Abstract
Odorant receptors (ORs) in olfactory sensory neurons (OSNs) mediate detection of volatile odorants. Divalent sulfur compounds, such as thiols and thioethers, are extremely potent odorants. We identify a mouse OR, MOR244-3, robustly responding to (methylthio)methanethiol (MeSCH2SH; MTMT) in heterologous cells. Found specifically in male mouse urine, strong-smelling MTMT [human threshold 100 parts per billion (ppb)] is a semiochemical that attracts female mice. Nonadjacent thiol and thioether groups in MTMT suggest involvement of a chelated metal complex in MOR244-3 activation. Metal ion involvement in thiol–OR interactions was previously proposed, but whether these ions change thiol-mediated OR activation remained unknown. We show that copper ion among all metal ions tested is required for robust activation of MOR244-3 toward ppb levels of MTMT, structurally related sulfur compounds, and other metal-coordinating odorants (e.g., strong-smelling trans-cyclooctene) among >125 compounds tested. Copper chelator (tetraethylenepentamine, TEPA) addition abolishes the response of MOR244-3 to MTMT. Histidine 105, located in the third transmembrane domain near the extracellular side, is proposed to serve as a copper-coordinating residue mediating interaction with the MTMT–copper complex. Electrophysiological recordings of the OSNs in the septal organ, abundantly expressing MOR244-3, revealed neurons responding to MTMT. Addition of copper ion and chelator TEPA respectively enhanced and reduced the response of some MTMT-responding neurons, demonstrating the physiological relevance of copper ion in olfaction. In a behavioral context, an olfactory discrimination assay showed that mice injected with TEPA failed to discriminate MTMT. This report establishes the role of metal ions in mammalian odor detection by ORs.
Humans are extremely sensitive to the smell of thiols, for example, the natural gas odorant 2-methyl-2-propanethiol (Me3CSH), detectable at 0.3 parts per billion (ppb) (1). Spider monkeys are yet more sensitive, detecting 0.001 ppb ethanethiol (2). Malodorous volatile thiols and amines are protein degradation products found in putrid food, so sensitive identification of these compounds is crucial to avoiding intoxication (2, 3). The molecular mechanisms underlying olfactory detection of thiols are yet to be elucidated. Strong-smelling volatiles are usually good metal-coordinating ligands: thiols are also named “mercaptans,” from mercurium captans (Latin: capturing mercury). Odorant receptors (ORs) mediating odor perception have been suggested to function as metalloproteins, whereby transition metal ions (e.g., Zn2+, Ni2+, Cu2+, and Cu+) may be required for activation of ORs by thiol and amine ligands (4–7). In 1977 Crabtree (4) proposed that “these substances bind chemically to a nasal receptor … containing a transition metal at the active site,” that Cu(I) is “the most likely candidate for a metallo-receptor site in olfaction,” and that “the Cu(I) centre would be stabilized by coordination, perhaps to a protein thiolato-group, and … two or three additional protein S or N neutral donor groups.” Crabtree supported his hypothesis observing that, compared with unstrained olefins, strained olefins like strong-smelling trans-cyclooctene, “give much more stable complexes, e.g., [Cu2Cl2-(trans-cyclooctene)3]” (4). In 1969 Henkin and Bradley (8) suggested that the physiology of taste involved copper. In 1996 Turin (7) proposed a key role for zinc in olfaction. In 2003 Wang et al. (6) reported that the short synthetic pentapeptide HACKE corresponding to a conserved sequence in the extracellular loop of ORs could effectively bind to metal ions and may form the basis for sensitive activation of ORs by thiols. However, it has not been shown that intact OR proteins rely on interaction with metal ions for function.
(Methylthio)methanethiol (MeSCH2SH; MTMT), a strong-smelling volatile semiochemical found specifically in male mouse urine, activates certain olfactory sensory neurons (OSNs), mediating attraction in female mice (9). Here we show that copper ion is an essential cofactor for detection of MTMT and other thiols, and trans-cyclooctene. Identification of an OR activated by thiols and elucidation of metal ion involvement represent an important step forward in deciphering the molecular mechanism of olfaction, especially that of sensitive detection of thiols and other metal-coordinating odorants.
Resuts
MOR244-3 Is Selectively Activated by MTMT.
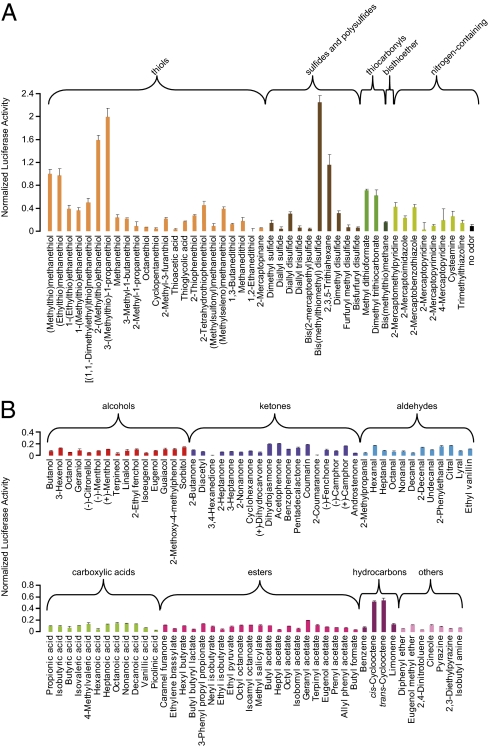
We first screened a previously defined library of 219 mouse ORs for MTMT using an HEK293T-based OR heterologous expression system (10–12). Candidate ORs were further tested, and MOR244-3 (alternatively, MOR83 or Olfr1509) robustly responded to MTMT. To determine the receptor specificity of MOR244-3, we tested for its response against a panel containing MTMT, 19 analogs, and 24 other sulfur-containing compounds (Fig. 1A and Table S1), and a panel of 89 diverse odors (Fig. 1B and Table S2). MOR244-3 also selectively responded to several analogs of MTMT, including certain thiols, disulfides, and thiocarbonyl compounds. Simple thiols (e.g., methanethiol and octanethiol) did not elicit a response.
