Abstract
When animals are infected with helminthic parasites, resistant hosts show type II helper T immune responses to expel worms. Recently, natural helper (NH) cells or nuocytes, newly identified type II innate lymphoid cells, are shown to express ST2 (IL-33 receptor) and produce IL-5 and IL-13 when stimulated with IL-33. Here we show the relevant roles of endogenous IL-33 for Strongyloides venezuelensis infection-induced lung eosinophilic inflammation by using Il33−/− mice. Alveolar epithelial type II cells (ATII) express IL-33 in their nucleus. Infection with S. venezuelensis or intranasal administration of chitin increases in the number of ATII cells and the level of IL-33. S. venezuelensis infection induces pulmonary accumulation of NH cells, which, after being stimulated with IL-33, proliferate and produce IL-5 and IL-13. Furthermore, S. venezuelensis infected Rag2−/− mice increase the number of ATII cells, NH cells, and eosinophils and the expression of IL-33 in their lungs. Finally, IL-33–stimulated NH cells induce lung eosinophilic inflammation and might aid to expel infected worms in the lungs.
Keywords: helminth, Th2 cytokine, Loeffler syndrome
Animals, infected with intestinal nematodes, develop type II helper T (Th2) immune responses, which induce high level of IgE production, systemic eosinophilia, and local eosinophilic infiltration, particularly in the lung (1–4). We still do not know why only lungs develop such severe eosinophilic inflammation (Löffler syndrome) under helminth infection. We recently reported IL-33 is important for acute eosinophilic inflammation by using allergic conjunctivitis model (5).
IL-33, a member of IL-1 family cytokine, is a ligand of ST2 (IL-1RL1) (6). IL-33 was originally reported as a nuclear factor protein in endothelial cells of high endothelial venules (7). IL-33 is synthesized as an active full-length form and the processing by caspases abrogates its function (8–10). It is well documented that IL-33 is important for innate-type mucosal immunity in the lungs and gut (11) and for airway inflammation and peripheral antigen-specific responses (12). Th2 cells and various types of innate cells including basophils, mast cells, eosinophils, natural helper (NH) cells, and nuocytes express ST2 and produce Th2 cytokines in response to IL-33 (5, 13–16). Thus, IL-33–stimulated Th2 cells and innate cells play a critical role in various allergic inflammation by production of IL-4, IL-5, IL-13, and chemokines (5, 6, 14–19). Among innate cells, as we previously reported, only basophils and eosinophils produce IL-4 in response to IL-33, and as Moro et al. (15) and Neill et al. (16) showed, only NH cells and nuocytes produce IL-5 in response to IL-33. Helminthic parasite infection induces Th2 immune response. However, it remains uncertain how parasite infection stimulates innate cells to produce Th2 cytokines. Chitin, a widespread environmental biopolymer, provides structural rigidity to fungi, crustaceans, helminths, and insects (20). Because intranasal administration of chitin induces pulmonary eosinophilia (21), we focused on the function of chitin as a stimulator for IL-33 production.
In this article, we first demonstrated the alveolar epithelial type II cells (ATII) express IL-33 and Strongyloides venezuelensis infection or intranasal administration of chitin markedly increases the number of ATII cells. Second, we demonstrated that S. venezuelensis infection induces severe eosinophilic inflammation and goblet-cell hyperplasia in the lungs almost dependently on IL-33. Third, this parasite infection induces pulmonary eosinophilia even in Rag2−/− mice, suggesting the contribution of IL-33–stimulated NH cells. Fourth, this infection strongly and IL-33–dependently increases the number of NH cells. Finally, IL-33–stimulated NH cells induce lung eosinophilic inflammation through their production of IL-5 and IL-13.
Result
IL-33 Is Induced in the Lungs after S. venezuelensis Infection.
