or the scaffold protein PSD-95 to induce synapse maturation (27, 28) (Fig. 1D). Both conditions greatly diminished global AMPAR diffusion (Fig. 1F). These effects were reproduced in the model, the former by doubling obstacle density and the latter by increasing simultaneously PSD size and kon. Overall, the model could interpret SPT experiments in a wide range of biological conditions.
AMPAR Dynamics at Synapses Compared with FRAP and Peptide Competition Data.
To refine the estimation of kinetic parameters, we challenged the model against FRAP experiments performed on primary neurons expressing recombinant pHluorin-tagged GluA1 or GluA2 subunits (16). We superimposed 500 individual trajectories to build AMPAR maps resembling fluorescence images, over time. After a 10-s baseline, photobleaching was modeled by setting an intensity parameter irreversibly to zero when AMPARs reached one particular synapse (Fig. 2A and Movie S2). The simulated FRAP curves using the parameter set kon = 1.5 s−1 and koff = 0.1 s−1 matched very well experimental data at DIV 10 (Fig. 2B). However, FRAP curves in older neurons (DIV 24) showed a lower recovery than at DIV 10. To fit those data, we had to decrease koff = 0.02 s−1 for the whole AMPAR population, or to introduce a 30% AMPAR fraction with a zero koff, this second option better accounting for the fast and saturatable experimental data. Similarly, we also had to consider immobile AMPARs to fit the slow decay at synapses observed in fluorescence loss in photobleaching experiments (12, 15) (Fig. S5). This reduction in koff observed upon neuronal maturation with ensemble measurements of AMPAR dynamics could not be well detected in SPT experiments, which tend to underestimate long synaptic dwell times due to short trajectories and difficulty of Qdots to access the core of the PSD (Fig. S2B).
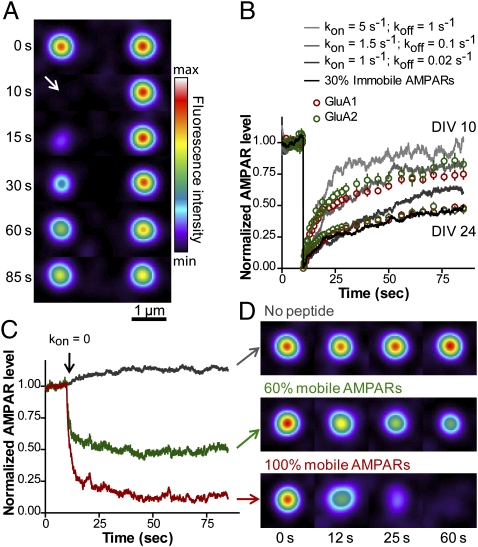
Fig. 2.
Comparison of the model with FRAP and peptide competition experiments. (A) Time-lapse images of simulated FRAP experiments. AMPARs were treated as light point sources represented by Gaussian intensity profiles. The synapse on the left was photobleached at t = 10 s (arrow), and fluorescence recovery was monitored for 75 s. AMPAR levels at control synapses (Right) slightly decrease due to redistribution of bleached AMPARs. (B) Simulated recovery curves generated using several (kon, koff) pairs (gray scale) plotted against experimental FRAP data obtained for DIV 10 and DIV 24 neurons expressing pHluorin-tagged GluA1 or GluA2 (red and green circles, respectively). The black curve represents 70% mobile AMPARs (kon = 1.5 s−1 and koff =0.1 s−1) plus 30% immobile AMPARs (kon = 1.5 s−1 and koff = 0 s−1). (C and D) To mimic disruption of AMPAR/scaffold interactions by intracellular peptides, kon was set to zero at t = 10 s, resulting in rapid AMPAR loss from the synapse. All AMPARs were considered either mobile (koff = 0.1 s−1; red) or 60% mobile and 40% immobile (koff = 0 s−1; green). AMPAR levels at control synapses (gray) slightly increase because AMPARs displaced by peptide competition redistribute among neighboring synapses.
