Abstract
Development of type 1 diabetes in the nonobese diabetic (NOD) mouse is preceded by an immune cell infiltrate in the pancreatic islets. The exact role of the attracted cells is still poorly understood. Chemokine CCL2/MCP-1 is known to attract CCR2+ monocytes and dendritic cells (DCs). We have previously shown that transgenic expression of CCL2 in pancreatic islets via the rat insulin promoter induces nondestructive insulitis on a nonautoimmune background. We report here an unexpected reduction of diabetes development on the NOD background despite an increased islet cell infiltrate with markedly increased numbers of CD11c+ CD11b+ DCs. These DCs exhibited a hypoactive phenotype with low CD40, MHC II, CD80/CD86 expression, and reduced TNF-α but elevated IL-10 secretions. They failed to induce proliferation of diabetogenic CD4+ T cells in vitro. Pancreatic lymph node CD4+ T cells were down-regulated ex vivo and expressed the anergy marker Grail. By using an in vivo transfer system, we show that CD11c+ CD11b+ DCs from rat insulin promoter-CCL2 transgenic NOD mice were the most potent cells suppressing diabetes development. These findings support an unexpected beneficial role for CCL2 in type 1 diabetes with implications for current strategies interfering with the CCL2/CCR2 axis in humans, and for dendritic cell biology in autoimmunity.
Keywords: monocyte chemotactic protein-1, immune tolerance, BDC2.5, myeloid DC, B-7
Cellular infiltration of the insulin-producing islets of Langerhans precedes the onset of diabetes in nonobese diabetic (NOD) mice (1, 2). Destruction of the islet β-cells accompanies progression to overt disease and is associated with a shift from a Th2 to Th1 CD4+ T-cell response (1, 3). Antigen-presenting cells (APCs) are necessary for antigen-specific activation of T cells. Macrophages and dendritic cells (DCs) are the first hematopoietic cells detected around islets (2, 4), but the precise role of APCs in the pathogenesis of type 1 diabetes (T1D) is not fully understood. APCs from peripheral blood have been shown to be defective in patients with T1D (5). Chemotaxis to sites of inflammation is remarkably impaired in NOD mice (6). NOD APCs display also various other functional abnormalities, including maturation, cytokine production, and signaling (7–11). Furthermore, in vivo modulation of APC function, e.g., via TNF-α, TGF-β, or GM-CSF, alters diabetes susceptibility depending on the expression and duration of the modulating factor (12–15). More recent studies from Katz and coworkers showed that unmanipulated DCs, depending on the subset, can positively or negatively influence diabetes development (16, 17). Interestingly, a recently described DC subtype called merocytic DC was shown to be pathogenic in the NOD mouse (16).
APCs express high levels of the chemokine receptor 2 CCR2 (18, 19). CCR2 induces monocyte emigration from the bone marrow (20). CCL2 (also known as monocyte chemotactic protein-1 or MCP-1) is one of several ligands for CCR2. CCL2 efficiently recruits monocytes in vitro and in vivo, and reduces IL-12 secretion during infection (21). CCR2 is also necessary for DC maturation (22) and accumulation of inflammatory DCs in lymph nodes (23). The various functions of the CCR2/CCL2 system in APCs appear therefore to be cell type- and context-dependent.
In addition to its pleiotropic roles in APCs, CCL2 has been shown to stimulate IL-4 secretion from activated, CCR2-expressing T cells (24). Thus, CCL2 creates an environment that favors Th2 development by suppressing monocyte-derived IL-12 and enhancing T cell-derived IL-4 production. This notion has been supported by analyses of CCL2-deficient mice (25, 26), but not CCR2-deficient mice (27), possibly because there are several ligands for this receptor. The CCL2/CCR2 axis is also known to promote Th1-driven experimental autoimmune encephalitis via recruitment of an inflammatory infiltrate into the central nervous system (28–30). Thus, the function of CCR2/CCL2 in T helper cell differentiation and its disease states is also context- and model-dependent as summarized in an earlier work (18).
This is further illustrated by the fact that neutralization of CCL2 protects mice from mortality in a model of lethal endotoxemia (31), whereas systemic expression causes susceptibility to intracellular pathogens in a transgenic (tg) model (32). It is thought that disruption of natural chemokine gradients or desensitization of monocytes is responsible for the marked differences between local and systemic expression of CCL2 (33), further adding to the complex biology of CCL2.
The role of the CCL2/CCR2 system in the pathogenesis of T1D is incompletely understood. IL-1 was shown to up-regulate CCL2 in rat and human islet β-cells (34, 35). NOD mouse-derived β-cells exhibit an age-related increase in CCL2 mRNA expression peaking at 8 wk of age (34), which contrasts with the nadir of CCL2 expression in pancreatic LNs of NOD mice at 8 wk of age (roadmap of T1D NOD microarray, http://fathmanlab.stanford.edu). The functional significance of these correlative data remain unknown. Genetic ablation of the receptor CCR2 delays onset of diabetes in NOD mice but does not significantly reduce disease incidence at 30 wk (36).
