Abstract
The world's oceans are undergoing profound changes as a result of human activities. However, the consequences of escalating human impacts on marine mammal biodiversity remain poorly understood. The International Union for the Conservation of Nature (IUCN) identifies 25% of marine mammals as at risk of extinction, but the conservation status of nearly 40% of marine mammals remains unknown due to insufficient data. Predictive models of extinction risk are crucial to informing present and future conservation needs, yet such models have not been developed for marine mammals. In this paper, we: (i) used powerful machine-learning and spatial-modeling approaches to understand the intrinsic and extrinsic drivers of marine mammal extinction risk; (ii) used this information to predict risk across all marine mammals, including IUCN “Data Deficient” species; and (iii) conducted a spatially explicit assessment of these results to understand how risk is distributed across the world's oceans. Rate of offspring production was the most important predictor of risk. Additional predictors included taxonomic group, small geographic range area, and small social group size. Although the interaction of both intrinsic and extrinsic variables was important in predicting risk, overall, intrinsic traits were more important than extrinsic variables. In addition to the 32 species already on the IUCN Red List, our model identified 15 more species, suggesting that 37% of all marine mammals are at risk of extinction. Most at-risk species occur in coastal areas and in productive regions of the high seas. We identify 13 global hotspots of risk and show how they overlap with human impacts and Marine Protected Areas.
Keywords: International Union for the Conservation of Nature Red List, threatened and endangered species, life history, random forest models
Oceans occupy 71% of the earth's surface and harbor much of its biodiversity. Despite the vast expanse of the oceans, no area remains unaffected by humans (1). Human activities are polluting, warming, and acidifying the oceans, melting sea ice, overharvesting fisheries, and altering entire food webs (1–4). Fisheries bycatch causes deaths of more than 650,000 marine mammals each year (5). Overfishing has depleted food supplies by reducing fish populations by 50–90%, and industrial-scale krill harvesting will likely further deplete food resources (6–8). In addition, polar oceans are warming at rates twice as fast as the global average (3); this has already altered whale migrations, reduced benthic prey populations, and caused declines in seals and polar bears (Ursus maritimus) whose lifestyles are dependent on sea ice (9). The International Union for the Conservation of Nature (IUCN) Red List currently classifies 25% (32 of 128 species) of marine mammals as threatened with extinction. Examination of the threats on the basis of the Red List shows that nearly half of all species are threatened by two or more human impacts, with pollution being the most pervasive, followed by fishing, invasive species, development, hunting, and climate change (Fig. S1).
However, our understanding of which marine mammals are most at risk remains poor because many species are difficult to study, changes in their populations can be hard to detect, and their natural histories have not been well documented (10–12). Indeed, the conservation status of about 40% of marine mammal species has not been categorized by the Red List, mostly because of insufficient information (i.e., “Data Deficient” species), and with ever-increasing human impacts on the oceans, many more species likely will become threatened in the near future. Predictive, spatially explicit models that can identify which species are most likely to be at risk are urgently needed to address the rapid changes impacting marine mammal biodiversity (13, 14). Such quantitative models have been developed for terrestrial mammals (14–17) and for some marine species (16, 18), but are lacking for marine mammals as a whole at the global scale.
Here, we provide a predictive, spatially explicit assessment of global marine mammal extinction risk. We combined spatial analyses with a powerful machine-learning technique and an ecoinformatic database to determine (i) which marine mammal species are at greatest risk; (ii) why they are threatened; and (iii) where risk is greatest globally. Because extinction results from the combination of species’ attributes, geographic settings, and human threats, we developed a predictive model of extinction that considers the important interactions between intrinsic species’ traits and extrinsic environmental variables, including spatially explicit human impacts on the world's oceans (1). Using this information, we then identified major geographic hotspots of extinction risk and showed how these regions overlap with human activities to inform marine conservation.