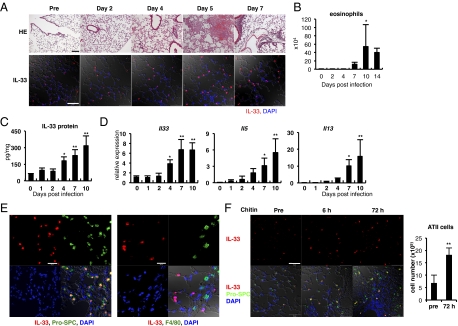
We examined histological differences of the lungs before and after S. venezuelensis infection. C57BL/6 (B6) WT mice, infected with third-stage larvae (L3) of S. venezuelensis, developed eosinophil-dominated leukocyte infiltration at days 5 and 7 (Fig. 1 A and B). Immunohistochemical analysis of lung tissues revealed that there were a small number of cells that expressed IL-33 in their nucleus even before infection (Fig. 1A). S. venezuelensis infection increased the number of these IL-33+ cells particularly at days 5 and 7 (Fig. 1A). These kinetics seemed to be proportional to that of induction of eosinophil infiltration in the bronchoalveolar lavage fluid (BALF) and of IL-33 protein production in the lung (Fig. 1 B and C). Next, we performed kinetic study of Il33 mRNA expression in the lungs after infection. We found S. venezuelensis infection increased the expression of mRNA for Il33 at day 4 and elevated further this expression at day 7 (Fig. 1D). These kinetics parallel well with that for Il5 or Il13, and the appearance of lung inflammation after S. venezuelensis infection (Fig. 1A). Nippostrongylus brasiliensis infection induced a similar kinetics of induction of Il33, Il5, and Il13 mRNA in BALB/c mice (Fig. S1A). Next we sought the IL-33–producing cells in the lung. DAPI staining data confirmed that IL-33 is present in the nucleus. These IL-33+ cells are ATII cells because they were also positively stained for prosurfactant protein C, a specific marker for ATII cells (22) (Fig. 1E, Left). To determine whether other types of cells, such as macrophages, also express IL-33, we stained macrophages with anti–IL-33 antibody and anti-F4/80 antibody, and found that they do not express IL-33 (Fig. 1E, Right). As chitin is a component of the outer membrane of parasites (20), and is included in soluble extracts from Strongyloides stercoralis (23), we examined the capacity of chitin to induce IL-33 production in the lungs. Immunohistochemical analysis of lung tissues revealed that an intranasal administration of chitin promptly increased the number of IL-33+-ATII cells in the lungs (Fig. 1F). This treatment promptly increased the IL-33 level in the BALF (Fig. S2A). We examined whether administration of chitin indeed increased the number of ATII cells. We counted the number of cells negative for T1α, CD16/32, and CD45.2 and positive for MHC class II as ATII cells (22, 24) (Fig. S3) and concluded that chitin treatment increased the number of ATII cells at least 72 h after treatment (Fig. 1F). Taken together, these results indicated that S. venezuelensis infection induces IL-33 production in the lung possibly by the action of chitin.
Fig. 1.
S. venezuelensis infection increases IL-33 expression in the lungs. (A–E) WT mice were infected with S. venezuelensis at day 0. (A) Histological analysis of lungs was performed at indicated days. (Upper) HE, stained with H&E. (Scale bar, 100 μm.) (Lower) Confocal microscopic analysis of the IL-33 expression. Red, IL-33; blue, DAPI. (Scale bar, 50 μm.) (B) The number of eosinophils in BALFs at indicated days (n = 3 ∼4). (C and D) IL-33 concentration in the lung lysates was examined by ELISA. The amounts of IL-33 were normalized by the total protein concentration (C). Quantitative RT-PCR (qPCR) analysis of the expression levels of mRNA for Il33, Il5, or Il13 in the lungs (D). Data are representative of two independent experiments and expressed as the means ± SD (n = 5) *P < 0.01, **P < 0.001 (B–D; one-way ANOVA with Dunnett's post test). (E) IL-33 (red) in the lungs at day 7 postinfection was costained with pro-SPC (green, Left) or F4/80 (green, Right). Blue, DAPI. (Scale bars, 20 μm.) (F) Chitin was intranasally administered into B6 mice. (Left) Confocal microscopic analysis of the lungs at indicated time points. Red, IL-33; blue, DAPI; green, pro-SPC. (Scale bar, 50 μm.) (Right) Lung cells were prepared from nontreated mice or mice treated with chitin 72 h before (n = 3). The numbers of ATII cells were calculated as described in Fig. S3.
Generation and Immunological Investigation of Il33−/− Mice.