To better understand the biology of locally expressed CCL2, we genetically engineered this chemokine to be selectively expressed in the insulin-producing pancreatic β-cells from nonautoimmune mice (33). Tg CCL2 expression using the rat-insulin promoter (RIP) recruited a monocyte-rich infiltrate in the islets. Chronic islet infiltrates were composed of F4/80+ monocytes with minor populations of CD4+, CD8+, and B220+ cells (33). Despite persistent transgene expression, the insulitis never progressed, and blood glucose levels remained normal (33). Islet-specific CCL2 expression on the C57BL/6 × DBA background does lead to diabetes (37), but this strain does not reflect the functional abnormalities of APCs from NOD mice and patients with T1D as described earlier. We show here that locally produced CCL2 in the pancreatic islets of NOD mice induces a prominent immune cell infiltrate but unexpectedly prevents disease development. We demonstrate further that the potential mechanism of protection is mediated by tolerogenic CD11c+ CD11b+ DCs in vivo that are hypoactive and inhibit diabetogenic T-cell proliferation in vitro.
Results
RIP-CCL2 tg NOD Mice Develop Less Diabetes Despite a Denser Islet Cell Infiltrate.
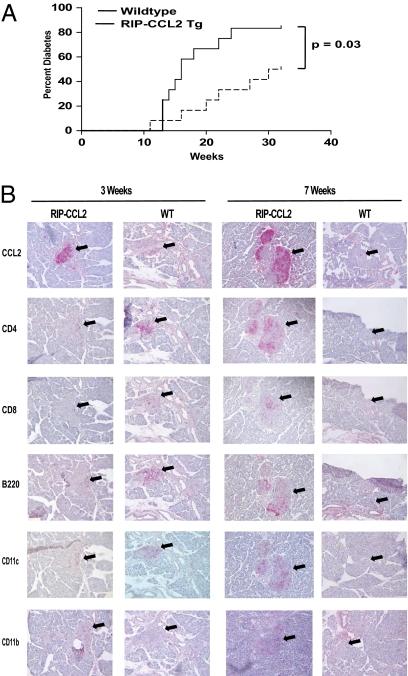
To investigate the impact of islet cell-specific expression of CCL2 on the pathogenesis of T1D, we have fully crossed the RIP-CCL2 tg mice onto the NOD background. We first monitored the incidence of diabetes in NOD RIP-CCL2 tg mice vs. NOD littermate controls. We found that tg expression of CCL2 unexpectedly exerted a protective effect on the disease process. NOD RIP-CCL2 tg mice developed significantly less diabetes in comparison with WT littermate controls (Fig. 1A).
Fig. 1.
Diabetes incidence and histology of islet infiltrates in RIP-CCL2 tg NOD mice. (A) Diabetes incidence of female RIP-CCL2 tg and WT NOD mice; 12 mice were monitored for diabetes development until 32 wk of age (x axis). The percentage of mice that turned diabetic is depicted on the y axis. Cumulative incidences of WT and tg mice were 83.3% and 50%, respectively (P = 0.03, log-rank test). (B) Immunohistochemistry of pancreatic islets from RIP-CCL2 tg and WT NOD mice. Pancreata were prepared and stained as described in Materials and Methods. CCL2, CD4, CD8, B220, CD11c, and CD11b staining is shown at 3 wk of age (Left) and 7 wk of age (Right). Black arrows point to the area where islets are located.
We next examined the pancreata of WT and tg NOD mice for histologic differences at various time points during evolution of insulitis, that is, at 3, 5, 7, and 9 wk of age. As expected, CCL2 staining was very bright at any time point within the islets of tg compared with WT littermate controls (Fig. 1B). As seen on a nonautoimmune background expressing the RIP-CCL2 transgene (33), the islet cells of tg NOD mice were more heavily infiltrated than in control NOD mice (Fig. 1B). However, the infiltrate was more diverse, comprised of T cells, B cells, macrophages, and DCs. Insulitis scores were consistently higher in tg NOD mice at every time point tested despite the decreased incidence of diabetes (Fig. 1A). Absolute cell numbers from pancreatic and lymphoid tissues of 7- to 8-wk-old mice were also counted. Total numbers of mononuclear cells were increased threefold in tg pancreata (Table 1), whereas total cell numbers in spleen and inguinal, axillary, and pancreatic lymph nodes were only marginally elevated (∼1.1–1.4 times; Table 1). Whereas pancreatic CD4+ T cells were increased only threefold, the numbers of APCs in pancreatic tissue were markedly increased compared with controls. CD11c+ cells were increased more than 10 times in tg NOD mice (from 4,000 to 46,000; average of 10 pooled mice). CD11b+ and CD11b/Gr-1 (Ly-6C)+ cells were increased fivefold (41,000 vs. 205,000, and 9,000 vs. 46,000, respectively; Table S1). Relative numbers of pancreatic DCs were also markedly increased (Fig. S1A).
Table 1.