Interestingly, such a stable AMPAR population was also apparent in patch-clamp recordings, where a quick and persistent 50% drop in AMPA-mediated EPSCs was observed upon application of peptides that acutely disrupt the stargazin/PSD-95 interaction (20). To mimic these experiments, we set kon to zero at a given time. If AMPARs were all considered mobile, AMPAR level at the postsynapse dropped to almost zero (Fig. 2 C and D and Movie S3). To reach a 50% reduction in synaptic AMPAR level as observed in experiments, we introduced a 40% immobile AMPAR fraction compatible with FRAP data. The characteristic time of decrease in AMPAR level (10 s) was roughly the inverse of koff, corresponding to AMPAR detachment from the PSD. Fitting the model with those different experiments thus allowed a fine-tuning of the kinetic parameters, which were used for further predictions.
Mapping AMPAR Distribution and Fluctuations at Synapses.
We first examined AMPAR distribution in extrasynaptic and synaptic compartments. AMPARs starting from initial random locations progressively accumulated at PSDs in a diffusion-limited manner (Fig. S6 and Movie S4). The steady-state AMPAR number at synapses was linearly related to the PSD area (Fig. 3 A and C), in quantitative agreement with EM data (29). Furthermore, the synaptic AMPAR enrichment (AMPARs bound to scaffold vs. free AMPARs) at equilibrium was strictly proportional to kon/koff (Fig. 3 B and D), which characterizes the binding affinity. Under basal conditions, the AMPAR density in the PSD was about 100-fold higher than in the extrasynaptic membrane, consistent with experimental estimates (30). To map the occupancy of PSD scaffolds, the 300 × 300-nm area corresponding to a single PSD was further decomposed into a grid of 20 × 20 pixels of 15 nm each, representing an array of 400 scaffold molecules. On average, 88% of the pixels were occupied by zero AMPAR, 11% by one AMPAR, and only 1% by two AMPARs (Fig. 3E), consistent with our hypothesis that scaffolds were not saturated by AMPARs.
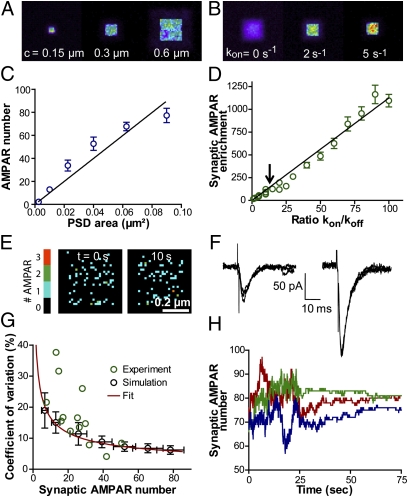
Fig. 3.
Mean and fluctuations of AMPAR number at synapses. (A and B) Heat maps of AMPAR distribution in the plasma membrane, calculated for different PSD sizes and kon values, respectively. The intensity corresponds to the number of times each pixel was visited by an AMPAR during a 100-s period. (C) AMPAR number per synapse as a function of PSD area (mean ± SEM, 10 PSDs). For larger PSDs, there is an inflection in the curve because of limited numbers of available AMPARs to populate synapses in the simulations. (D) Ratio between AMPAR density at the PSD vs. extrasynaptic space as a function of kon/koff (mean ± SEM, 10 PSDs). The arrow points to the AMPAR enrichment corresponding to kon = 1.5 s−1 and koff = 0.1 s−1. (E) Fluctuations of AMPAR number at a single PSD over time. The number of AMPARs per pixel is color coded. (F) AMPAR-mediated EPSCs upon ionotophoretic application of glutamate at synapses. Several recordings are superimposed. Note a larger scatter for the synapse showing the smaller average AMPAR current. (G) The mean and variance in AMPAR number at synapses were calculated for different AMPAR densities in the simulations (25–125 AMPAR/μm2). CV plotted vs. the mean synaptic AMPAR number (black circles). The red curve represents a fit with the equation √P(1 − P)/n, expected from a binomial distribution, where n is the average synaptic AMPAR number and P is the probability for an AMPAR to be in a synapse (P = 0.65, taken as the ratio between synaptic and total AMPAR numbers). Green circles represent electrophysiology data. (H) Reduction in the fluctuations of AMPAR number at PSDs upon mimicking cross-linking by lowering koff (0.02 s−1) and Dout (0.0002 μm2/s) simultaneously (at t = 25 s). Three representative curves are shown.