We wished to determine whether endogenous IL-33 is critically required for establishment of lung eosinophilic inflammation in S. venezuelensis infected mice. For this purpose, we generated Il33 gene-deficient mice (Fig. S4 A and B) and examined their immunological properties. RT-PCR and Western blot analysis showed that the expression of IL-33 was completely abrogated in their lung tissues (Fig. S4 C and D). Proportions of T cells, B cells, dendritic cells, neutrophils, and eosinophils in the spleens of Il33−/− mice were comparable to those of Il33+/+ and Il33+/− mice (Fig. S4E). Anti-CD3–induced cytokine production responses revealed no skewing of splenic CD4+ T cells into Th1 or Th2 phenotype (Fig. S4F). Next, we examined the susceptibility of Il33−/− mice to S. venezuelensis infection. We simultaneously measured their systemic Th2/IgE response and mucosal mast cell activation in vivo. CD4+ T cells prepared from mesenteric lymph nodes of Il33−/−mice exhibited normal differentiation into Th2 cells (Fig. S5A). However, the measurement of serum levels of IgE and mouse mast cell protease 1, an activation marker of mucosal mast cells, indicated that the absence of IL-33 partly but significantly diminished these responses (Fig. S5 B and C). Furthermore, their capacity to expel S. venezuelensis was also modestly impaired (Fig. S5D). Thus, IL-33 is partly involved in the host defense against S. venezuelensis infection.
Il33−/− Mice Show Reduced Accumulation of Eosinophils in the Lungs After S. venezuelensis Infection.
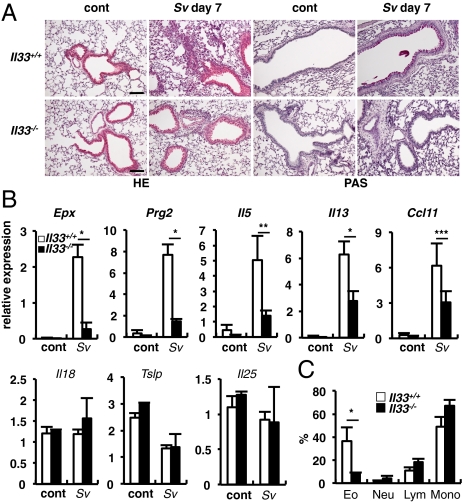
After S. venezuelensis infection, Il33+/+ mice developed eosinophilic inflammation and goblet-cell hyperplasia in the lungs at day 7, but Il33−/− mice only modestly developed these changes (Fig. 2A), suggesting critical involvement of IL-33 in these responses. Consistent with this modest eosinophilic inflammation, expressions of mRNA for eosinophil markers (25) Epx (eosinophil peroxidase) and Prg2 (major basic protein) in the lungs were significantly lower in Il33−/− mice than those in Il33+/+ mice (Fig. 2B). Because the development and the recruitment of eosinophils are regulated by IL-5, IL-13, and chemokines (e.g., CCL11) (26), respectively, we measured their mRNA expressions. We also measured the mRNA expression for the epithelial cells-derived cytokines, IL-18, IL-33, thymic stromal lymphopoietin and IL-25, all of which are shown to up-regulate allergic inflammation (27, 28). The expressions of Il5, Il13, and Ccl11 were strongly increased in the lungs of Il33+/+ mice, but significantly diminished in those of Il33−/− mice, suggesting that IL-33 is responsible for the productions of IL-5, IL-13, and CCL11, which in turn stimulate eosinophils to grow and infiltrate into the lung (Fig. 2B). Simultaneous measurement of epithelial cytokines revealed that S. venezuelensis infection selectively increased the expression of Il33 mRNA among these cytokines (Figs. 1D and 2B). These results strongly indicated the IL-33–dependent production of IL-5, IL-13, and CCL11 is essential for goblet-cell hyperplasia and eosinophilic inflammation in the lung after S. venezuelensis infection. Next we examined the proportion of eosinophils in the BALFs. There were very few eosinophils in the BALFs of uninfected mice. However, at day 7 after S. venezuelensis infection, we observed high proportion of eosinophils (Fig. 2C and Fig. S5E) in the BALFs from Il33+/+ mice. In contrast, this proportion in the BALFs from Il33−/− mice was relatively low (Fig. 2C and Fig. S5E). As chitin is shown to induce IL-33 production in the lung (Fig. 1E and Fig. S2A), we examined whether Il33+/+ mice developed eosinophilia after treatment with chitin. We found that mice treated with chitin, displayed infiltration of inflammatory cells around the chitin particles and marked goblet-cell hyperplasia at 72 h (Fig. S2B). In contrast, chitin-treated Il33−/− mice failed to develop these changes (Fig. S2C). Furthermore, chitin treatment increased the number of eosinophils in the BALF and the expression of Il5 and Il13 mRNA by BALF cells in an IL-33–dependent manner (Fig. S2 D and E).