We compiled a species-level database for 125 extant marine mammals, including cetaceans, pinnipeds, sirenians, polar bears, and two species of otters. Our database consisted of two kinds of predictor variables: (i) intrinsic biological traits (adult body mass, geographic range size, life-history traits, social group size, trophic group, habitat, foraging location, taxonomic order, diet breadth, and migratory behavior) and (ii) extrinsic environmental variables [mean annual net primary production (ANPP) (19) and mean human impact index (1)] within each species’ geographic range (SI Materials and Methods). For the intrinsic life-history variables, we included traits that determine the speed of life history (20, 21). Specifically, we used the components of mass-specific production, p, where p = (mw/mA) ⋅ l ⋅ n, where mA is adult body mass, mw is offspring weaning mass, l is litter size, and n is number of births per year (22). We then used a dichotomous response variable to represent extinction risk: species classified as Vulnerable, Endangered, Critically Endangered, or Extinct by the IUCN were considered “threatened”; species classified as Near Threatened or of Least Concern were considered “non-threatened” (23).
We quantified relationships between predictor variables and extinction risk using a random forest model of 500 classification trees (24, 25). This is a powerful machine learning technique that combines the predictions of multiple independent decision tree models into a robust composite model with high predictive accuracy (24, 26, 27). Decision trees are able to disentangle complex ecological phenomena, such as extinction risk, by identifying nonlinear, context-dependent interactions among multiple, correlated predictor variables (13, 24). Moreover, these models are non-parametric techniques that provide viable alternatives to phylogenetic contrasts (28). To assess the role of phylogeny, we included taxonomic group in our models. We used the random forest model to estimate the relative importance of each predictor variable and to predict threat status for each species, including Data Deficient species. We provide further details of methodology in Materials and Methods and in SI Materials and Methods, and a list of species predicted to be at risk in Table S1.
Results and Discussion
Our random forest model classified species on the Red List with 92% accuracy (Cohen's kappa = 0.8, P < 0.0001; see Table S2 for all goodness-of-fit metrics). Our model identified 27 of the 32 species currently recognized as Vulnerable or Endangered on the Red List plus an additional 15 species (Table S2). Of the latter 15, 2 are currently listed as Least Concern and the remaining 13 are Data Deficient on the Red List. Summing the 27 species that were both predicted by our model and on the Red List, the 5 species on the Red List but not predicted by our model, and the 15 species predicted by our model but not on the Red List gives a total of 47 species, or 37% of extant marine mammals, likely to be at risk of extinction.
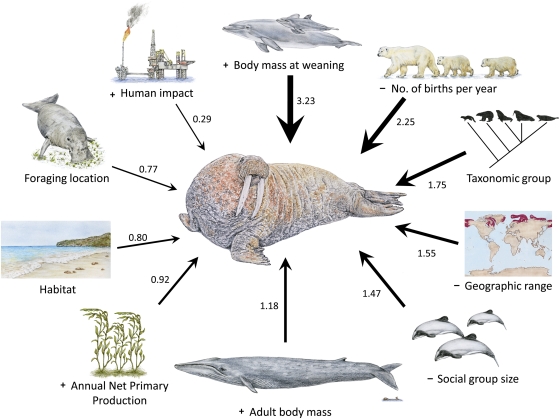
In decreasing order of importance, the primary predictors of risk identified by our random forest model were body mass at weaning, number of births per year, taxonomic group, geographic range area, and social group size (Fig. 1 and Fig. S2). The first two predictors, mean body mass at weaning and number of births per year, highlight the influence of life history. Because all marine mammals except polar bears give birth to only one offspring per reproductive cycle, size of offspring at weaning multiplied by the frequency of breeding gives productivity, or rate of biomass production via reproduction (22). So, together, these two variables index the speed of life history and are the primary determinants of rmax, the maximum or intrinsic rate of population increase and the capacity for species to recover from reduced populations after threats have been removed. Rate of population increase after depletion is important to marine conservation (29–31). For example, baleen whales have fast life histories for their body size, and several species, including humpbacks and gray whales (Megaptera novaeangliae and Eschrichtius robustus), have shown strong recoveries following the international ban on commercial whaling (4, 30). Other taxa, including sea otters (Enhydra lutris) and northern elephant seals (Mirounga angustirostris), have increased exponentially after protection (30). The latter had been reduced to 20–30 individuals by 1900, and despite very low genetic diversity, elephant seals increased at an estimated 8.3% per year to a population of ∼170,000 today (23, 32). These results imply that when species with high productivities fail to rebound rapidly after protection, they have not achieved the near-maximal rates of population growth expected on the basis of their life histories. This suggests that the original environmental threats have not been alleviated or that new threats, such as climate change, have arisen to inhibit recovery (e.g., 33). Similar issues apply to species with low productivities, but more time may be required to assess whether failure to recover after protection is due to intrinsic life-history characteristics or extrinsic environmental factors.