We also looked at the fluctuations of AMPAR numbers at synapses per unit of time, due to continuous diffusional exchange. First, we varied the density of receptors in the simulations (10–125 μm−2) and predicted that the coefficient of variation (CV) decreased inversely to the square root of the synaptic AMPAR number (Fig. 3G). Assuming that AMPA EPSCs are proportional to AMPAR number at PSDs, this result is consistent with glutamate iontophoresis experiments, where higher scatter was seen for weaker synapses (Fig. 3 F and G). Second, to mimic the reduction in the fluctuations of synaptic AMPA currents induced by antibody cross-link of surface AMPARs (21), we decreased Dout and Din according to experimental values (0.0002 μm2/s) (26, 31) and simultaneously lowered koff by fivefold (0.02 s−1) to take into account the fact that apparent bond lifetimes increase upon multimerization (32). Under these conditions, the average AMPAR number was unchanged, but the CV dropped from 7.0 ± 0.4% to only 1.2 ± 0.2% (mean ± SEM of five PSDs, P < 0.0001 by paired Student t test; Fig. 3H and Movie S5). This difference corresponds closely to the drop in the CV of AMPA EPSCs, without a change in amplitude, as observed upon cross-linking (21). Taken together, these data suggest that a significant fraction of the scatter in AMPAR synaptic transmission can be attributed to fluctuations in the number of surface-diffusing AMPARs. Other sources of fluctuations might include postsynaptic changes in channel conductance and open probability, as well as presynaptic variations in vesicle size and glutamate content (33).
Effects of Exocytosis and Enhanced Trapping on Synaptic AMPAR Level.
To reveal the impact of vesicular recycling on AMPAR dynamics both in basal conditions and in response to synaptic stimulation, we introduced discrete exo/endocytic events into the simulations. To describe synaptic potentiation, we took into account increases in AMPAR exocytosis and AMPAR binding to scaffold proteins, both linked to the phosphorylation of GluA1 and stargazin C-terminal domains, as well as increases in synapse size (1, 34).
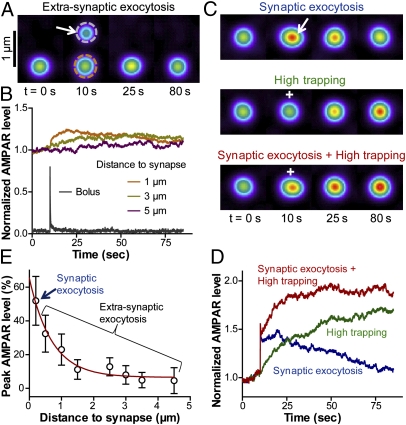
To simulate enhanced exocytosis, a bolus of 50 AMPARs, which lies in the range of experimental estimates (4, 7), was superimposed at a given time to an already populated 2D space containing five synapses. When AMPARs were inserted at extrasynaptic locations (0.5–5 μm from synapses), they instantly diffused throughout the membrane, and showed slight accumulation at nearby synapses in a distance-dependent manner (Fig. 4 A and B and Movie S6). These predictions are in agreement with small increases in GluA1-pHluorin signal (10–20%) at synapses located near the exocytic spot (4, 6). In contrast, when the same AMPAR bolus was inserted within the synapse, AMPAR density at the PSD was immediately much higher, and persisted for a fairly long time (Fig. 4 C and D and Movie S7). If binding parameters were kept constant, the signal eventually went back to baseline, corresponding to detachment of AMPARs from the PSD. Overall, the relative increase in synaptic AMPAR level contributed by exocytosis decreased exponentially with respect to the distance between the exocytic locus and the PSD (Fig. 4E).
Fig. 4.
Effect of exocytosis and trapping on AMPAR accumulation at the PSD. (A) Time-lapse images of extrasynaptic exocytosis, where a bolus of 50 AMPARs was delivered 1 μm from a synapse (arrow). (B) The distance between the locus of exocytosis and synapses was varied between 1 and 5 μm, and AMPAR level was measured in synapses (color) or at the exocytosis location (gray), both normalized by the basal synaptic AMPAR level. (C) Time-lapse images of simulated experiments, where the AMPAR bolus was delivered in the synapse 0.2 μm from the PSD (arrow), kon was doubled (plus sign), or both modifications were applied simultaneously. (D) Normalized synaptic AMPAR level over time, for the three conditions indicated in C. (E) Initial increase in synaptic AMPAR level as a function of the distance between the exocytic zone and the PSD (mean ± SD, 16 simulations). The red curve is an exponential decay fitting the data.