Fig. 1.
MOR244-3 is selectively activated by MTMT and other sulfur-containing odors. Responses of MOR244-3 to (A) MTMT and a sulfurous odor panel containing 19 MTMT analogs and 24 other sulfur-containing compounds and (B) a diverse odor panel containing 89 compounds. The concentration of all odors used was 30 μM, except when cell toxicity was observed, the maximally tolerant concentrations were used, including 0.3 μM for 2-(methylthio)ethanethiol and 10 μM for 2-mercaptobenzothiazole and 2-mercaptopyridine. All responses are normalized to the response to MTMT. For all figures, normalized luciferase responses are shown as mean ± SEM (n = 3). Odorants are color-coded by structural features and functional groups. For odorants with multiple functional groups, the functional group with the highest oxidation state is used for categorization.
Copper Ion Enhances the Activation of MOR244-3 in Response to MTMT.
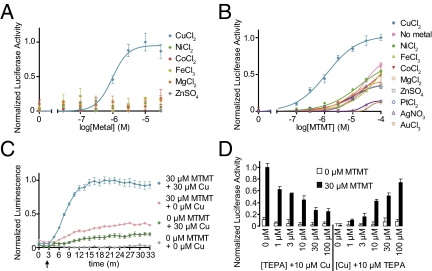
We examined the effects of metal ions on MOR244-3 activation. First, we supplemented the stimulation medium used for odorant dilution with various metal ions. The addition of Cu2+ induced a stronger response of MOR244-3 to 30 μM MTMT (Fig. 2A). This effect intensified with increasing Cu2+ concentrations up to 30 μM. The total copper concentration in mouse nasal mucus measured by inductively coupled plasma-mass spectroscopy is also in the micromolar range [41.7 ± 2.8 μM (mean ± SD, n = 2)], similar to that of the cell culture system. Although we introduce extracellular copper as Cu2+, owing to the reducing environment of most cells, intracellular copper may exist mainly as Cu1+, presumably the form in which it reduces S–S bonds and forms odorant complexes (13). Other metal ions tested (e.g., Ni2+, Co2+, Fe3+, Mg2+, and Zn2+) did not affect receptor activation (Fig. 2A). When the Cu2+ concentration was fixed at 30 μM, the response of MOR244-3 against increasing concentrations of MTMT had a >20-fold decrease in EC50 values and higher saturation responses compared with the negative control with basal level of copper [≈0.6 μM (Table S3)], indicating higher potency and efficacy of MOR244-3 in the presence of Cu2+ (Fig. 2B). In contrast, adding 30 μM Ni2+, Co2+, Fe3+, Mg2+, Zn2+, Au3+, 10 μM Pt2+, or 5 μM Ag+ did not enhance receptor activation compared with the control. Although Au3+, Pt2+, and Ag+ do not occur naturally, they are found in pharmaceuticals such as the chemotherapeutic agent cisplatin (Pt2+).
Fig. 2.
Copper ion enhances the activation of MOR244-3 in response to MTMT. (A) Dose–response curves of MOR244-3 against 30 μM MTMT with increasing concentrations of various metal ions supplemented in the cell culture medium. (B) Dose–response curves of MOR244-3 against increasing concentrations of MTMT with the concentration of different metals held constant at 30 μM, except for PtCl2 and AgNO3, which were 10 μM and 5 μM, respectively. (C) Real-time measurement of MOR244-3 activation in the presence of 30 μM Cu2+ and/or 30 μM MTMT as measured by a GloSensor assay within 30 min of odorant addition. Arrow indicates odorant addition. (D) Responses of MOR244-3 to 0 and 30 μM MTMT with 10 μM Cu2+ or TEPA and increasing the amount of TEPA or Cu2+, respectively. In this and subsequent figures, all responses are normalized to the highest response to MTMT. For C, y axis represents luminescence, shown as mean ± SEM (n = 6).
We used the GloSensor assay to measure real-time changes in cAMP levels caused by receptor activation upon ligand addition. MOR244-3 was robustly activated only in the presence of both Cu2+ and MTMT. MTMT alone elicited a much diminished response, and adding Cu2+ alone did not elicit a response (Fig. 2C). This effectively excluded the possibility of MOR244-3 acting as a copper receptor. Finally, we directly measured the change in levels of cAMP after the activation of the receptor by MTMT. We observed an increase in cAMP concentration only in the presence of 30 μM Cu2+ (Fig. S1A).