Fig. 1.
Relative importance, in rank order, of intrinsic and extrinsic predictors of marine mammal extinction risk. Numerical values of importance for each predictor variable were calculated as the decrease in classification accuracy after predictor removal in a random forest of 500 trees. Model accuracy was 92% (Cohen's kappa = 0.8, P < 0.0001; Table S2 and Fig. S2). “+” or “−” indicates the direction of correlation for the continuous variables. Drawings are by Sharyn N. Davidson.
At the high-risk end of the spectrum were species with low rates of production and so with slow life histories. These species often belonged to specific taxa (orders, families, and genera), suggesting constraints of intrinsic traits inherited from common ancestors and therefore related to phylogeny. Interestingly, slow speed of life history also has been shown to be a strong predictor of risk in ungulates and terrestrial carnivores (34). Sirenians (Order Sirenia: manatees and dugongs) are a good example of marine mammals at the high-risk end of the spectrum. They have low productivities and are the only herbivorous marine mammals. All five extant species in the order are at risk (23), and the giant Steller's sea cow (Hydrodamalis gigas) was hunted to extinction within a few decades after discovery by Europeans. Most toothed whales also have low production rates, but they have large geographic ranges and often form large social groups, which helps offset risk. Pinnipeds, on the other hand, have relatively high rates of production; however, walruses (Odobenus rosmarus) and eared seals (Otariidae) generally have slower rates than true seals (Phocidae), which can make them more vulnerable. Nevertheless, some true seals, such as monk seals (Monachus spp.), are critically endangered due to high human impacts within their small geographic ranges (5). So, although speed of life history is the most important predictor of extinction risk overall, decision tree analyses emphasize that there are multiple pathways to extinction, and risk usually cannot be attributed to a single intrinsic or extrinsic variable (16) (Fig. 1).
Other intrinsic traits, including small geographic range area and small social group size, were also important predictors, consistent with traits identified for terrestrial mammals in general (16) (Fig. 1 and Fig. S3). Small geographic range is a robust predictor of risk across many vertebrate groups (16, 34–36), and this includes species whose ranges have contracted significantly due to human impacts (16, 37). In marine mammals, social group size may reduce risk because of the advantages of sociality in reducing predation and enhancing foraging. The endangered, endemic Galápagos fur seal (Arctocephalus galapagoensis) (5, 23) is a good example; it has one of the smallest ranges of all marine mammals and a small social group size. Extrinsic environmental variables were generally poorer predictors than were intrinsic traits, perhaps in part because they are indirect and affect extinction by interacting with life history and other biological traits and in part because environmental variables were obtained from global databases that may be too coarse-grained to capture localized human threats.
Our analysis predicted that 13, or about one-third, of all Data Deficient species may be at risk of extinction. One of these is the boto (Amazon River dolphin; Inia geoffrensis). It and other river dolphins are especially vulnerable because they have not only slow life histories, but also small social group sizes and extremely small geographic ranges. Although our analysis was not able to evaluate extrinsic predictors for river dolphins (see Materials and Methods), they do face intense human pressures from pollution, fishing, and damming (e.g., by Brazil's recently approved Belo Monte hydroelectric dam, which will be the third largest in the world). The walrus is another Data Deficient species predicted to be at risk. It is threatened by ocean warming, which is reducing sea ice used for breeding, feeding, and resting and leading to increased shipping traffic, pollution, and development (38). Several Data Deficient beaked whales (Ziphiidae) and other whale and dolphin species were also predicted to be at risk. In fact, none of the beaked whales have a designated conservation status under the Red List (23). They are elusive, deep-sea mammals that occur in low abundances and depend on critical habitat like isolated deep-sea canyons (39). Because of their deep-diving behavior, they appear to be especially vulnerable to decompression sickness triggered by naval sonar (39).