Absolute cell numbers in pancreas, pancreatic LN, inguinal LN, and spleen of 7- to 8-wk-old RIP-CCL2 tg NOD mice and littermate controls
| Location | WT | Tg | Fold change |
| Pancreas, ×104 | 4.13 ± 0.95 | 12.5 ± 2.22 | 3.0* |
| Pancreatic LN, ×106 | 4.47 ± 0.11 | 6.27 ± 0.18 | 1.4 |
| Inguinal LN, ×106 | 9.46 ± 0.10 | 11.97 ± 0.06 | 1.3 |
| Spleen, ×106 | 85.46 ± 6.14 | 96.33 ± 11.73 | 1.1 |
Six to eight RIP-CCL2 tg NOD mice and littermate controls (WT) were pooled in each experiment. Numbers represent averages from absolute cell counts of three or four independent experiments ± SEM. Fold change represents relative increase of absolute cell numbers in tg vs. WT.
*Statistically significant change.
CD11c+ CD11b+ DCs Are Markedly Increased in Pancreatic Lymph Nodes of RIP-CCL2 tg NOD Mice.
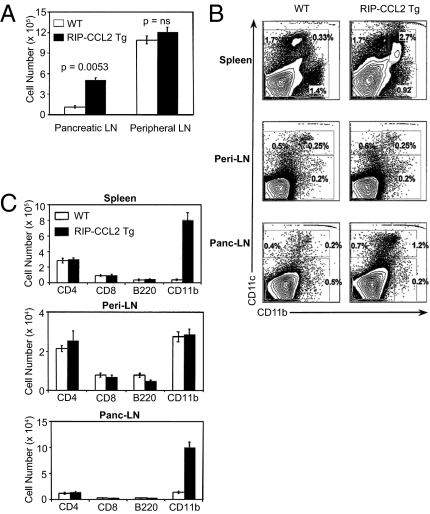
Given the 10-fold increase of CD11c+ cells in the pancreatic tissue, we examined DC numbers in the pancreatic lymph nodes of tg animals, the site of autoreactive T-cell priming (38), and of DC presentation of pancreatic antigens that are transported to this draining lymph node (39). Assuming that these APCs are important for disease protection, we expected a similar disproportional increase despite the marginal increase of total cell numbers in pancreatic lymph nodes compared with pancreatic tissue (1.5 vs four times). Indeed, absolute numbers of DCs were also 10 times increased in pancreatic, but not peripheral, lymph nodes (Fig. 2A). Interestingly, CD11c+ CD11b+ DCs accumulated also in spleens of tg NOD mice (Fig. 2 B and C), suggesting a partially systemic effect of islet cell-expressed CCL2. When examining DC subsets other than the myeloid CD11b+ subtype, we did not find significant differences between tg NOD mice and littermate controls in pancreatic LNs, peripheral LNs, or spleens (Fig. 2C and Fig. S1B). Specifically, absolute and relative numbers of CD11c+ CD8α+ or B220+ cells were not increased in tg NOD mice (Fig. 2C and Fig. S1B), suggesting that these DC subsets are not involved in inhibition of autoimmunity.
Fig. 2.
DC numbers and subtypes in pancreatic LNs, peripheral LNs, and spleens of RIP-CCL2 tg NOD mice. (A) Absolute cell numbers of CD11c+ CD11b+ DCs from pancreatic and peripheral LNs of WT (white columns) and RIP-CCL2 tg (black columns) NOD mice. Bars represent absolute numbers ×105 from an average of three independent experiments. Error bars represent SEM. P values are shown above each bar. (B) Relative numbers of CD11c+ CD11b+ DCs from spleen, peripheral LN (peri-LN), and pancreatic LN (panc-LN). Tissues were disrupted, and total cells were isolated and stained as described in SI Materials and Methods. FACS contour plots of CD11b and CD11c double staining of cells from WT (Left) and RIP-CCL2 tg (Right) NOD mice are shown. Plots from one representative experiment among three from each group are shown. Percentages of CD11b+/CD11c−, CD11b+/CD11c+, and CD11b−/CD11c+ cells are depicted within each quadrant. (C) Absolute numbers of CD11c+ CD11b+ DCs are selectively increased in RIP-CCL2 tg NOD mice. CD11c+ cells from spleen, peripheral LNs (peri-LN), and pancreatic LNs (panc-LN) from WT (white columns) and RIP-CCL2 tg (black columns) NOD mice were double-stained with CD4, CD8α, B220, and CD11b. Each column represents the average absolute numbers from three independent experiments. Error bars represent SEMs.
CD11c+ CD11b+ DCs from RIP-CCL2 tg NOD Mice Are Hypoactive and Fail to Induce Autoreactive T-Cell Proliferation in Vitro.