To further validate the effect of copper in MOR244-3's response to MTMT, we next determined whether copper deficiency would reduce receptor activation. We supplemented the stimulation medium with the membrane-permeable, high-affinity copper chelator tetraethylenepentamine [(H2NCH2CH2NHCH2CH2)2NH; TEPA]. When the concentration of Cu2+ was fixed at 10 μM, increasing TEPA decreased the response of MOR244-3 to 10 μM MTMT (Fig. 2D). Notably, adding 10 μM TEPA to the medium without exogenous Cu2+ entirely abolished the receptor's response to 30 μM MTMT, indicating that basal levels of Cu2+ in the stimulation medium are required for MOR244-3 activation by MTMT. Furthermore, when the TEPA concentration was fixed at 10 μM, receptor activity was rescued by increasing Cu2+ concentration. This suggests that excess Cu2+ reversed TEPA activity, reinforcing the role of copper ion in the enhancement of receptor activity (Fig. 2D). The effect of TEPA was also confirmed in the more direct measurement of cAMP (Fig. S1B).
Specificity and Selectivity of the Copper Ion Enhancement Effect.
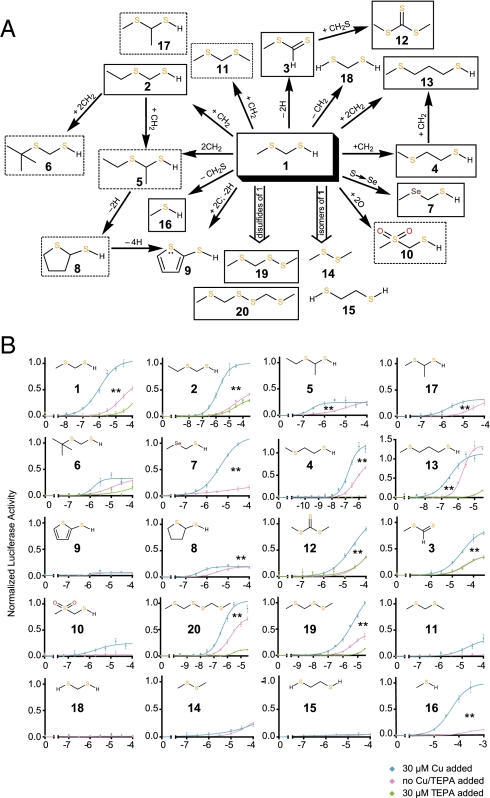
We investigated the structural requirement for the copper effect by profiling MOR244-3's response against a panel of 19 MTMT analogs (Fig. 3 and Tables S1 and S4). A previous study showed that conformers and isomers selectively activate an OR (14). Because of the simplicity of MTMT (1; Fig. 3A), a limited number of structurally related compounds are possible; for example, isomeric with MTMT or differing (i) by addition of one or two carbon atoms and associated hydrogens, or of two oxygens (giving a sulfone), (ii) by deletion of two hydrogen atoms, or (iii) by replacing the thioether sulfur with selenium. Isomerization or atom addition, deletion, or substitution could alter the number of thiol and thioether groups, change the steric crowding at the sulfur atoms and the bulk of resulting copper complexes, modify the ligand “bite angle,” alter the S-H acidity, change the availability of thioether electron pairs, and affect the compound lipophilicity, in turn, potentially modifying the corresponding copper complex and its protein docking ability.
Fig. 3.
Structural requirements of the copper ion enhancement effect of MOR244-3. (A) Structural relationship between MTMT and its analogs. Odors boxed with solid lines are those with prominent responses in the presence of 30 μM Cu2+, and odors boxed with dashed lines are those with less prominent responses, as defined by a more than 50% reduction in efficacy compared with MTMT. Unboxed odors did not elicit MOR244-3 response. (B) Dose–response curves of MOR244-3 to representative sulfur-containing compounds with and without 30 μM exogenous Cu2+addition. For odors with a significant response in the absence of exogenous Cu2+, as defined arbitrarily by a top value greater than 0.32, dose–response curves with 30 μM of TEPA are also shown. The chemical structures of the respective compounds are shown in the upper left-hand corners. F tests were used to compare the pairs of dose–response curves with or without Cu2+. Asterisks represent significance of P values after Bonferonni corrections. **P < 0.01 (n = 3).
Replacement of the MTMT methyl group by ethyl in 2 has no effect on activity (modest steric effect), whereas addition of a methyl group on the carbon between the sulfur atoms (17), addition of both the ethyl in 2 and methyl in 17 (5), or replacing ethyl with tert-butyl (6) diminishes the activity (greater steric effects, more lipophilic). Interestingly, methanethiol (16) shows a significant copper effect (Fig. 3B), albeit at much higher concentrations than 1, whereas octanethiol remains unreactive with or without copper. Although copper(I) thiolates derived from unhindered thiols such as 16 are polymeric or form polynuclear clusters (15, 16) [e.g., (MeSCu)n], displacement of methylthio groups on copper by available OR cysteine thiol groups (the small size of MeS should favor such known rapid ligand exchange) (17) would allow the resultant copper methanethiolate to be accommodated in the OR, although not as comfortably as the MTMT complex. The confined space within the OR active site, where Cu1+ is ligated by OR amino acids, disfavors conversion of OR-bound (MeS)2L2Cu to (MeSCu)n. Both of the above processes would fail with the larger octanethiolate complex: octanethiolate would be less easily displaced from copper and when complexed in the OR, less easily accommodated in the binding site.