Fig. 2.
Il33−/− mice showed reduced accumulations of eosinophils in the lungs after S. venezuelensis infection. (A) Histological analysis of lungs of Il33+/+ and Il33−/− was performed before (cont) and after S. venezuelensis infection (Sv). PAS, periodic acid-Schiff stain. (Scale bars, 100 μm.) (B) Total RNA was prepared from lungs and the levels of mRNA expressions for Epx, Prg2 and indicated cytokines were determined by qPCR. Data are representative of two independent experiments and expressed as the means ± SD (n = 3 ∼5) *P < 0.01, **P < 0.001, ***P < 0.05 (Student's t test). (C) Proportions of eosinophils (Eo), neutrophils (Neu), lymphocytes (Lym), or monocytes (Mono) in the BALF were calculated from flow cytometric analysis of CD45+ BALF cells from S. venezuelensis infected mice (Sv). Data are representative of two independent experiments and expressed as the means ± SD (n = 3 ∼5) *P < 0.05, (Student's t test).
S. venezuelensis Infection Induces Pulmonary Eosinophilia Even in the Absence of Acquired Immune Cells.
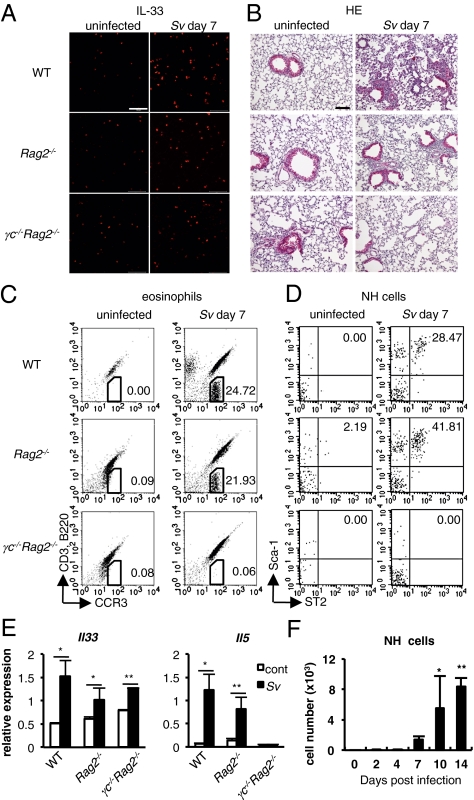
We wished to directly demonstrate that S. venezuelensis infection induces pulmonary eosinophilia without help from Th2 cells. We infected WT, Rag2−/−, or γc−/−Rag2−/− mice with S. venezuelensis. All of these mice increased the number of IL-33+ ATII cells in their lungs (Fig. 3A). Expectedly, like WT mice, Rag2−/− mice developed pulmonary eosinophilia (Fig. 3B). WT mice and Rag2−/− mice also increased the number of eosinophils in the BALFs and the expression of Il33 mRNA and Il5 mRNA in their lungs (Fig. 3 C and E). As innate cells, such as NH cells, were reported to produce IL-5 in response to IL-33 stimulation (29), we tried to show the presence of these innate cells in the BALF cells. Expectedly, these cells appeared as Sca-1+ST2+ cells in the FSClowSSClowLin− cells in the BALF cells from WT and Rag2−/− mice after infection (Fig. 3D). To further identify the phenotype of Lin−ST2+ cells, we examined the expression of other surface markers; then, we found they expressed Sca-1, Thy1.2, IL-7Rα, CD25, c-Kit, and ICOS and had limited expression of MHC class II, as described in NH cells (Fig. S6) (30). In contrast to WT and Rag2−/−, γc−/−Rag2−/− mice, which have no NH cells in mesenteric tissues (15), failed to develop these changes, suggesting the importance of the expression of the γc chain for the induction of pulmonary eosinophilia, NH cell proliferation, and IL-5 expression. NH cells emerged around day 7 after infection and expanded at least until day 14 in WT mice (Fig. 3F). Along with their expansion, degree of eosinophilia and expression of IL-5 and IL-13 are simultaneously up-regulated (Fig.1 B and D). Similar increases in the number of NH cells in the lungs were also observed at day 7 after N. brasiliensis infection (Fig. S1B).