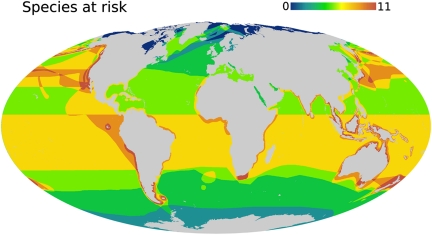
Using the at-risk species identified by our model and the Red List, we created maps showing the global distribution and hotspots of risk (Figs. 2–4). Hotspots were defined as cells with six or more at-risk species, corresponding to the top 2% of geographic grid cells (totaling about 12,950,000 km2). Globally, the marine grid cells contained from 0 to 11 at-risk marine mammal species (Fig. 2); cells with 6 or more at-risk species represent the 75th percentile of at-risk marine mammal richness. We then mapped marine mammal species richness (Fig. S4A), marine productivity (ANPP) (19) (Fig. S4B), human impacts (1) (Figs. 3 and 4), and Marine Protected Areas (MPAs) (40) (Fig. 4D) to relate the geographic distribution of risk predicted by our model to the distributions of marine mammal species, extrinsic environmental factors included in our model, and protected areas. We determined the correlations between risk and species richness and ANPP, and calculated mean and range of the human impact index (1) within each risk hotspot in ArcGIS v9.3 (Fig. S5). We also overlapped our hotspots with the geographic distributions of total human impacts (1), specific human impacts (including commercial fishing, shipping, pollution, and sea-surface temperature anomalies indicating recent climate change) (1), and MPAs (40) (Figs. 3 and 4; Fig. S6).
Fig. 2.
Global distribution of marine mammal species at risk (model-predicted plus IUCN Red List species).
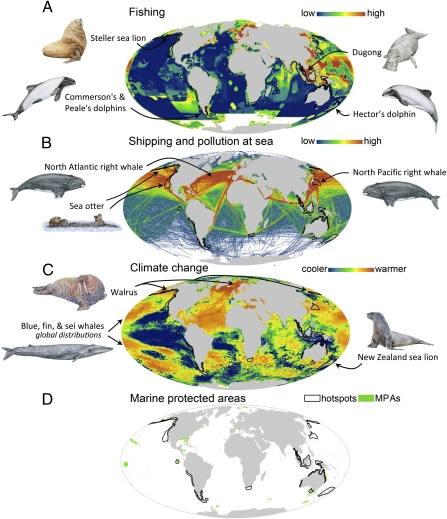
Fig. 4.
Global hotspots of marine mammal species extinction risk, overlaid with the geographic distributions of the leading human impacts (1) on marine mammals and with Marine Protected Areas (MPAs) (39). (A) Fishing intensity. (B) Ship traffic and pollution. (C) Sea-surface temperature change: 1985–2005. (D) World distribution of MPAs (see also Fig. S6 for magnified view of D). Hotspots show the top 2% of geographic grid cells for at-risk species (model-predicted plus IUCN Red List species). Maps A, B, and C show examples of species predicted to be at risk by our model that occur within the hotspots, or other highly impacted regions, and whose populations are threatened by fishing, shipping, pollution, or climate change. Drawings are by Sharyn N. Davidson.