To mimic enhanced AMPAR/scaffold binding, we increased kon (Fig. 4 C and D and Movie S8) or decreased koff (Fig. S7 A and B), resulting in a higher steady-state AMPAR enrichment at the PSD within a longer time course than the one obtained with exocytosis alone. Importantly, a rapid and stable increase in AMPAR content at the PSD was obtained only if exocytosis was triggered at the synapse and kon was simultaneously increased (Movie S9). Thus, the model suggests that the rapid phase of synaptic potentiation is accounted for by transient exocytosis within or very close to the synapse, whereas the plateau is dictated by a persistent increase in trapping strength. Alternatively, long-term synaptic potentiation could also be simulated by increasing the size of the PSD (Fig. S7 C and D) to mimic an increase in the number of AMPAR binding slots (1).
Effects of Basal Endocytosis on AMPAR Distribution.
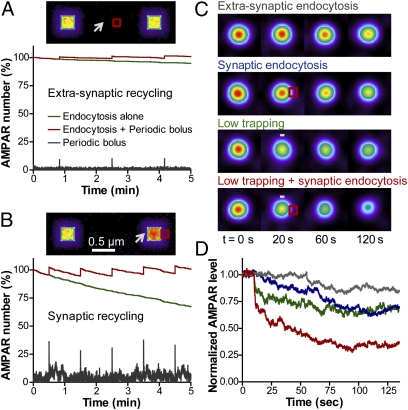
To address the role of AMPAR endocytosis in basal conditions and upon synaptic depression, we introduced regularly spaced EZs of dimensions 150 × 150 nm, one adjacent to the PSD and one between two synapses (10, 11) (Fig. 5 A and B and Fig. S8A). Endocytosis was considered as a sink governed by the rate constant kendo (s−1). AMPAR density at the cell surface decreased linearly over time, at a speed dictated by kendo (Fig. S8 B and C). The curve obtained with kendo = 0.1 s−1 closely corresponded to AMPAR internalization rates measured by antibody-feeding experiments (i.e., 10–20% in 5 min) (10, 11, 35, 36). Strikingly, AMPAR loss from the cell surface was about fivefold more rapid when endocytosis was induced within synapses than extrasynaptically (Fig. 5 A and B and Fig. S8 B and C), consistent with long-range AMPAR diffusion being rate limiting. To assess the importance of the localization of AMPAR recycling events, we compensated constitutive AMPAR endocytosis by periodic exocytosis with a frequency adjusted to experimental values (4, 6), thus restoring steady-state AMPAR density (Fig. 5 A and B). For synaptic endocytosis, which caused a rapid loss in surface AMPARs, the bolus was adjusted to 33 AMPARs, whereas for extrasynaptic endocytosis, which induced a slower decrease in AMPARs, the bolus contained only 10 AMPARs. Synaptic recycling resulted in a 24 ± 1% greater AMPAR enrichment (n = 6) compared with nonrecycling synapses, suggesting that local synaptic recycling is important for AMPAR accumulation at PSDs.
Fig. 5.
Effect of endocytosis and trapping on AMPAR distribution at the PSD. (A and B) AMPAR endocytosis at EZs (red squares) was counterbalanced by periodic exocytic events (arrows). Images show the AMPAR distribution for extrasynaptic (A) or synaptic recycling (B). Note AMPAR enrichment at the PSD in the latter case. The graphs show the overall decrease of AMPAR surface density by endocytosis alone (green), and the steady-state AMPAR level (red) restored by periodic exocytosis (gray peaks). (C) Images illustrating changes in AMPAR level in four different conditions, imposed at t = 10 s: kendo was increased 10-fold above baseline either at extrasynaptic (gray) or synaptic (blue) EZs; kon was decreased twofold (minus sign, green); or both kendo was increased and kon decreased simultaneously (red). (D) Synaptic AMPAR level vs. time for the four conditions illustrated in C.
Effect of Acute Endocytosis and Reduced Trapping on Synaptic AMPAR Level.
