We each have particular strengths—some of us are good at math and others are good at language—that vary from one person to another. Less appreciated is the fact that individual differences also extend to performing what appear on the surface to be simple perceptual tasks, like learning to detect one visual form among an array of visually similar forms (1, 2). Neurophysiological studies in awake monkeys and functional MRI (fMRI) studies in humans have provided considerable information about the neural changes that accompany visual perceptual learning—including that learning is highly specific to the retinotopic location of the trained stimulus—making it an ideal model system for studying cortical plasticity (3). Typically, there is also a large amount of intersubject performance variability, making it an ideal model for exploring the neural correlates of individual differences. However, other than good learners showing greater neural activity during the first learning trials than poor learners (2), little is known about the neural underpinnings of individual differences in perceptual learning. In PNAS, Baldassarre et al. (4) present a unique approach to this topic by evaluating the relationship between visual perceptual learning and slowly (<0.1 Hz) fluctuating, spontaneous neural activity recorded during an fMRI resting-state scan 1–2 days before learning.
It has been well-established that distinct cortical networks are revealed by the covariation of spontaneous activity, as measured by fMRI during the so-called resting-state (typically, subjects lie in a scanner while staring at a centrally located cross). This covarying, spontaneous activity is relatively stable across a wide range of cognitive states, ranging from fully awake to light sleep and anesthesia (5). It is also behaviorally relevant, showing learning-dependent plasticity that is indexed by changes in the strength of a network's correlation structure after learning (6–8).
The authors of the current paper (4) had previously reported a striking example of the specificity of these learning-related changes in spontaneous activity resulting from perceptual learning (6). In addition to changes in task-evoked fMRI activity, comparison of the spontaneous activity recorded after training vs. before training revealed changes in the correlation structure (and thus, in the functional connectivity). In particular, functional connectivity changed between regions of both the trained and untrained visual cortex and the regions of parietal and frontal cortex that were modulated by the task. Moreover, the magnitude of these changes correlated with task performance, suggesting that a novel learning experience can “sculpt” spontaneous neural activity in a highly specific fashion (6).
The perceptual learning task required subjects to focus their attention on the lower left quadrant of a visual display and press a button when they detected a briefly presented target shape—an inverted T—embedded among a radial display of randomly oriented T distracter stimuli. Although the inverted T was always presented in the lower left quadrant, the task was difficult: The stimulus array was visible for a short duration (150 ms), the exact location of the target within the quadrant as well as the orientation of the distracters varied from trial to trial, and the subjects’ eyes had to remain fixated on the center of the display, which was confirmed by an eye tracker, while the stimuli appeared in the periphery (5° from fixation). Under these constraints, successful perceptual learning (80% accuracy over 10 consecutive blocks of 45 trials) required, on average, close to 5,600 trials spread over 4 days with 2–3 hours of practice per day. Initial performance varied markedly from one subject to another, with detection accuracy ranging from a low of 13% to a high of nearly 70% during the first 10 training blocks. Baldassarre et al. (4) capitalize on this large intersubject variability in performance to ask whether the state of the task-relevant neural circuitry, as evaluated in the resting-state data recorded before learning, would be predictive of subsequent performance.
As one would expect, the subjects who were highly accurate during the earliest trials reached criterion for successful performance earlier and showed a shallower learning curve than subjects who started out poorly. Baldassarre et al. (4) use a principle components analysis to combine these three correlated measures of learning (initial accuracy, trials to criterion, and learning slope) to create a single measure—task fitness—to serve as a behavioral index for evaluating the relationship between pretraining fluctuations in spontaneous neural activity and performance. Correlational analysis reveals highly specific associations between task performance and the status of the task-relevant neural circuitry before any exposure to the task. Specifically, task fitness is strongly correlated with the strength of functional connectivity between areas in early visual cortex, which is identified by retinotopic mapping, including regions outside the fovea representing the near periphery where the stimuli were to be presented. Moreover, task fitness is positively correlated with spontaneous activity in pairs of regions representing different heterotopic visual quadrants, both within one hemisphere (e.g., left ventral and left dorsal) and between hemispheres (e.g., left ventral with right dorsal), rather than between homotopic quadrant pairs (e.g., left ventral and right ventral) or between different regions within a single quadrant. This pattern of relationships between task fitness and functional connectivity between heterotopic visual quadrants is particularly noteworthy because it is essentially opposite to the pattern of absolute strength of baseline functional connectivity within and between the visual quadrants. Thus, whereas local (within quadrant) and homotopic baseline functional connectivity is stronger than the connectivity between heterotopic regions, the relationships between visual quadrant functional connectivity and subsequent behavior are strongest between the heterotopic quadrants.
This finding is intriguing because it suggests that the predictive functional connections are those that are necessary to coordinate communication between the different quadrants during performance of the task. Accordingly, subjects with the strongest coupling across visual quadrants before learning are apparently most able to focus attention to the lower right quadrant while suppressing information from the other visual quadrants. This view is also consistent with the other major finding from this study (4). Specifically, task fitness is negatively correlated with functional connectivity between visuotopic cortex and cortical regions associated with top-down attentional control (Fig. 1) (see also 9). Baldassarre et al. (4) suggest the possibility that stronger decoupling before learning between visual cortex and areas involved in attentional control may facilitate the suppression of distracting information during the earliest learning trials. Additional work is needed to test this interesting hypothesis.