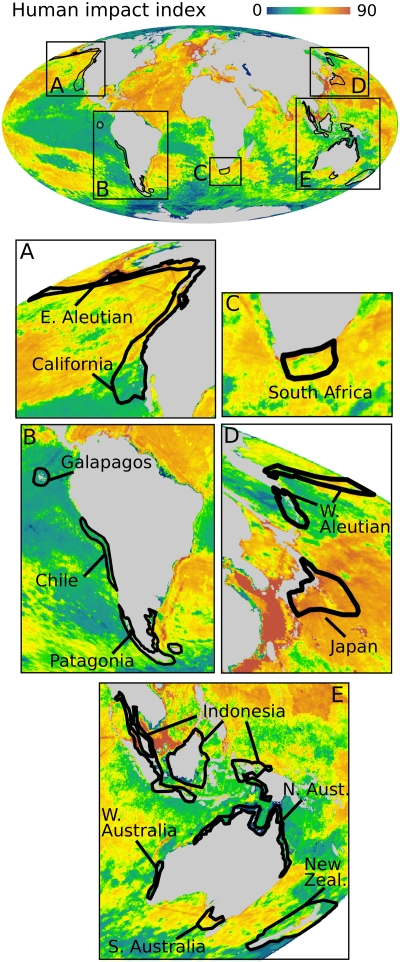
Fig. 3.
Global hotspots of marine mammal species extinction risk, overlaid with human impact on the world's oceans (1). Hotspots show the top 2% of geographic grid cells for at-risk species (model-predicted plus IUCN Red List species). Panels A–E provide a magnified view of hotspots.
Not surprisingly, there was broad agreement in the geographic distribution of species on the Red List and those that our model predicted to be at risk. Importantly, however, our model identified additional high-risk areas in the Indo-Pacific, around South Africa, New Zealand, and Patagonia, and along the western coasts of South America and central Africa (Fig. 2) that are not currently recognized (12). In general, the spatial distribution of risk correlated strongly with that of marine mammal species richness (r = 0.58, n = 50,927, P < 0.0001) and, to a lesser extent, with net primary productivity (r = 0.18, n = 50,927, P < 0.0001) (Fig. S4). Most at-risk species and all 13 hotspots were distributed along coastlines (Figs. 2 and 3). Coastal areas with cold currents and upwelling are highly productive and support large populations and diverse species of marine mammals (12). However, most coastal areas also experience high levels of human impact (1), and our random forest model indicated that species that live, forage, or breed along coasts are at higher risk. Consequently, other coastal regions, in addition to the specific hotspots identified here, should be considered as high priority areas for conservation. Some regions of the open ocean, such as the North Pacific Transition Zone, also warrant protection because they are highly productive, have high abundance and species diversity of large predators (including marine mammals), and have been heavily impacted by human activities (41, 42).
Our analysis showed that 74% of marine mammal species experience high levels of human impact [impact index >60 (1)] within their geographic ranges (Fig. S7). These reflect the cumulative effects of many factors, including fishing, shipping, pollution, sea-surface temperature change, ocean acidification, invasive species, oil rigs, and human population density (1). However, levels of human impact varied spatially across most species’ geographic ranges and even within hotspots (Fig. 3 and Fig. S5). Localized human impacts were extremely high in the Indonesian, Japanese, Californian, and northern Australian hotspots (Fig. 3 and Fig. S5).
Overfishing and bycatch are among the leading anthropogenic threats to marine mammals worldwide (5) and were particularly high in the hotspots of the eastern Aleutian Islands and Patagonia, and especially in the Indonesian hotspot in the biologically rich Indo-Pacific (Fig. 4A). Shipping and pollution are widespread throughout the Northern Hemisphere (1) and impact marine mammals through direct ship strikes, noise (e.g., ship, military, and industrial activities), and other forms of pollution (e.g., oil spills, chemical wastes, entanglement in abandoned fishing gear, ingestion of plastic debris) (43). These impacts are especially high in the Californian and Japanese hotspots, where there are major human population centers and shipping routes (Fig. 4B) (1). Climate and oceanographic changes are widespread and escalating throughout the world's oceans (1, 3) and figure importantly in some of the higher latitude hotspots (e.g., western and eastern Aleutian Islands, Japan, and South Australia) where temperature changes have been more dramatic (Fig. 4C). The prospect of a warming ocean is especially serious for marine mammals, such as polar bears, walruses, and several species of seals, which occur at high latitudes and depend on sea ice for feeding, breeding, and/or resting (38).