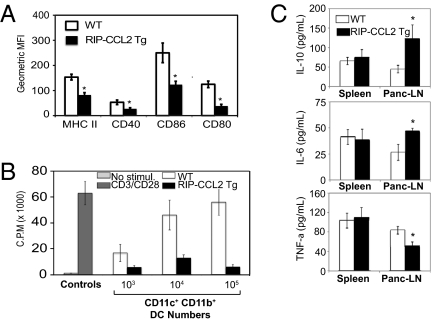
As we observed a significantly decreased incidence of diabetes in RIP-CCL2 tg NOD mice, we speculated that the CD11c+ CD11b+ DCs in pancreatic LNs might not be functionally active. Indeed, phenotypic markers of DC activation (CD40, MHC II) were down-regulated in RIP-CCL2 tg NOD mice (Fig. 3A and Fig. S2A). The costimulatory molecules CD80 and CD86 were also not up-regulated on DCs from pancreatic LNs of tg animals (Fig. 3A and Fig. S2A). On the contrary, the activation state of B220+ and CD8α+ DC subsets were equal to controls (Fig. S2B). In addition, expression of activation and costimulatory markers on CD11c− CD11b+ macrophages from pancreatic LNs, spleens, or inguinal LNs were also not different between WT and tg (Fig. S2C). These findings suggested that specifically CD11c+ CD11b+ DCs are hypoactive and might be incapable of stimulating diabetogenic CD4+ T cells. We therefore examined their stimulatory capacity in an in vitro proliferation assay using TCR-tg BDC2.5 CD4+ T cells and their cognate antigen, the MHC class II-restricted BDC2.5 peptide (40, 41). Whereas DCs isolated from WT pancreatic LNs induced robust proliferation of T cells in a dose-dependent manner, DCs from tg mice failed to do so at every cell number tested (Fig. 3B). Importantly, in vitro activated CD11c+ CD11b+ DCs from pancreatic LNs of tg NOD mice secreted significantly higher amounts of the immunosuppressive cytokine IL-10 (Fig. 3C). These DCs were also defective in TNF-α secretion but secreted more IL-6 (Fig. 3C), suggesting an overall dysfunctional state.
Fig. 3.
CD11c+ CD11b+ DCs from RIP-CCL2 tg NOD mice are phenotypically and functionally hypoactive. (A) Activation and costimulatory markers on DCs from WT and tg NOD mice. CD11c+ CD11b+ DCs from PLNs of 12-wk-old WT and RIP-CCL2 tg NOD mice were stained ex vivo for MHC class II, CD40, CD80, and CD86, respectively. All markers are consistently down-regulated in RIP-CCL2 tg NOD mice. Shown are bar graphs of the average geometric mean fluorescence intensity (MFI) from three independent experiments. Error bars represent SEM. P values less than 0.05 are marked with an asterisk. (B) DCs from RIP-CCL2 tg NOD mice are functionally impaired in vitro. Cocultures of BDC2.5 TCR tg CD4+ T cells with NOD CD11c+ CD11b+ DCs and BDC2.5 peptide were performed as described in SI Materials and Methods. BDC2.5 CD4+ T cells (1 × 105) were incubated with 103, 104, and 105 DCs obtained from pancreatic LN of 12-wk-old RIP-CCL2 tg (black columns) and WT (white columns) NOD mice. Control DCs and T cells from C57BL/6 mice (gray columns) were stimulated with αCD3 and αCD28 as described in SI Materials and Methods. Cell proliferation was measured by [3H]thymidine incorporation and is shown as cpm on the y axis. Each column represents the average of three independent experiments. Error bars represent SEM. (C) Cytokine secretion of LPS-stimulated CD11c+ CD11b+ DCs from WT and RIP-CCL2 tg NOD mice. Splenic and pancreatic LN DCs were isolated and stimulated in vitro with LPS as described in SI Materials and Methods. IL-10 (Top), IL-6 (Middle), and TNF-α (Bottom) levels were measured in the supernatant after 36 h of stimulation by ELISA. Each column represents the average of three independent experiments. Error bars represent SEM. P values less than 0.05 are marked with an asterisk.
CD4+ T Cells from Pancreatic LNs of RIP-CCL2 tg NOD Mice Express Reduced IL-2 and Detectable RNA Levels of Anergy Marker Grail.