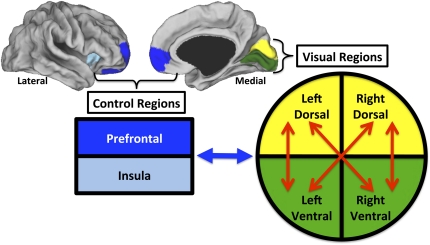
Fig. 1.
Functional connectivity of visual regions predicts performance. Individual differences in pretraining resting-state functional connectivity predicted task fitness. Colored regions projected onto the lateral and medial cortical surfaces approximate visual (yellow, dorsal; green, ventral) and control (dark blue, prefrontal cortex; light blue, insula) regions. Task fitness was positively correlated with pretraining functional connectivity (red arrows) between heterotopic (i.e., dorsal to ventral within and across hemispheres) but not homotopic (ventral to ventral and dorsal to dorsal across hemispheres) or local (within visual quadrant) visual regions. Conversely, task fitness was negatively correlated with pretraining functional connectivity (blue arrow) between visual regions and both prefrontal and insular areas involved in cognitive control.
To serve as a control, Baldassarre et al. (4) also evaluate the relationship between task fitness and functional connectivity of primary and secondary regions of auditory cortex. As typically found during visual processing tasks, auditory cortex is deactivated during visual perceptual learning, and, as found with visual regions, strong functional connectivity is observed between different regions of auditory cortex in the pretraining, resting-state scans. However, in marked contrast to visual regions, functional connectivity within and between auditory regions is unrelated to task performance. Thus, the predictive power of pretraining functional connectivity is modality-specific and limited to visual regions and their interaction with other regions of cortex engaged during perceptual learning.
Taken as a whole, these findings provide striking evidence that the strength of functional connectivity within a task-relevant network is predictive of subsequent task performance (4). A compelling feature of this finding is that the resting-state data were collected a full 1–2 days before the subjects’ exposure to the task (4). As a result, differences in subjects’ attentional and/or motivational state just before learning can be ruled out as major explanatory factors.
At present, our understanding of the predictive value of resting-state connectivity is constrained by our limited understanding of the functional role of spontaneous neural fluctuations and their underlying neurophysiological mechanisms (10). One possibility, however, that has gained some traction is the idea that the continuous, slow fluctuations recorded with fMRI reflect synchronized coupling of neural activity in much faster frequencies—including but likely not limited to γ-range oscillations (11)—necessary for the ongoing maintenance of behaviorally relevant neural circuits. Neurophysiological recording studies in animals (12) as well as magnetoencephalography in humans (13) have linked increased synchronization of neural oscillations across nodes of a network to changes in attention (14) and learning (15). The findings by Baldassarre et al. (9) suggest an additional but related possibility—that the strength of ongoing synchronous activity within highly specific networks reflects an individual subject's history of task-relevant experience and skill. This possibility is broadly consistent with an ever-increasing literature on differences in resting-state functional connectivity between normal and neuropsychiatric conditions (16, 17). Clearly, considerable work lies ahead to establishing the limits on the predictive value of resting-state functional connectivity for understanding individual variation in basic sensory motor as well as higher-order cognitive tasks. The data presented by Baldassarre et al. (4) extend the potential usefulness of resting-state functional connectivity in an innovative and unexpected direction (cf. ref. 18).
Acknowledgments
This work was supported by the National Institute of Mental Health, Division of Intramural Research Programs.
Footnotes
The authors declare no conflict of interest.
See companion article on page 3516.
References
- 1.Fahle M, Henke-Fahle S. Interobserver variance in perceptual performance and learning. Invest Ophthalmol Vis Sci. 1996;37:869–877. [PubMed] [Google Scholar]
- 2.Mukai I, et al. Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. J Neurosci. 2007;27:11401–11411. doi: 10.1523/JNEUROSCI.3002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasaki Y, Nanez JE, Watanabe T. Advances in visual perceptual learning and plasticity. Nat Rev Neurosci. 2010;11:53–60. doi: 10.1038/nrn2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldassarre A, et al. Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci USA. 2012;109:3516–3521. doi: 10.1073/pnas.1113148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 6.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20:1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tambini A, Ketz N, Davachi L. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron. 2010;65:280–290. doi: 10.1016/j.neuron.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigman M, et al. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46:823–835. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leopold DA, Maier A. Ongoing physiological processes in the cerebral cortex. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.10.059. Oct 25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105:16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Ghuman AS, Bar M, Dobbins IG, Schnyer DM. The effects of priming on frontal-temporal communication. Proc Natl Acad Sci USA. 2008;105:8405–8409. doi: 10.1073/pnas.0710674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 15.Gotts S, Carson C, Martin A. Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cogn Neurosci. 2012 doi: 10.1080/17588928.2012.670617. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uddin LQ, Supekar K, Menon V. Typical and atypical development of functional human brain networks: Insights from resting-state FMRI. Front Syst Neurosci. 2010;4:21. doi: 10.3389/fnsys.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Q, Zhang J, Luo YLL, Dilks DD, Liu J. Resting-state neural activity across face-selective cortical regions is behaviorally relevant. J Neurosci. 2011;31:10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]