Fig. 3.
WT, Rag2−/−, and γc−/−Rag2−/− (n = 3~5) mice were uninfected (cont) or infected with S. venezuelensis (Sv). (A) Confocal microscopic analysis of the IL-33 (red) expression in the lungs. (Scale bars, 50 μm.) (B) Histological analysis (H&E) of lungs. (Scale bar, 100 μm.) (C and D) Flow cytometric analysis of the BALF cells. Numbers indicate proportion of eosinophils (C) or NH cells (D). Cells were gated on the CD45+ fraction (C) or FSClowSSClowLin− fraction (D). (E) qPCR analysis of the expression levels of mRNA for Il33 and Il5 in the lungs. Data are expressed as the means ± SD *P < 0.01, **P < 0.05 (Student's t test). (F) The numbers of NH cells in the BALFs from mice in Fig. 1B are shown. Data are expressed as the means ± SD *P < 0.01, **P < 0.001 (one-way ANOVA with Dunnett's post test). Data are representative of two independent experiments.
NH Cells Are Induced in S. venezuelensis Infected Mice in an IL-33–Dependent Manner.
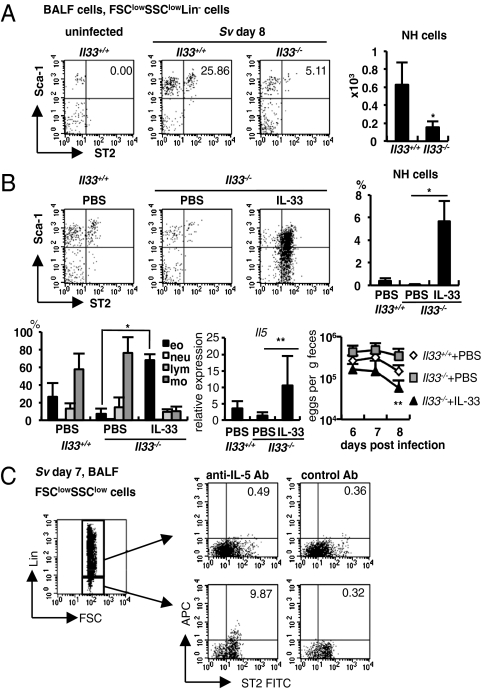
We demonstrated that S. venezuelensis infected Il33+/+ mice but not Il33−/− mice markedly increased the expression of Il5 and Il13 mRNA in their lungs (Fig. 2B). Thus, we examined whether S. venezuelensis infection increased the number of NH cells by induction of IL-33 production in the lungs. Compared with Il33+/+ mice, Il33−/− mice exhibited significantly reduced number of NH cells in the BALFs (Fig. 4A), suggesting the importance of endogenous IL-33 for the induction of NH cells. To determine whether IL-33 is directly responsible for increasing NH cells and IL-5 expression, we examined the effects of IL-33 on these responses by intranasal administration. Intranasal administration of IL-33 strongly increased the number of NH cells and eosinophils in the BALF of Il33−/− mice (Fig. 4B). At the same time, this treatment strongly increased the expression of Il5 mRNA in the lungs.
Fig. 4.
IL-33-dependent induction of NH cells in S. venezuelensis infected mice. (A) Flow cytometric analysis of NH cells in BALF cells from uninfected or S. venezuelensis infected (Sv) Il33+/+ or Il33−/− mice. Cells were gated on the FSClowSSClowLin− fraction. (Right) The numbers of NH cells in total BALF cells from Il33+/+ (n = 11) or Il33−/− (n = 5) mice. Data are expressed as the means ± SD *P < 0.0005 (Student's t test). (B) Il33+/+ (n = 7) and Il33−/− mice (PBS; n = 4, IL-33; n = 6) were infected with S. venezuelensis at day 0 and treated daily with 30 μL of PBS or 4 μg rhIL-33 intranasally from day −1 to 3. (Upper) Flow cytometry of NH cells in FSClowSSClowLin− fraction of BALF cells and the proportion of NH cells in total BALF cells at 8 d postinfection. (Lower Left) The proportion of eosinophils (eo), neutrophils (neu), lymphocytes (lym), or monocytes (mo) in BALF cells. (Lower Center) The expression of Il5 mRNA in the lungs. (Lower Right) The numbers of eggs per gram feces from each group at day 6, 7, or 8 postinfection. Data are expressed as the means ± SD; *P < 0.001, **P < 0.05 versus corresponding values for Il33−/− mice with PBS treatment (Student's t test). (C) Intracellular staining of IL-5 in Lin−ST2+ cells in BALF cells. Cells were stained as described in SI Materials and Methods. Numbers indicate the proportion of ST2+IL-5+ cells. Data are representative of five mice and of two independent experiments.