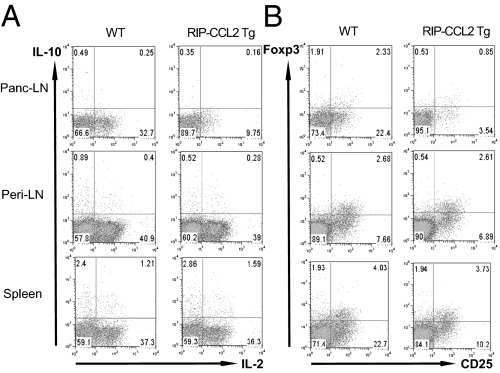
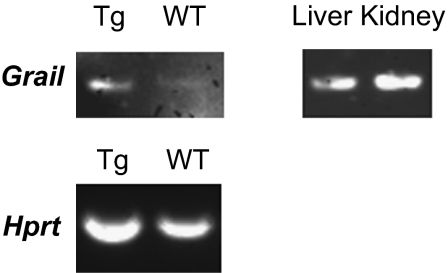
Based on the markedly insufficient stimulatory capacity of DCs from RIP-CCL2 tg NOD mice, we hypothesized that T cells from pancreatic LNs would also display a hypoactive phenotype. To this end, we performed flow cytometric analysis of various intracellular cytokines in CD4+ T cells from pancreatic LNs, peripheral LNs, and spleens. IL-2 was expressed at lower levels in tg than WT NOD mice (Fig. 4A). We could not identify a significant shift in Th1, Th2, or Th17 cytokine profiles in pancreatic LNs, suggesting that the helper-type phenotype remained unaffected (Fig. S3A; CD8+ T cell phenotype is shown in Fig. S3B). However, consistent with suppressed IL-2 secretion, its high-affinity receptor, the IL-2Rα chain CD25, was also down-regulated in CD4+ T cells from pancreatic LNs, and to a lesser degree also from spleens (Fig. 4B and Fig. S3C). In addition, T cells appear also less activated within the pancreatic islets as CD62L, a marker least altered by collagenase treatment (42), is increased on cells from RIP-CCL2 tg NOD mice (Fig. S3D). Of note, the regulatory T cell marker Foxp3 was expressed at slightly lower levels in both PLN CD25+ and CD25− CD4+ T cells (Fig. 4B) suggesting that Foxp3+ regulatory T cells are not induced by the IL-10–secreting, immature-appearing DCs. Instead, messenger RNA of the T-cell anergy and quiescence marker Grail (43–45) was found to be expressed in pancreatic LN CD4+ T cells of RIP-CCL2 tg NOD mice (Fig. 5 and Fig. S4). These findings suggested that the hypoactive DCs likely induced an anergic or quiescent T-cell state in situ.
Fig. 4.
CD4+ T cells from pancreatic LNs of RIP-CCL2 tg NOD mice are less activated than WT CD4+ T cells. (A and B) CD4+ T cells from pancreatic LN (panc-LN), peripheral LN (peri-LN), and spleen of WT (Left) and RIP-CCL2 tg (Right) NOD mice were stained intracellularly for IL-10 and IL-2 (A), and Foxp3 together with surface staining of CD25 (B) after PMA/ionomycin stimulation for 4 h as described in SI Materials and Methods. FACS scatter plots are shown for each tissue. Percentages of relative numbers from three to six pooled mice each are depicted within each quadrant.
Fig. 5.
Grail expression in CD4+ pancreatic LN T cells from RIP-CCL2 NOD mice. RT-PCR from pancreatic LN CD4+ T cells of WT and RIP-CCL2 tg NOD mice was performed as described in SI Materials and Methods. Anergy gene Grail and housekeeping gene Hprt were amplified. Grail mRNA was detectable only in T cells from RIP-CCL2 tg mice after 40 cycles of amplification. Liver and kidney control tissues that constitutively express Grail mRNA are shown on the right. Each band is representative of 10 pooled mice. Densitometric analyses are shown in Fig. S4.
Transfer of CD11c+ CD11b+ DCs from RIP-CCL2/BDC2.5 TCR tg NOD Mice into NOD Mice Delays Diabetes Onset in Vivo.
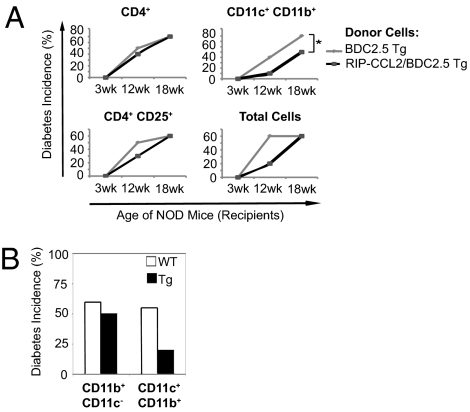
Given the functionally impaired DCs and consecutively down-regulated CD4+ T cells in RIP-CCL2 tg NOD mice, we speculated that CD11c+ CD11b+ DCs are suppressive upon transfer in vivo. To this end, we used the BDC2.5 TCR tg system and crossed the RIP-CCL2 tg NOD mice with BDC/NOD tg mice to transfer various sorted cell populations into female NOD mice including BDC2.5 antigen-specific T cells. NOD mice were monitored after i.v. transfer for diabetes development over time. Although CD4+ CD25+ T cells had a partially protective effect, transfer of CD11c+ CD11b+ DCs from tg mice conferred the most profound and longest-lasting protection in NOD mice (Fig. 6A). Of note, CD11b+ CD11c− macrophages/monocytes did not mediate protection upon transfer (Fig. 6B). In vivo titration experiments revealed a biologic, dose-dependent protective effect of CD11c+ CD11b+ DCs on diabetes development (Fig. S5). These results support the notion that the attracted DCs in RIP-CCL2 tg NOD mice are mechanistically the crucial cell type, as they are not only down-regulatory in vitro but also suppressive in vivo. Treatment of WT NOD CD11c+ CD11b+ DCs in vitro with CCL2 did not induce the immature phenotype described earlier. This suggests that the in vivo tolerance in the RIP-CCL2 tg NOD mice might be a result of the chemotactic properties of CCL2 on immature DCs and not an immunomodulatory effect of CCL2 that directly acts on mature DCs, although such mechanisms are not mutually exclusive and need to be tested in future studies.