Disulfides 19, found in male mouse urine, and 20, an MTMT oxidation product (9), elicited strong responses by the receptor. For these two compounds there was also a dramatic reduction in the response by basal level of copper ion in the medium when 30 μM TEPA was added. These observations are consistent with reports on the ability of a neighboring electron-donor in disulfides “that enhances the electron transfer from Cu(I) to the disulfide leading to S–S bond scission” (18) (e.g., the methylthio groups in 19 and 20), because dimethyl disulfide (14) is not reduced under these same conditions. The latter observations require that (i) thiolate (not its oxidation product) itself is present in the OR, leading to the strong copper effect; (ii) disulfides derived from oxidation of thiols and the precursor thiols themselves give a common copper complex when docked in the OR. Most activity is retained when the thioether sulfur is replaced by selenium in 7 and on removal of two hydrogens giving dithioester 3; good activity also remains with trithiocarbonate 12. Consideration of compounds 4, 9–11, 13–15, and 18 is provided in SI Results and Discussion.
Taken together, our data suggest that the most active complexes involve sulfur compounds of type RX(CH2)nS (X = S or Se; n = 1–3; R = Me or Et), with one terminal thiolate (C–S–) or thiocarbonyl (C=S) sulfur. Interestingly, although the addition of 30 μM TEPA reduced the responses of most of these compounds owing to the presence of basal level of copper ion in the medium, the two thiocarbonyl compounds, 3 and 12, elicited unchanged levels of response with TEPA. This observation suggests that the binding of 3 and 12 to MOR244-3 may be different from that of all of the other sulfur compounds, possibly because they involve a thionyl ligand (C=S-Cu), as seen in the bacterial copper acquisition compound methanobactin (19), rather than the more commonly found thiolate (C-S-Cu). Interactions between the thiocarbonyl groups and OR protein aromatic rings (20, 21) and/or OR hydrogen bond donors are possible with 3 and 12.
A few other compounds containing nitrogen, such as cysteamine and trimethylthiazoline (TMT; Fig. S2A), which did not elicit a response without copper, evoked a prominent response after Cu2+ addition. In addition, we found that a variety of less potent ligands structurally and functionally distinct from MTMT emerged in the larger odorant panel upon Cu2+addition. In particular we found a positive copper effect with trans-cyclooctene, conducting the experiments in the dark to avoid trans-to-cis isomerization (Fig. S2B). Other ligands showing copper effects included heptanal, lyral, cineole, and phenylethanal, representing chemicals of diverse structural features containing aldehyde and ether moieties. We generated dose–response curves for all of these ligands and confirmed that eight of them were bona fide, yet weaker, ligands of MOR244-3. All of these nonthiol odorants exhibited the copper ion enhancement effect on receptor activation (Fig. S2C). A common feature of these nonsulfurous ligands is the presence of one or more oxygen atoms with lone pairs capable of metal coordination, suggesting that these molecules can potentially coordinate copper ions.
Finally, we also tested random OR–ligand pairs, including the known human OR for a thiol, all showing no observable difference with or without Cu2+ (Fig. S2D), indicating that the copper ion enhancement effect is specific to MOR244-3. The above results collectively showed that although MOR244-3 is a broadly tuned receptor for sulfurous and other potentially metal-coordinating compounds, the copper ion enhancement effect is only observed for ligands within certain structural limits.
Modeling MOR244-3 Receptor–Ligand Interaction with a Candidate Copper-Binding Site.
Copper, a micronutrient playing an essential role in biology, is a cofactor for various enzymes. The copper ion involved in OR activation could originate from inside the cell, where it could be assembled with the receptor during protein biosynthesis, or from outside the cell, where it could bind to the cell-surface receptor or to the ligand before activation. Our data suggest that copper is probably not required for the biosynthesis of functional MOR244-3 (SI Results and Discussion) but may rather originate from an extracellular source. If an OR relies on the copper ion as a cofactor for activation, the copper ion may bind to the OR itself and/or to the ligand. To address this, we combined a site-directed mutagenesis approach with a homology model of MOR244-3. First, to elucidate the mechanism underlying the copper enhancement effect of MOR244-3, we attempted to map the potential copper-binding site in this OR. Wang et al. (6) proposed a metal-binding site consisting of metal-coordinating amino acids, histidine, and cysteine, in a consensus sequence HXXC[DE] in the second extracellular loop in ORs, X being any amino acid. However, MOR244-3 lacks this motif in the EC2 domain, instead having the sequence SYFCD. Turin (7) proposed another candidate pentapeptide metal binding site, CGSHL, in the cytoplasmic end of TM6.
Given these speculations and the facts that amino acid residues histidine, cysteine, and methionine frequently coordinate copper in cuproenzymes (22–25) and that the amino acids distant from each other in the primary structure may be closely interacting in actual spatial arrangement, we constructed a series of single-site mutants, changing all methionines to alanines, all histidines to arginines, lysines, tyrosines, leucines, valines, phenylalanines, asparagine, and/or alanines, and all cysteine residues to serines, valines, and/or phenylalanines in MOR244-3 on the basis of structural or evolutionary conservation and asked how the respective mutations affected MOR244-3 activation by MTMT in the luciferase assay (Fig. S3). Some of the mutations did not significantly affect the Cu2+-induced enhancement when stimulated by three concentrations of MTMT, excluding these sites as copper- and/or ligand-binding, whereas some of the sites reduced the response of MOR244-3 both with and without Cu2+. The rest of the sites, including C97, H105, H155, C169, C179, and H243, abolished responses to MTMT completely when mutated, regardless of Cu2+ (Fig. S3B). Of these, we found that mutants C97S, H155R, C169S, C179S, and H243R, but not H105K, have little or no cell-surface expression (Fig. S3C), suggesting that these mutants lost their functions as a result of defects in receptor folding/trafficking. The H105K loss-of-function mutation may either disrupt copper/ligand binding or lead to general receptor defects, by affecting downstream signaling. Notably, this mutant retains the ability to respond to some of MOR244-3's nonsulfurous ligands (e.g., cineole) and to ligands with no copper effect (e.g., dimethyl sulfide), indicating that the mutant receptor is intact and that this residue might be at the interface of MTMT and/or copper binding (Fig. S4A).