This treatment also significantly accelerated the worm expulsion in Il33−/− mice (Fig. 4B, Right). Finally, we tried to identify the cells that produce IL-5 in response to IL-33 in the lungs of S. venezuelensis infected mice. We prepared BALF cells from WT mice at day 7 after infection and divided them into two fractions: lineage marker- (CD3, CD4, CD8, CD19, NK1.1, Gr-1, siglec F, IgE) positive and negative fractions. We could not find IL-5–producing cells in Lin+ cells, thus excluding the presence of IL-5–producing Th2 cells in the BALF (Fig. 4C). However, we found a substantial proportion of ST2+ cells in Lin− fraction produced IL-5 (Fig. 4C). Taking these data together, IL-33 contributes to the induction of NH cells, which in turn protect host against S. venezuelensis infection by inducing lung eosinophilia through their production of IL-5 and IL-13.
Discussion
We demonstrated that S. venezuelensis infection of mice induced severe eosinophilic inflammation, goblet-cell hyperplasia, and accumulation of NH cells, and increased the number of IL-33–producing ATII cells and the expressions of mRNA for Il5 and Il13 in the lungs, even without help from acquired immune cells. Intranasal administration of chitin also induced similar pulmonary changes, suggesting that S. venezuelensis infection induced these alterations principally by the action of chitin. In contrast, Il33−/− mice infected with S. venezuelensis failed to develop these pathological changes. Thus, IL-33 plays a critical role in induction of eosinophilia and goblet-cell hyperplasia in the lung.
In this article, we showed that S. venezuelensis infection normally induced the development of Th2 cells in mesenteric lymph nodes of Il33−/− mice, which did not develop severe pulmonary eosinophilia, confirming that there are some innate cells that produce IL-5 and IL-13 in response to IL-33 in the lungs. We could demonstrate that S. venezuelensis infection increased the number of NH cells by inducing IL-33 production in the lungs. Because we could not detect NH cells in the BALF before infection, we speculated that they existed in parenchyma and proliferated in response to IL-33. Consistent with this theory, NH cells were recently found in the normal lung and increased by infection with H3N1 subtype of influenza A virus (30), and were shown to proliferate in response to IL-33 and IL-2 in vitro (15). Influenza virus infection induces NH cells/IL-13–dependent airway hyper-reactivity in the lung, but cannot induce pulmonary eosinophilia. This finding is quite different from our case, that S. venezuelensis infection induces IL-33–dependent eosinophilia. We suspected that this discrepancy could come from IL-10 production in influenza virus-infected mice because IL-10 is shown to inhibit eosinophil accumulation (31, 32).
Pulmonary eosinophilia is a very common complication of helminth infections, such as Strongyloides, Ascaris, Toxocara, and Ancylostoma species (33). Nevertheless, the physiological relevance and the pathogenesis of pulmonary eosinophilia are still not thoroughly understood. Because helminth infection strongly induces Th2 immune response (4), it is generally accepted that Th2 cells are responsible for inducing both systemic eosinophilia and local pulmonary eosinophilia (Löffler syndrome) (34). On the other hand, it is well documented that most of the nematodes cause pulmonary eosinophilia during larval migration through the lungs (35, 36). Indeed, we observed several hemorrhagic areas in the lung tissues of mice at day 5 after S. venezuelensis infection. Furthermore, nematode infection might induce pulmonary eosinophilia without systemic eosinophilia (37). In this article, we demonstrated lung eosinophilic inflammation seen with Löffler syndrome is induced by the action of NH cells even in the absence of conventional Th2 cells. However, compared with that in S. venezuelensis infected WT mice, the degree of lung eosinophilic inflammation in S. venezuelensis infected Rag2−/− mice seems relatively mild. Thus, acquired immune cells might partly be responsible for increasing degree of lung inflammation by producing Th2 cytokines.