Fig. 6.
CD11c+ CD11b+ DCs from RIP-CCL2/BDC2.5 tg NOD mice protect from diabetes in vivo upon transfer into female NOD mice. (A and B) Pancreatic LN cells from 10 pooled, 3-wk old BDC2.5 (gray lines) and RIP-CCL2/BDC2.5 double tg (black lines) NOD mice were isolated, and 1 million cells of each subpopulation was transferred into 3-wk-old NOD mice as described in SI Materials and Methods and Fig. S6. (A) Total pancreatic LN cells and subpopulations of CD11c+ CD11b+ DCs, CD4+ T cells, and CD4+ CD25+ T cells from BDC2.5 (gray lines) or RIP-CCL2/BDC2.5 double tg (black lines) NOD mice were injected i.v. into 3-wk-old WT NOD mice. Diabetes incidence was assessed at 12 and 18 wk, respectively. Incidences (in percentages) are shown for 3-, 12-, and 18-wk time points from a total of nine or 10 recipient mice in each group (*P = 0.0126, log-rank test). (B) CD11b+ CD11c− macrophages and CD11c+ CD11b+ DCs isolated from 3-wk-old BDC2.5 (white bars; WT) and RIP-CCL2/BDC2.5 double tg (black bars; Tg) NOD mice were injected into 3-wk-old WT NOD mice in separate experiments performed as described for A. Diabetes incidence at 12-wk time point is shown for a total of nine or 10 recipient mice in each group.
Discussion
We have shown here that constitutive pancreatic expression of CCL2 in NOD mice reduces, as opposed to aggravates, disease development despite a rich cellular infiltrate in the islets. A heavy cellular infiltrate induced by tg expression of CCL2 in the pancreatic islets of mice on a nonautoimmune background (33) led us originally to hypothesize that RIP-CCL2 tg NOD mice would suffer from more frequent and more severe diabetes. However, we observed the opposite when following fully backcrossed tg NOD mice until development of glucosuria. In this study, we dissected the immunologic mechanisms involved in the protective effects of locally expressed CCL2 in the pancreatic islets of NOD mice. A similar absence of autoimmune destruction despite the presence of an intense infiltrate has previously been observed in mice expressing immunomodulatory cytokines locally in the islets (e.g., refs. 46–48). Remarkably, CCL2 expression increased DC numbers by 10 times in situ but was associated with reduced levels of costimulatory molecules and activation markers like MHC class II and CD80/CD86. This marked DC infiltrate suggested that this cell type is functionally impaired or tolerogenic. Indeed, pancreatic LN CD11c+ CD11b+ DCs secreted reduced TNF-α, but elevated IL-6 and the immunoregulatory cytokine IL-10. Furthermore, these DCs inhibited proliferation of pathogenic CD4+ T cells in vitro that were stimulated with a diabetogenic self-peptide. The tolerogenic phenotype of CD11c+ CD11b+ DCs ex vivo supports also that they inhibit autoreactive T cells in vivo at the site of antigenic exposure, that is, in the pancreatic LN and pancreas. Overall, these data suggest that T cells attracted to the target organ are likely inhibited locally by the immunosuppressive milieu created by these tolerogenic DCs, leading to functional down-regulation. This is also indicated by reduced intracellular IL-2 expression in T cells and induction of detectable levels of T-cell anergy and homeostasis marker Grail (43–45). Furthermore, the CD11c+ CD11b+ DCs suppressed autoimmune diabetes in an in vivo transfer model, supporting that this cell type is responsible for the protection against T cell-mediated autoimmune destruction of pancreatic islets in the NOD mouse. These attracted tolerogenic DCs are in contrast to recently identified pathogenic DCs, which are CD11b−/low and termed merocytic (16). Those DCs appear to be attracted normally to the pancreatic islets and are responsible for breaking peripheral tolerance. On the contrary, the CCL2-attracted, hypoactive CD11c+ CD11b+ DCs described in this study maintain peripheral tolerance and reduce diabetes incidence. Thus, the functional status and subtype of DCs migrating to the target organ in NOD mice are key in deciding between autoimmunity and tolerance to islet antigens.