To describe synaptic depression, one has to consider increases in AMPAR endocytosis triggered by phosphatase signaling and decreases in AMPAR binding to AMPA receptor binding protein (ABP)/GRIP and PSD-95—induced by phosphorylation of GluA2 and stargazin, respectively—as well as reductions in synapse size (1, 34). To simulate enhanced AMPAR endocytosis, we raised kendo by 10-fold above the basal rate (37). When endocytosis was induced extrasynaptically, the decrease in AMPAR level at the PSD was fairly slow (Fig. 5 C and D), as observed for basal endocytosis. Strikingly, the loss in AMPAR level was more pronounced and synapse specific when endocytosis was induced within the synapse. To mimic a sudden reduction in AMPAR/scaffold binding affinity, kon was decreased (Fig. 5 C and D) or koff was increased (Fig. S7 A and B), resulting in a drop of AMPAR level within seconds, to a plateau below baseline. This effect was due to the fact that AMPARs detaching from the PSD can escape the synapse by diffusion. Interestingly, an increase in the diffusion of synaptic AMPARs has been observed by SPT upon induction of chemical LTD (17), possibly reflecting AMPAR/scaffold detachment. When synaptic endocytosis was combined with a decrease in kon, the effects added to each other and resulted in both rapid and sustained decrease in AMPAR level, to about 50% below baseline (Fig. 5 C and D and Movie S10). Thus, the model predicts that the rapid loss of AMPAR from synapses is due to a decrease in trapping force, whereas the persistent decrease in AMPARs is due to endocytosis. Alternatively, synaptic depression could also be simulated by reducing the size of the PSD (Fig. S7 C and D) to mimic a decrease in the number of AMPAR binding slots.
Discussion
This framework allows spatial and temporal discrimination of the roles of AMPAR diffusion, trapping, and exo/endocytosis in regulating AMPAR dynamics at synapses. The model shows that AMPAR surface diffusion is an obligatory link between internal recycling and trapping at the PSD, and clarifies the impact of recycling events compared with changes in AMPAR/scaffold affinity or PSD size in synaptic plasticity. The main prediction is that synapses endowed with internal endocytic and exocytic recycling mechanisms are privileged over other synapses, because they can efficiently regulate their AMPAR level in response to potentiation or depression stimuli.
Intrinsic AMPAR Dynamics at Synapses.
This model allowed a clear distinction of diffusion and binding events, which are generally hidden in FRAP curves (23). By fitting simulations to both SPT and FRAP data, we estimated the AMPAR/scaffold reaction rates. The overall AMPAR/scaffold dissociation rate (koff = 0.1 s−1) is about 10-fold lower than the value measured by surface plasmon resonance between single PDZ domains and PDZ binding motifs (38). This longer lifetime measured in live neurons is likely to reflect an increased avidity coming from the simultaneous binding of AMPARs or TARPs to multiple PDZ domains (20, 32).
The model predicted that in young neurons a majority of AMPARs exchanged within tens of seconds between synaptic and extrasynaptic compartments. In contrast, older neurons were characterized by a lower koff (0.02 s−1), or the appearance of a stable AMPAR population. Because stargazin peptides that specifically compete with class I PDZ scaffold proteins, including PSD-95 and SAP-97, did not completely abolish AMPA EPSCs (20), stable AMPAR anchoring might involve higher valencies or other molecules, e.g., class II PDZ scaffold proteins such as ABP/GRIP or PICK1 (18), the actin-binding protein 4.1N (6), or the adhesion protein N-cadherin involved in synaptic maturation (39). Stable AMPARs may also bear specific subunit composition: GluA1 and GluA2 subunits exhibited similar recovery curves, but it is possible that GluA3- or GluA4-containing AMPARs have slower kinetics. The appearance of a stable fraction may also be linked to geometrical effects of dendritic spine maturation, in which the neck acts as a diffusion barrier (15, 23).
Our model also predicts that under basal conditions, AMPARs are about 100-fold enriched at PSDs compared with the extrasynaptic space. This result is consistent with glutamate iontophoresis experiments (21, 40) and with immunogold labeling of surface AMPARs after freeze fracture electron microscopy (30). However, these values are higher than other estimates using immunolabeling (7) or overexpression of fluorescently tagged AMPAR subunits (15, 16). Most likely, those techniques are underestimating synaptic AMPAR enrichment, the former because of the difficulty for antibodies to access the crowded synaptic space, and the latter because it biases the stoichiometry of AMPARs to scaffold molecules. An alternative explanation would be the existence of AMPAR nanoclusters at the synapse (2). In addition, the model predicts that fluctuations in synaptic AMPAR numbers due to diffusional exchange are smaller for synapses containing more AMPARs, reflecting more reliable synaptic transmission.