MOR244-3 was modeled on the basis of the closest-matching structure turkey β1 adrenergic receptor [Protein Data Bank entry 2VT4, resolution 2.7 Å] (26). We searched for candidate ligand pockets as implemented in POCASA (http://altair.sci.hokudai.ac.jp/g6/service/pocasa/), which predicted a binding pocket formed by the loops and transmembrane domains facing the extracellular side of the receptor (Fig. S4B), consistent with the location of a possible ligand-binding pocket in ORs (27–30). Notably, H105 is found between the second to fifth transmembrane domains close to the extracellular side, representing a plausible copper coordinating residue. Thus, MTMT and copper ion may share a binding site in this region. Alternatively, the pocket could harbor an allosteric copper-binding site, important for ligand binding.
MTMT-Responsive OSNs Are Abundantly Present in the Septal Organ (SO).
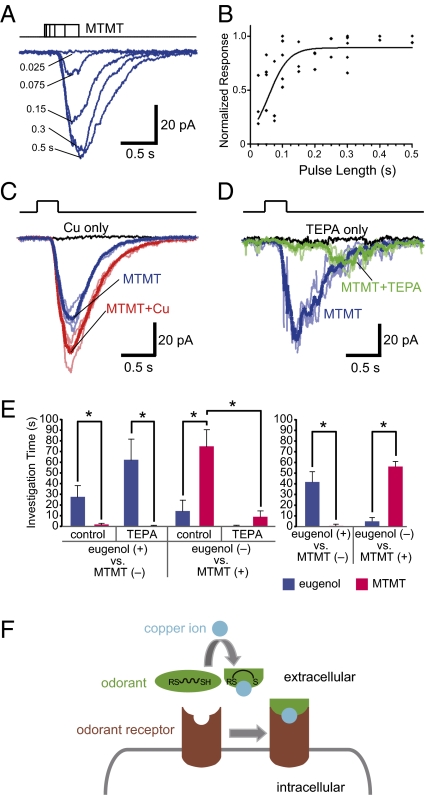
To confirm that in situ OSNs can respond to MTMT, we performed perforated patch clamp recordings in the SO, which expresses SR1 and MOR244-3 in high abundance (31, 32) (Fig. S5A). Because copper is present in the olfactory mucosa, we expected to observe MTMT responses among the non-SR1 cells. In fact, of 132 cells recorded, 76 cells or 58% responded to MTMT in a dose-dependent manner (Fig. 4 A and B). This percentage is higher than that of MOR244-3 cells (27%) in the non-SR1 cells of the SO, suggesting that additional ORs in the SO are responsive to MTMT. We used in vitro screening to test this possibility and found that another OR, MOR256-17, showed robust responses to MTMT (Fig. S5B). Interestingly, the MTMT responses of MOR256-17 were not modulated by copper addition. These results indicate that MTMT-responsive cells in the SO are heterogeneous in their OR choice and in their modulation by copper. Consequently, we expect to observe different modulations of MTMT responses by copper. From 32 cells tested by adding copper together with MTMT, the MTMT responses were enhanced in 12 cells (38%) (Fig. 4C), unchanged in 15 cells (47%), and, curiously, decreased in five cells (16%). Similarly, MTMT responses of the SO cells showed different modulation by TEPA; TEPA reduced the MTMT responses in 36% of the cells (n = 50) (Fig. 4D) but had no effect in other cells (64%). The patch clamp data indicate that many cells in the SO are capable of responding to MTMT in the absence of exogenous copper, suggesting that mice can readily detect these compounds in the urine. Depending on the OR identity and the endogenous copper concentration in the olfactory epithelium, these cells show heterogeneous modulation by copper and its chelator.
Fig. 4.
Physiological relevance of copper in the detection of MTMT. (A–D) MTMT responsive OSNs are present in the SO. (A) In a single non-SR1 cell, inward currents were elicited by MTMT pulses with varying length from 0.025 to 0.5 s. (B) The dose–response curve was fitted by grouping the data from six cells tested by MTMT pulses with different lengths. For each cell, the peak current induced by a pulse was normalized to the maximum response in that cell. (C) In some cells, the MTMT (10 μM) response was facilitated by adding 30 μM Cu2+. (D) In some cells, the MTMT response (10 μM) was reduced by adding 30 μM TEPA. In C and D, the thick trace was averaged from three trials under that condition (thin lines). All recordings were performed under voltage clamp mode with a holding potential of −60 mV. (E) Mice were trained to associate either eugenol or MTMT with sugar reward. On the test day, mice were injected with dH2O or TEPA and then tested for the ability to discriminate the two odors. Left: TEPA injection specifically abolishes olfactory detection of MTMT. Right: Recovery of olfactory discrimination ability for eugenol and MTMT 2 d after TEPA injection. Two days after the initial testing, mice were retested for the recovery of the ability to discriminate the two odors. The y axis represents time spent investigating each odorant during the 9-min test period, shown as mean ± SEM. Paired t test was used to compare the investigation times between groups. *P < 0.05 (n = 4). (F) A model for OR activation involving copper ion as a cofactor with the copper ion binding to the ligand for subsequent binding of the copper ion–odor complex to the OR.