We propose that persistent expression of CCL2 in pancreatic islets overcomes the relative deficiency of CCL2 during early insulitis in NOD mice, leading to recruitment of tolerogenic APCs. We speculate that such tolerogenic DCs could migrate possibly from the gastrointestinal tract to the islets, as constant down-regulation of immune responses to commensal bacteria at epithelial surfaces is necessary at a steady state (49, 50). This scenario is consistent with the emerging role of CCL2 as a chemokine involved in tissue homeostasis/repair at mucosal or injured sites (51–54). Along these lines, it might be interesting to investigate whether male NOD mice might have higher numbers of these tolerogenic DCs, given that they are preferentially protected from T1D compared with female mice, which is thought to be due to a different set of beneficial commensals colonizing mucosal sites (55). Importantly, APC migration to CCL2 is inherently defective in female NOD mice (6), suggesting that this deficiency is possibly a pathogenic feature that can be overcome by high local expression in RIP-CCL2 tg NOD mice. Interestingly, CCL2 levels in humans with T1D are decreased compared with control subjects based on a large study that was recently published (56). These data point toward a possible protective role in humans as well. The beneficial effects of CCL2 expression within the target organ of murine autoimmune diabetes have potential implications for future treatment strategies in human T1D and related autoimmune diseases. Additionally, the attracted tolerogenic DCs warrant further study to evaluate them for potential immunomodulatory therapies in autoimmune and other immune-mediated diseases.
Materials and Methods
Mice, Pancreatic Immunohistochemistry, and Assessment of Insulitis and Diabetes.
RIP-CCL2 tg NOD and BDC2.5/NOD mice, pancreatic histology, staining, and diabetes assessment are described in SI Materials and Methods.
Cell Isolations, Flow Cytometric Analysis, and in Vivo Transfer Experiments.
Cell isolations, FACS, and in vivo transfer experiments are described in SI Materials and Methods. Fig. S6 shows an illustration of the in vivo transfer experiments.
Cell Culture, in Vitro Experiments, and Stimulation of DCs.
In vitro cocultures of BDC2.5 TCR tg CD4+ T cells with NOD CD11c+ DCs and in vitro stimulated DCs are described in SI Materials and Methods.
RNA Extraction and RT-PCR.
RNA isolation and reverse transcription was performed as described previously (44) and as described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Li Wen and the Yale Diabetes Endocrinology Research Center Islet Isolation Sub-core for pancreatic islet cell isolation, Anthony Ferrandino for technical assistance, and Fran Manzo for manuscript preparation. This work was supported by National Institutes of Health Grant DK47535 (to R.A.F.), the Emmy Noether Programme of the German Research Foundation (Deutsche Forschungsgemeinschaft) (to M.A.K.), a grant from the Arthritis National Research Foundation (to M.A.K.), and National Institutes of Health K08 Grant AI095318-01 (to M.A.K.). C.R. was supported by a fellowship from the American Diabetes Association. R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115308109/-/DCSupplemental.
References
- 1.Adorini L, Gregori S, Harrison LC. Understanding autoimmune diabetes: Insights from mouse models. Trends Mol Med. 2002;8:31–38. doi: 10.1016/s1471-4914(01)02193-1. [DOI] [PubMed] [Google Scholar]
- 2.Solomon M, Sarvetnick N. The pathogenesis of diabetes in the NOD mouse. Adv Immunol. 2004;84:239–264. doi: 10.1016/S0065-2776(04)84007-0. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 4.Jansen A, et al. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994;43:667–675. doi: 10.2337/diab.43.5.667. [DOI] [PubMed] [Google Scholar]
- 5.Jansen A, van Hagen M, Drexhage HA. Defective maturation and function of antigen-presenting cells in type 1 diabetes. Lancet. 1995;345:491–492. doi: 10.1016/s0140-6736(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 6.Bouma G, et al. NOD mice have a severely impaired ability to recruit leukocytes into sites of inflammation. Eur J Immunol. 2005;35:225–235. doi: 10.1002/eji.200425513. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Beller D. Aberrant production of IL-12 by macrophages from several autoimmune-prone mouse strains is characterized by intrinsic and unique patterns of NF-kappa B expression and binding to the IL-12 p40 promoter. J Immunol. 2002;169:581–586. doi: 10.4049/jimmunol.169.1.581. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Beller DI. Distinct pathways for NF-kappa B regulation are associated with aberrant macrophage IL-12 production in lupus- and diabetes-prone mouse strains. J Immunol. 2003;170:4489–4496. doi: 10.4049/jimmunol.170.9.4489. [DOI] [PubMed] [Google Scholar]
- 9.Marleau AM, Summers KL, Singh B. Differential contributions of APC subsets to T cell activation in nonobese diabetic mice. J Immunol. 2008;180:5235–5249. doi: 10.4049/jimmunol.180.8.5235. [DOI] [PubMed] [Google Scholar]
- 10.Nikolic T, Bunk M, Drexhage HA, Leenen PJ. Bone marrow precursors of nonobese diabetic mice develop into defective macrophage-like dendritic cells in vitro. J Immunol. 2004;173:4342–4351. doi: 10.4049/jimmunol.173.7.4342. [DOI] [PubMed] [Google Scholar]
- 11.Sen P, et al. NF-kappa B hyperactivation has differential effects on the APC function of nonobese diabetic mouse macrophages. J Immunol. 2003;170:1770–1780. doi: 10.4049/jimmunol.170.4.1770. [DOI] [PubMed] [Google Scholar]
- 12.Green EA, Eynon EE, Flavell RA. Local expression of TNFalpha in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity. 1998;9:733–743. doi: 10.1016/s1074-7613(00)80670-6. [DOI] [PubMed] [Google Scholar]