AMPAR Exocytosis and Trapping in Synaptic Potentiation.
The issue of where AMPAR exocytosis takes place is controversial. Some studies reported essentially extrasynaptic AMPAR exocytosis (4–7), whereas others described AMPAR exocytosis intrasynaptically or near the synapse (8, 41, 42). In this debated context, our model shows that extrasynaptic exocytosis is relatively inefficient and unspecific in delivering AMPARs to synapses compared with synaptic exocytosis, because AMPARs rapidly dilute in the extrasynaptic membrane. In contrast, if AMPAR exocytosis occurs within or close to the synapse (<1 μm), trapping is efficient and fast (within seconds). Moreover, AMPAR accumulation persists for a relatively long time (1 min) because of slow AMPAR diffusion in the synapse, such that newly exocytosed AMPARs stay available for trapping. If, in addition to exocytosis, the AMPAR/scaffold affinity is increased, then the initial burst persists for minutes. Both types of kinetics were reported by observing GluA1-pHluorin accumulation at synapses upon chemical LTP (8, 42). The synaptic response could involve a selective exocytosis of GluA1-containing AMPARs providing the rapid enhancement in synaptic strength (4, 6), followed by a long-term trapping of GluA2-containing subunits through enhanced scaffold interactions (43). The model thus predicts a repertoire of LTP responses dictated by the relative contributions of AMPAR exocytosis, trapping, and changes in PSD size.
AMPAR Endocytosis and Trapping in Synaptic Depression.
AMPAR internalization was predicted to be threefold more efficient within the synapse than in the extrasynaptic space, consistent with the expression of dominant-negative dynamin-3, which displaces EZs away from synapses (10). In addition, constitutive recycling of AMPARs within the synapse resulted in stronger AMPAR enrichment compared with synapses with no recycling, matching the loss in synaptic AMPARs in neurons expressing the dynamin-3 mutant (10) or treated with endocytosis inhibitors (12). When extrasynaptic endocytosis was acutely triggered, it was again relatively inefficient in removing AMPARs from nearby synapses. This result matched the progressive decline of GluA1-pHluorin signal at synapses, following AMPAR endocytosis in the shaft upon NMDA treatment (44). Thus, an intrinsic limitation of NMDAR-dependent LTD may be a saturation of the endocytic capability, unable to capture fast-diffusing extrasynaptic AMPARs. In contrast, synaptic endocytosis resulted in a more-pronounced loss of AMPARs from synapses, in agreement with NMDAR-dependent LTD induced by electrical stimulation (36). Finally, when AMPAR/scaffold affinity was simultaneously reduced, the decrease was more rapid and reached a lower steady state than with endocytosis alone. This behavior is close to the drop in AMPA EPSCs observed within 1 min upon mGluR-dependent LTD (35). Together with changes in PSD size, the fine-tuning between these processes potentially gives rise to a panel of synaptic responses, according to the LTD stimulation protocol.
Materials and Methods
Experiments and model are described in SI Materials and Methods. Briefly, SPT, FRAP, and patch-clamp methods were described previously (16, 26, 31). Computer simulations of individual AMPAR movement, including diffusion, trapping at the PSD, and exo/endocytic events, were programmed using Mathematica software. Superimposition of trajectories to visualize AMPAR distribution over time was made using a custom program written in Metamorph.
Supplementary Material
Acknowledgments
We thank P. Scheiffele, S. Okabe, and K. Keinänen for plasmids; B. Tessier for molecular biology; J. Delgado, G. Giannone, Y. Humeau, D. Jullié, D. Perrais, and M. Sainlos for discussions; and C. Breillat, D; Bouchet, A. Frouin, and N. Retailleau for primary neurons. Funding for this study was provided by European Union Seventh Framework Program Grant 232942 Nano-Dyn-Syn, the Centre National de la Recherche Scientifique, Agence Nationale de la Recherche Projects Neuroligation and Synapse-2Dt, Conseil Régional Aquitaine, Fondation pour la Recherche Médicale, and ERA-Net NEURON (Moddifsyn).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109818109/-/DCSupplemental.