We demonstrated that mouse ATII cells, which express the surfactant proteins required for the expansion of the lung and produce cytokines and chemokines for immune regulation (24), began to express IL-33 in their nucleus at day 2 after infection with S. venezuelensis or at 6 h after treatment with chitin. It has been reported that bronchial epithelial cells or alveolar macrophages also express IL-33 (30, 38). Here we demonstrated that macrophages from S. venezuelensis infected mice did not express IL-33. We speculated that, because influenza virus activates Toll-like receptor 7 (TLR7) signal (39), macrophages may require TLR signaling for IL-33 expression.
There are two types of alveolar epithelial cells: ATI and ATII. ATII cells only cover 5% of the surface and the remaining 95% of the surface is covered by ATI cells (24). It may therefore be hypothesized that ATI cells might be more likely to be damaged by microbes, such as S. venezuelensis, than ATII cells. ATII cells have the potential to proliferate and develop into ATI cells eventually, suggesting that damaged ATI cells are replaced by ATII cells. In this article, we demonstrated that administration of chitin induces an increase in the number of ATII cells in the lung. Thus, along with the increased number of ATII cells, the production of IL-33 in the lung is rapidly increased. Subsequently, IL-33 stimulates NH cells to proliferate and to produce IL-5 and IL-13, which in combination induce severe eosinophilic inflammation and goblet-cell hyperplasia in the lung.
We demonstrated that NH cells increased in the lungs of S. venezuelensis infected mice. NH cells and nuocytes have similarity in their expression patterns of surface antigens or the capacity for cytokine production. It has been demonstrated that nuocytes expand in response to both IL-25R–mediated and ST2-mediated signalings in N. brasiliensis infected mice (16). Here we reported that IL-33–deficient mice showed severely impaired accumulation of NH cells in the lungs after S. venezuelensis or N. brasiliensis infection compared with WT mice, although the expression of mRNA for Il25 did not differ in the lungs between WT and Il33−/− mice or before and after S. venezuelensis infection. At present, we cannot demonstrate that the expanded cells that produce IL-5 and IL-13 in the lungs of S. venezuelensis infected mice are either NH cells or nuocytes. On the other hand, we can exclude the contribution of multipotent progenitor (MMP) cells (40) or innate type-2 helper (Ih2) cells (19), because MMP or Ih2 cells have been shown to lack ST2 and Sca-1 expression, respectively (41).
Previous reports have shown that IL-33 is involved in eosinophils expansion (6). In agreement with this finding, IL-33–deficient mice showed significantly impaired accumulation of eosinophils in their lungs after S. venezuelensis infection. It has been also demonstrated that IL-33 can stimulate eosinophils to increase their survival, adhesion and production of cytokines and chemokines, and to produce superoxide anion and degranulation (5, 42, 43). These effects of IL-33 for the expansion and the activation of eosinophils might aid to expel infected worms in the lungs.
In conclusion, IL-33 is important not only for the expansion of NH cells but also for their production of IL-5, IL-13, and CCL11, which in turn induce the accumulation of eosinophils in S. venezuelensis infected mice.
Materials and Methods
Helminths Infection.
In vivo passage and animal infection of S. venezuelensis were shown previously (44). N. brasiliensis (Nb) has been maintained in male SD rat. Mice were inoculated subcutaneously with 500 L3 Nb. Details of analyses are described in SI Materials and Methods.
Statistics.
All data are shown as the mean ± SD. The numerical data were analyzed using either Student's t test or one-way ANOVA with Dunnett's post test. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank Dr. F. D. Finkelman for his critical reading and valuable comments; A. Usumoto and C. Minemoto for secretarial assistance; K. Kamei-Kobayashi and M. Nagata for technical assistance; and M. Yamada (Kyoto Prefectural University of Medicine) for providing Nippostrongylus brasiliensis. This work was supported by The Japanese Ministry of Education, Culture, Sports, Science and Technology (Grant-in-Aid for Scientific Research on Priority Areas 18073016) and The Japan Society for the Promotion and Science (Grants-in-Aid for Scientific Research 23249022 and Grant-in-Aid for Young Scientists 23790450).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201042109/-/DCSupplemental.