- 13.Judkowski V, et al. Increased islet antigen presentation leads to type-1 diabetes in mice with autoimmune susceptibility. Eur J Immunol. 2004;34:1031–1040. doi: 10.1002/eji.200324563. [DOI] [PubMed] [Google Scholar]
- 14.King C, et al. TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity. 1998;8:601–613. doi: 10.1016/s1074-7613(00)80565-8. [DOI] [PubMed] [Google Scholar]
- 15.Lee LF, et al. The role of TNF-alpha in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: Analysis of dendritic cell maturation. Proc Natl Acad Sci USA. 2005;102:15995–16000. doi: 10.1073/pnas.0508122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz JD, Ondr JK, Opoka RJ, Garcia Z, Janssen EM. Cutting edge: Merocytic dendritic cells break T cell tolerance to beta cell antigens in nonobese diabetic mouse diabetes. J Immunol. 2010;185:1999–2003. doi: 10.4049/jimmunol.1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 18.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 19.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 20.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 21.Chensue SW, et al. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: Relationship to local inflammation, Th cell expression, and IL-12 production. J Immunol. 1996;157:4602–4608. [PubMed] [Google Scholar]
- 22.Jimenez F, et al. CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J Immunol. 2010;184:5571–5581. doi: 10.4049/jimmunol.0803494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano H, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpus WJ, et al. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 25.Gu L, et al. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 26.Lu B, et al. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 31.Zisman DA, et al. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99:2832–2836. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutledge BJ, et al. High level monocyte chemoattractant protein-1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J Immunol. 1995;155:4838–4843. [PubMed] [Google Scholar]
- 33.Grewal IS, et al. Transgenic monocyte chemoattractant protein-1 (MCP-1) in pancreatic islets produces monocyte-rich insulitis without diabetes: Abrogation by a second transgene expressing systemic MCP-1. J Immunol. 1997;159:401–408. [PubMed] [Google Scholar]
- 34.Chen MC, Proost P, Gysemans C, Mathieu C, Eizirik DL. Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1 beta-exposed human and rat islet cells. Diabetologia. 2001;44:325–332. doi: 10.1007/s001250051622. [DOI] [PubMed] [Google Scholar]
- 35.Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52:348–355. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 36.Solomon M, Balasa B, Sarvetnick N. CCR2 and CCR5 chemokine receptors differentially influence the development of autoimmune diabetes in the NOD mouse. Autoimmunity. 2010;43:156–163. doi: 10.3109/08916930903246464. [DOI] [PubMed] [Google Scholar]
- 37.Martin AP, et al. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes. 2008;57:3025–3033. doi: 10.2337/db08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Höglund P, et al. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Judkowski V, et al. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 41.Stadinski BD, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faveeuw C, Gagnerault MC, Lepault F. Isolation of leukocytes infiltrating the islets of Langerhans of diabetes-prone mice for flow cytometric analysis. J Immunol Methods. 1995;187:163–169. doi: 10.1016/0022-1759(95)00180-i. [DOI] [PubMed] [Google Scholar]
- 43.Anandasabapathy N, et al. GRAIL: An E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 44.Kriegel MA, Rathinam C, Flavell RA. E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance. Proc Natl Acad Sci USA. 2009;106:16770–16775. doi: 10.1073/pnas.0908957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurieva RI, et al. The E3 ubiquitin ligase GRAIL regulates T cell tolerance and regulatory T cell function by mediating T cell receptor-CD3 degradation. Immunity. 2010;32:670–680. doi: 10.1016/j.immuni.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grewal IS, et al. Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in nonobese diabetic (NOD) mice by preventing the development of auto-reactive islet-specific T cells. J Exp Med. 1996;184:1963–1974. doi: 10.1084/jem.184.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Insulitis in transgenic mice expressing tumor necrosis factor beta (lymphotoxin) in the pancreas. Proc Natl Acad Sci USA. 1992;89:10036–10040. doi: 10.1073/pnas.89.21.10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta transgenic mice. J Immunol. 1993;150:4136–4150. [PubMed] [Google Scholar]
- 49.Strober W. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity. 2009;31:377–388. doi: 10.1016/j.immuni.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Varol C, Zigmond E, Jung S. Securing the immune tightrope: Mononuclear phagocytes in the intestinal lamina propria. Nat Rev Immunol. 2010;10:415–426. doi: 10.1038/nri2778. [DOI] [PubMed] [Google Scholar]
- 51.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 52.Medzhitov R. Inflammation 2010: New adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Lu H, et al. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25:358–369. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takada Y, et al. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–2676. doi: 10.4049/jimmunol.0804012. [DOI] [PubMed] [Google Scholar]
- 55.Kriegel MA, et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci USA. 2011;108:11548–11553. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan R, et al. Chemokine (C-C motif) ligand 2 (CCL2) in sera of patients with type 1 diabetes and diabetic complications. PLoS ONE. 2011;6:e17822. doi: 10.1371/journal.pone.0017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.