The risk hotspots cover only 1.7% of the global oceans, but they include at least parts of the geographic ranges of 88 (70%) marine mammal species. These hotspots do not capture all regions and habitats in need of protection, however, because high levels of human impact threaten populations and species of marine mammals well beyond the hotspots. For example, the vaquita (Phocoena sinus), perhaps the single most endangered marine mammal species, is threatened by localized artisanal fishing activity in inadequately protected areas in the Gulf of California (5, 44). Ship strikes also are the primary threat to the world's remaining ∼350 North Atlantic right whales (Eubalaena glacialis), whose geographic range overlaps with intensive shipping activity (45) (Fig. 4B). Climate change is likely to have wide-ranging, disruptive impacts on many species throughout the world's oceans, but these are only beginning to be understood (38, 46). Nevertheless, the distribution of hotspots of at-risk species in relation to human impacts provides information that can be used to manage key areas for marine mammal protection.
Importantly, the hotspots of risk overlap little with current Marine Protected Areas (Fig. 4D and Fig. S6). International efforts are underway to increase MPAs from 0.7% of the world's oceans currently to 10% by 2020 (39, 47). The magnitude and geographic distribution of extinction risk that we identify here is key to informing this process. Although previous studies have identified global patterns of marine diversity and current Red List status (12, 48–50), our study builds on this work not only by mapping the 32 species currently on the Red List, but also by adding the additional 15 species predicted to be at risk by our model. In addition, our maps (Figs. 2–4) provide insights into the geographic overlap of risk, human impacts, and protected areas across the world's oceans. Our results, coupled with previous studies, provide an important basis for specific conservation actions. Still needed, however, are more and better biological data, especially on migratory routes, and the location of feeding, calving/pupping, and breeding grounds to protect the geographic areas and networks of critical habitats on which highly mobile marine animals and other taxa depend (41, 51).
Conclusions
We show that the most important predictor of extinction risk is speed of life history because this captures the capacity of a species to rebound from human impacts. Our model also shows that intrinsic traits are more important predictors of risk than extrinsic factors because they are measures of the inherent susceptibility to human impacts and ability to recover from them. Therefore, our analysis emphasizes the importance of understanding the basic biology and ecology of marine mammals to assess the correlates and causes of extinction and to implement science-based conservation. Unfortunately, such basic information remains poorly known for most species, and not just for those considered Data Deficient, but new technologies are beginning to provide new and better data on both the biology of marine mammals and the ecology of the oceans (51). Incorporating this key information into scientifically sound, well-informed management of local and regional ecosystems has the potential to mitigate the threats facing many species. In addition, however, because of the large magnitude and spatial scale of anthropogenic impacts and the wide ranges of many species, conservation of marine mammals will require unprecedented global effort and political will. There is little time to avoid widespread declines and extinctions of marine mammals with large attendant ecological, economic, social, and political consequences.
Materials and Methods
Database.
Our database consisted of 125 (of 128) marine mammals for which sufficient species’ trait data were available. We collected data on intrinsic predictor variables: adult body mass, body mass at weaning, number of births per year, number of offspring per reproductive bout, geographic range size, social group size, trophic group, habitat (coastal, oceanic), foraging location (continental shelf, continental slope, epipelagic, mesopelagic/bathypelagic zones), taxonomic order, diet breadth (generalist, specialist), and migratory behavior. We also gathered data on extrinsic variables: mean ANPP (19) and mean human impact index (1) within each species’ geographic range. Our geographic range area data were from Geographic Information System maps used in Pompa et al. (12), which were based on Reeves et al. (52). We used a dichotomous response variable to represent extinction risk: species classified as Vulnerable, Endangered, Critically Endangered, or Extinct by the IUCN were considered “threatened”, and species classified as Near Threatened or Least Concern were considered “non-threatened” (23).