References
- 1.Finkelman FD, et al. Interleukin-4- and interleukin-13–mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 2.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakanishi K. Basophils are potent antigen-presenting cells that selectively induce Th2 cells. Eur J Immunol. 2010;40:1836–1842. doi: 10.1002/eji.201040588. [DOI] [PubMed] [Google Scholar]
- 4.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuba-Kitamura S, et al. Contribution of IL-33 to induction and augmentation of experimental allergic conjunctivitis. Int Immunol. 2010;22:479–489. doi: 10.1093/intimm/dxq035. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Baekkevold ES, et al. Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol. 2003;163:69–79. doi: 10.1016/S0002-9440(10)63631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talabot-Ayer D, Lamacchia C, Gabay C, Palmer G. Interleukin-33 is biologically active independently of caspase-1 cleavage. J Biol Chem. 2009;284:19420–19426. doi: 10.1074/jbc.M901744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lüthi AU, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Oboki K, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louten J, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol. 2011;23:307–315. doi: 10.1093/intimm/dxr006. [DOI] [PubMed] [Google Scholar]
- 13.Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo Y, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 15.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 16.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–2449. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 18.Ho LH, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 19.Price AE, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. Bioessays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 21.Reese TA, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–96. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demaio L, et al. Characterization of mouse alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1051–L1058. doi: 10.1152/ajplung.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein LH, et al. Eosinophils utilize multiple chemokine receptors for chemotaxis to the parasitic nematode Strongyloides stercoralis. J Innate Immun. 2009;1:618–630. doi: 10.1159/000233235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herzog EL, Brody AR, Colby TV, Mason R, Williams MC. Knowns and unknowns of the alveolus. Proc Am Thorac Soc. 2008;5:778–782. doi: 10.1513/pats.200803-028HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster PS, et al. Elemental signals regulating eosinophil accumulation in the lung. Immunol Rev. 2001;179:173–181. doi: 10.1034/j.1600-065x.2001.790117.x. [DOI] [PubMed] [Google Scholar]
- 27.Tsutsui H, Mizutani H, Nakanishi K. Contribution of interleukin 18 to the development of infection-associated atopic dermatitis. Curr Probl Dermatol. 2011;41:93–103. doi: 10.1159/000323302. [DOI] [PubMed] [Google Scholar]
- 28.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: Epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koyasu S, Moro K. Innate Th2-type immune responses and the natural helper cell, a newly identified lymphocyte population. Curr Opin Allergy Clin Immunol. 2011;11:109–114. doi: 10.1097/ACI.0b013e3283448808. [DOI] [PubMed] [Google Scholar]
- 30.Chang YJ, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sel S, et al. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL-12 and IL-10. J Immunol. 2007;178:7805–7813. doi: 10.4049/jimmunol.178.12.7805. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med. 2009;15:277–284. doi: 10.1038/nm.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chitkara RK, Krishna G. Parasitic pulmonary eosinophilia. Semin Respir Crit Care Med. 2006;27:171–184. doi: 10.1055/s-2006-939520. [DOI] [PubMed] [Google Scholar]
- 34.Culley FJ, Brown A, Girod N, Pritchard DI, Williams TJ. Innate and cognate mechanisms of pulmonary eosinophilia in helminth infection. Eur J Immunol. 2002;32:1376–1385. doi: 10.1002/1521-4141(200205)32:5<1376::AID-IMMU1376>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Silveira MR, et al. Infection with Strongyloides venezuelensis induces transient airway eosinophilic inflammation, an increase in immunoglobulin E, and hyperresponsiveness in rats. Infect Immun. 2002;70:6263–6272. doi: 10.1128/IAI.70.11.6263-6272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshino K, et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190:1541–1548. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Te Booij M, de Jong E, Bovenschen HJ. Löffler syndrome caused by extensive cutaneous larva migrans: A case report and review of the literature. Dermatol Online J. 2010;16:2. [PubMed] [Google Scholar]
- 38.Préfontaine D, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 40.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil-mediated airway inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki Y, et al. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J Exp Med. 2005;202:607–616. doi: 10.1084/jem.20042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.