In Vivo Injection of TEPA Abolishes Olfactory Recognition of MTMT.
To assess the role of copper in the perception of MTMT in a behavioral context, we performed an olfactory discrimination assay using food-restricted mice that were trained to associate either eugenol or MTMT with sugar reward. On the test day, we first performed bilateral injection of the copper chelator TEPA into the nasal cavities of the mice. The mice were then exposed to both odorants in a testing cage, and the time spent investigating the odorants was scored. Mice trained to associate MTMT with sugar reward spent significantly less time investigating the odor after TEPA injection, suggesting that they had lost the ability to recognize the odor (Fig. 4E, Left). The group of mice trained for the nonsulfurous odorant eugenol could always discriminate the odor with or without TEPA injection, indicating that the negative effect of TEPA on olfactory discrimination is specific to MTMT (Fig. 4E, Left). Consistent with metabolic clearance of TEPA, the group trained to recognize MTMT regained olfactory discrimination ability 2 d after TEPA injection (Fig. 4E, Right). The results from the behavioral experiment indicate that copper is required for the olfactory detection of MTMT.
Discussion
Humans have an exquisitely sensitive sense of smell toward low valent sulfur compounds. The possible involvement of metal ions in olfaction has long been postulated (4, 6). However, the definitive role of metal ions in odor-evoked activation of the ORs is unknown. In this study, to evaluate the hypothesis, we used MTMT, a thiol and also a semiochemical in male mice urine that attracts female mice and activates mitral cells in the main olfactory bulb (9). By screening a panel of mouse ORs, we identified a highly potent OR for MTMT; importantly, we found that the function of the MTMT OR was greatly enhanced in the presence of Cu2+, allowing ppb detection of MTMT. We thus hypothesized that a copper–ligand complex would present more stable interaction with the ORs than the ligands without copper.
Past studies have shown the involvement of metal ions at the interface of protein–ligand interaction, such as the Arabidopsis ethylene receptor ETR1 that has a copper ion bound to its transmembrane domain (33). Certain metal ions, such as Zn2+, can also modulate G protein-coupled receptor (GPCR) activation, including chemokine receptor CXCR4, the μ-opioid receptors, and the melarnocortin receptors (34–37). How does copper ion enhance MOR244-3 receptor activation? Our data are congruent with the model that the overall thiolate–copper complex is more conformationally constrained, or smaller and more spherical in overall extension, than the free “odor” structure. A wide range of structure types are known for complexes of Cu1+ and Cu2+ with thiol, thiocarbonyl, thioether, selenoether, and disulfide groups, alone or in various combinations. In the case of MOR244-3, Cu1+ could simultaneously bind to ligands 1–20 and OR protein's nitrogen, sulfur, or oxygen functionalities affording a variety of structures. As implied by Fig. 4F, the ligand–copper complex would have to be of an appropriate size to fit into a receptor pocket. Furthermore, the ligand should not bind to copper so tightly so as to preclude complexation by the receptor protein, as is the case with TEPA, and presumably 1,2- and 1,3-dithiols.
By way of background on copper complexes of thiolates, the following is known. (i) Thiolates can act as monodentate or as bridging ligands between two copper centers; very bulky thiolates favor mononuclear copper thiolates. (ii) Thiolates can act as reducing agents, forming disulfides, hampering synthesis of thiolate complexes of Cu2+; however, copper complexes of disulfides are also known, as are those of thioethers or selenoethers, all of which are relatively poor ligands. (iii) Cu1+ can reduce disulfides to thiolates when the disulfide contains additional chelating groups (38). (iv) Cu2+ uses coordination numbers 4 (square planar), 5 (trigonal bipyramid or square pyramid), or 6 (octahedron), whereas Cu1+ prefers coordination numbers 2, 3, 4 (tetrahedral), and occasionally 5 (square pyramid). Representative examples of various types of Cu1+ and Cu2+ complexes involving divalent sulfur ligands are described in Table S5. Future crystallization studies may elucidate the structure of the copper–MTMT complex. Our proposed model of copper simultaneously binding to OR protein residues and thiol/thiolate as well as thioether or selenoether groups in odorants MeX(CH2)nSH may be analogous to the binding that occurs in blue copper proteins, which involves simultaneous binding to cysteine and methionine (or selenomethionine) (39–41). Sophisticated molecular dynamics simulation studies may be required to resolve the precise mode of interaction among the receptor–ligand–metal trio associated with odorant docking.
Materials and Methods
Chemicals.
All odorants, except for those described in SI Materials and Methods, as well as CuCl2, NiCl2, and ZnSO4, were purchased from Sigma-Aldrich, Contech Inc. (TMT), Sinopharm (CoCl2, AgNO3), or Aladdin Reagent Database Inc. (FeCl3, AuCl3, and PtCl2). The chemicals were dissolved in DMSO or ethanol and diluted further into working concentrations before experiments.
Dual-Glo Luciferase, GloSensor, and cAMP-Glo Assays.