References
- 1.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 2.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lledo PM, Zhang X, Südhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 4.Yudowski GA, et al. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin DT, et al. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao-Cheng JH, et al. Trafficking of AMPA receptors at plasma membranes of hippocampal neurons. J Neurosci. 2011;31:4834–4843. doi: 10.1523/JNEUROSCI.4745-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lüscher C, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, et al. Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron. 2007;55:874–889. doi: 10.1016/j.neuron.2007.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrini EM, et al. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaskolski F, Mayo-Martin B, Jane D, Henley JM. Dynamin-dependent membrane drift recruits AMPA receptors to dendritic spines. J Biol Chem. 2009;284:12491–12503. doi: 10.1074/jbc.M808401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–374. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Ashby MC, Maier SR, Nishimune A, Henley JM. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J Neurosci. 2006;26:7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frischknecht R, et al. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009;12:897–904. doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- 17.Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 19.Opazo P, et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Sainlos M, et al. Biomimetic divalent ligands for the acute disruption of synaptic AMPAR stabilization. Nat Chem Biol. 2011;7:81–91. doi: 10.1038/nchembio.498. [DOI] [PubMed] [Google Scholar]
- 21.Heine M, et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shouval HZ. Clusters of interacting receptors can stabilize synaptic efficacies. Proc Natl Acad Sci USA. 2005;102:14440–14445. doi: 10.1073/pnas.0506934102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holcman D, Triller A. Modeling synaptic dynamics driven by receptor lateral diffusion. Biophys J. 2006;91:2405–2415. doi: 10.1529/biophysj.106.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earnshaw BA, Bressloff PC. Biophysical model of AMPA receptor trafficking and its regulation during long-term potentiation/long-term depression. J Neurosci. 2006;26:12362–12373. doi: 10.1523/JNEUROSCI.3601-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolle DP, Le Novère N. Brownian diffusion of AMPA receptors is sufficient to explain fast onset of LTP. BMC Syst Biol. 2010;4:25. doi: 10.1186/1752-0509-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondin M, et al. Neurexin-neuroligin adhesions capture surface-diffusing AMPA receptors through PSD-95 scaffolds. J Neurosci. 2011;31:13500–13515. doi: 10.1523/JNEUROSCI.6439-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 28.Nikonenko I, et al. PSD-95 promotes synaptogenesis and multiinnervated spine formation through nitric oxide signaling. J Cell Biol. 2008;183:1115–1127. doi: 10.1083/jcb.200805132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antal M, et al. Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J Neurosci. 2008;28:9692–9701. doi: 10.1523/JNEUROSCI.1551-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masugi-Tokita M, et al. Number and density of AMPA receptors in individual synapses in the rat cerebellum as revealed by SDS-digested freeze-fracture replica labeling. J Neurosci. 2007;27:2135–2144. doi: 10.1523/JNEUROSCI.2861-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heine M, et al. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci USA. 2008;105:20947–20952. doi: 10.1073/pnas.0804007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans EA, Calderwood DA. Forces and bond dynamics in cell adhesion. Science. 2007;316:1148–1153. doi: 10.1126/science.1137592. [DOI] [PubMed] [Google Scholar]
- 33.Lisman J, Raghavachari S. A unified model of the presynaptic and postsynaptic changes during LTP at CA1 synapses. Sci STKE. 2006;2006:re11. doi: 10.1126/stke.3562006re11. [DOI] [PubMed] [Google Scholar]
- 34.Henley JM, Barker EA, Glebov OO. Routes, destinations and delays: Recent advances in AMPA receptor trafficking. Trends Neurosci. 2011;34:258–268. doi: 10.1016/j.tins.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron. 2005;45:81–94. doi: 10.1016/j.neuron.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 38.Fournane S, et al. Surface plasmon resonance analysis of the binding of high-risk mucosal HPV E6 oncoproteins to the PDZ1 domain of the tight junction protein MAGI-1. J Mol Recognit. 2011;24:511–523. doi: 10.1002/jmr.1056. [DOI] [PubMed] [Google Scholar]
- 39.Saglietti L, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Cottrell JR, Dubé GR, Egles C, Liu G. Distribution, density, and clustering of functional glutamate receptors before and after synaptogenesis in hippocampal neurons. J Neurophysiol. 2000;84:1573–1587. doi: 10.1152/jn.2000.84.3.1573. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci USA. 2008;105:11388–11393. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci USA. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passafaro M, Piëch V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- 44.Ashby MC, et al. Removal of AMPA receptors (AMPARs) from synapses is preceded by transient endocytosis of extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.