HEK293T and its derived cell line Hana3A and that containing ATP7A/B RNAi constructs were grown in minimum essential medium (HyClone) containing 10% FBS at 37 °C with 5% CO2. Lipofectamine 2000 (Invitrogen) was used for transfection. Luciferase assays were performed as previously described (11). An F test was used to compare the best-fit values of EC50, top, and Hill slope between the pairs of dose–response curves with or without copper ion. Bonferroni corrections were applied to account for multiple comparisons. For GloSensor and cAMP-Glo assays, Hana3A cells were plated onto 96-well plates (Biocoat; Becton Dickinson Biosciences). After 18–24 h, OR and mRTP1S (and a GloSensor plasmid in the case of GloSensor assay) were transfected into cells. Twenty-four hours after transfection, the cells were stimulated with odorants plus various concentrations of metal ions dissolved in CD293 (Invitrogen). We used the GloSeneor (Promega) and cAMP-Glo (Promega) kits and followed the manufacturer's protocol for measuring chemiluminescence. Additional materials and experimental procedures are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. K. Karlin, L. Vosshall, H. Schugar, and M. Luo for comments on the manuscript; Drs. E. J. Corey, C. He, C. Yang, D. Lin, H. Amrein, K. J. Franz, D. Y. Lin, and R. Roberts for discussions; Dr. J. Mainland for selection of an odorant panel and discussions; and Drs. D. Thiele and Y. Nose for contribution of ATP7A and ATP7B knockdown cell lines and advices. This research is supported by National Natural Science Foundation of China Grants 30970981 and 31070972, Shanghai Pujiang Program Grant 09PJ1406900, by the Program for Innovative Research Team of Shanghai Municipal Education Commission, Grant 2009CG15 from the Chen Guang Project funded by Shanghai Municipal Education Commission and Shanghai Education Development Foundation, and Grant J50201 from the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, from the Leading Academic Discipline Project of Shanghai Municipal Education Commission (all to H.Z.); National Institutes of Health (NIH) Grant DC005782 (to H.M.); National Basic Research Program of China (973 Program) Grant 2011CB504001 (to J.Z.); National Science Foundation Grant CHE-0744578 (to E.B.); and NIH/National Institute on Deafness and Other Communication Disorders Grant DC006213 (to M.M.). This article is dedicated to the memory of Dr. Lawrence C. Katz.
Footnotes
Conflict of interest statement: The authors declare a potential conflict of interest. The authors plan to submit a patent application relevant to the work.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111297109/-/DCSupplemental.
References
- 1.Devos M, Patte F, Rouault J, Laffort P, Van Gemert LJ, editors. Standardized Human Olfactory Thresholds. New York: IRL Press; 1990. [Google Scholar]
- 2.Laska M, Bautista RM, Höfelmann D, Sterlemann V, Salazar LT. Olfactory sensitivity for putrefaction-associated thiols and indols in three species of non-human primate. J Exp Biol. 2007;210:4169–4178. doi: 10.1242/jeb.012237. [DOI] [PubMed] [Google Scholar]
- 3.Block E. Reactions of Organosulfur Compounds. New York: Academic Press; 1978. [Google Scholar]
- 4.Crabtree RH. Copper(I)—possible olfactory binding-site. J Inorg Nucl Chem. 1978;40:1453. [Google Scholar]
- 5.Day JC. New nitrogen bases with severe steric hindrance due to flanking tert-butyl groups. cis-2,6-Di-tert-butylpiperidine. Possible steric blocking of olfaction. J Org Chem. 1978;43:3646–3649. [Google Scholar]
- 6.Wang J, Luthey-Schulten ZA, Suslick KS. Is the olfactory receptor a metalloprotein? Proc Natl Acad Sci USA. 2003;100:3035–3039. doi: 10.1073/pnas.262792899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turin L. A spectroscopic mechanism for primary olfactory reception. Chem Senses. 1996;21:773–791. doi: 10.1093/chemse/21.6.773. [DOI] [PubMed] [Google Scholar]
- 8.Henkin RI, Bradley DF. Regulation of taste acuity by thiols and metal ions. Proc Natl Acad Sci USA. 1969;62:30–37. doi: 10.1073/pnas.62.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 10.Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal. 2009;2:ra9. doi: 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H. RTP family members induce functional expression of mammalian odorant receptors. Cell. 2004;119:679–691. doi: 10.1016/j.cell.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang H, Matsunami H. Synergism of accessory factors in functional expression of mammalian odorant receptors. J Biol Chem. 2007;282:15284–15293. doi: 10.1074/jbc.M700386200. [DOI] [PubMed] [Google Scholar]
- 13.Pujol AM, et al. Hepatocyte targeting and intracellular copper chelation by a thiol-containing glycocyclopeptide. J Am Chem Soc. 2010;133:286–296. doi: 10.1021/ja106206z. [DOI] [PubMed] [Google Scholar]
- 14.Peterlin Z, et al. The importance of odorant conformation to the binding and activation of a representative olfactory receptor. Chem Biol. 2008;15:1317–1327. doi: 10.1016/j.chembiol.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilworth JR, Hu J. Complexes of sterically hindered thiolate ligands. Adv Inorg Chem. 1993;40:411–459. [Google Scholar]
- 16.Groysman S, Holm RH. A series of mononuclear quasi-two-coordinate copper(I) complexes employing a sterically demanding thiolate ligand. Inorg Chem. 2009;48:621–627. doi: 10.1021/ic801836k. [DOI] [PubMed] [Google Scholar]
- 17.Pufahl RA, et al. Metal ion chaperone function of the soluble Cu(I) receptor Atx1. Science. 1997;278:853–856. doi: 10.1126/science.278.5339.853. [DOI] [PubMed] [Google Scholar]
- 18.Desbenoît N, Galardon E, Frapart Y, Tomas A, Artaud I. Reductive metalation of cyclic and acyclic pseudopeptidic bis-disulfides and back conversion of the resulting diamidato/dithiolato complexes to bis-disulfides. Inorg Chem. 2010;49:8637–8644. doi: 10.1021/ic101148c. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, et al. Methanobactin, a copper-acquisition compound from methane-oxidizing bacteria. Science. 2004;305:1612–1615. doi: 10.1126/science.1098322. [DOI] [PubMed] [Google Scholar]
- 20.Zauhar RJ, Colbert CL, Morgan RS, Welsh WJ. Evidence for a strong sulfur-aromatic interaction derived from crystallographic data. Biopolymers. 2000;53:233–248. doi: 10.1002/(SICI)1097-0282(200003)53:3<233::AID-BIP3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Yamada S, Misono T, Tsuzuki S. Cation-π interactions of a thiocarbonyl group and a carbonyl group with a pyridinium nucleus. J Am Chem Soc. 2004;126:9862–9872. doi: 10.1021/ja0490119. [DOI] [PubMed] [Google Scholar]
- 22.Mirica LM, Ottenwaelder X, Stack TDP. Structure and spectroscopy of copper-dioxygen complexes. Chem Rev. 2004;104:1013–1045. doi: 10.1021/cr020632z. [DOI] [PubMed] [Google Scholar]
- 23.Holm RH, Kennepohl P, Solomon EI. Structural and functional aspects of metal sites in biology. Chem Rev. 1996;96:2239–2314. doi: 10.1021/cr9500390. [DOI] [PubMed] [Google Scholar]
- 24.Bertini I, Cavallaro G, McGreevy KS. Cellular copper management—a draft user's guide. Coordin Chem Rev. 2010;254:506–524. [Google Scholar]
- 25.Harding MM. The architecture of metal coordination groups in proteins. Acta Crystallogr D Biol Crystallogr. 2004;60:849–859. doi: 10.1107/S0907444904004081. [DOI] [PubMed] [Google Scholar]
- 26.Warne T, et al. Structure of a β1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strader CD, Fong TM, Tota MR, Underwood D, Dixon RA. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 28.Cherezov V, et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen SG, et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 31.Tian H, Ma M. Molecular organization of the olfactory septal organ. J Neurosci. 2004;24:8383–8390. doi: 10.1523/JNEUROSCI.2222-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian H, Ma M. Differential development of odorant receptor expression patterns in the olfactory epithelium: A quantitative analysis in the mouse septal organ. Dev Neurobiol. 2008;68:476–486. doi: 10.1002/dneu.20612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez FI, et al. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science. 1999;283:996–998. doi: 10.1126/science.283.5404.996. [DOI] [PubMed] [Google Scholar]
- 34.Gerlach LO, et al. Metal ion enhanced binding of AMD3100 to Asp262 in the CXCR4 receptor. Biochemistry. 2003;42:710–717. doi: 10.1021/bi0264770. [DOI] [PubMed] [Google Scholar]
- 35.Holst B, Elling CE, Schwartz TW. Metal ion-mediated agonism and agonist enhancement in melanocortin MC1 and MC4 receptors. J Biol Chem. 2002;277:47662–47670. doi: 10.1074/jbc.M202103200. [DOI] [PubMed] [Google Scholar]
- 36.Zhorov BS, Ananthanarayanan VS. Signal transduction within G-protein coupled receptors via an ion tunnel: A hypothesis. J Biomol Struct Dyn. 1998;15:631–637. doi: 10.1080/07391102.1998.10508980. [DOI] [PubMed] [Google Scholar]
- 37.Kinouchi K, Standifer KM, Pasternak GW. Modulation of μ1, μ2, and δ opioid binding by divalent cations. Biochem Pharmacol. 1990;40:382–384. doi: 10.1016/0006-2952(90)90704-o. [DOI] [PubMed] [Google Scholar]
- 38.Neuba A, et al. The trinuclear copper(I) thiolate complexes [Cu3(NGuaS)3](0/1+) and their dimeric variants [Cu6(NGuaS)6](1+/2+/3+) with biomimetic redox properties. Angew Chem Int Ed. 2011;50:4503–4507. doi: 10.1002/anie.201008076. [DOI] [PubMed] [Google Scholar]
- 39.Sarangi R, et al. Spectroscopic and density functional theory studies of the blue-copper site in M121SeM and C112SeC azurin: Cu-Se versus Cu-S bonding. J Am Chem Soc. 2008;130:3866–3877. doi: 10.1021/ja076495a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang JF, Nadas IA, Kim MA, Franz KJ. A Mets motif peptide found in copper transport proteins selectively binds Cu(I) with methionine-only coordination. Inorg Chem. 2005;44:9787–9794. doi: 10.1021/ic051180m. [DOI] [PubMed] [Google Scholar]
- 41.Haas KL, Franz KJ. Application of metal coordination chemistry to explore and manipulate cell biology. Chem Rev. 2009;109:4921–4960. doi: 10.1021/cr900134a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.