Random Forest Modeling.
Following the modeling approach used in Davidson et al. (16), we tested for quantitative relationships between predictor variables and extinction risk using the randomForest package in R version 1.10.1 (24, 25, 53). For our main random forest model (Fig. 1), we included only those species that occur in the marine environment. Species found solely in rivers or lakes were excluded from the model because we were unable to obtain extrinsic data on ANPP and human impacts that were comparable to those of the marine system (1, 19). However, to provide predictions of threat status for freshwater species as well, we ran a separate random forest model that included only the intrinsic variables for all species (freshwater and marine) to predict risk for marine mammals occurring in river and lake environments. The intrinsic variables included were the same as those in the main model (Fig. 1); only the extrinsic variables of ANPP and human impact were excluded from this intrinsic model. To predict risk for Data Deficient species (Table S1), we used the main random forest model to predict risk for marine species and the intrinsic model for freshwater species. The intrinsic model was comparable in accuracy to the main model because the extrinsic variables were not especially strong predictors of risk. Variables that did not improve accuracy were not included in the final models, and differences in importance between predictors were quantified with pairwise two-tailed z-tests (α = 0.05) (53).
Spatial Analyses.
We used ESRI's ArcGIS v9.3 to calculate spatial statistics for the two extrinsic variables used in the main random forest model, ANPP (19) and human impact (1), within the geographic range of each species. We used the zonal statistics tool to overlay each species’ range on top of the extrinsic variable raster dataset and counted pixels that fell within each range. We then used this tool to calculate mean values of ANPP and human impact experienced by each marine mammal species.
Our map of species at risk was created by overlapping geographic ranges of marine mammal species identified as at risk by our model and those on the Red List, and then by counting how many of these species were found in each spatial grid cell (Fig. 2). Hotspots were defined as grid cells ≥75th percentile of at-risk species, which corresponds to about 2% of all geographic grid cells (Figs. 3 and 4). Hotspot cutoff values near 2% have been used widely in both marine and terrestrial conservation studies (12, 54, 55). Note that marine mammals occurring in rivers and lakes were not included in our risk maps because the extrinsic data for the marine and freshwater environments are not comparable. Also, included in our maps were five species not predicted by our main model, but listed by the IUCN as Vulnerable (i.e., at risk) (Table S1).
We created maps on the geographic distribution of species richness, environmental variables, and MPAs to understand how they relate to the distribution of risk. Our map of species richness was similar to those produced elsewhere (especially ref. 12) and was created by overlapping the geographic ranges of all marine mammal species and counting how many species occur in each spatial grid cell. Our map of ANPP was based on Oregon State University's map of ocean productivity (19), and our maps of human impacts were obtained from Halpern et al. (1). We measured correlation of risk with richness and ANPP and used the zonal statistics tool to calculate the mean human impact index (1) within each hotspot (Fig. S5). Note that data on commercial fishing are based on 2008 values (1) (Fig. 4A). Because fishing impacts are highly variable over time and space, the map showing global distribution of fishing impacts may not accurately reflect present or future impacts and should be interpreted cautiously (Fig. 4A).
Supplementary Material
Acknowledgments
We thank Boris Worm, Charles W. Fowler, and John L. Gittleman for valuable comments that helped improve the manuscript and Sharyn N. Davidson for the drawings of marine mammals in Figs. 1 and 4. This study was supported in part by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica Project No. IN211811 from the Universidad Nacional Autónoma de México (UNAM); A.D.D. was supported by a UNAM postdoctoral fellowship; A.G.B. was partially supported by National Science Foundation Grant DBI-0805669; M.J.H. was supported by The Rockefeller Foundation and by National Science Foundation Grant DEB 0541625; and D.P.C. was supported by the E&P Sound and Marine Life Joint Industry Program of the Oil Gas Producers, National Science Foundation Grant ANT-0838937, and Office of Naval Research Grant N00014-08-1-1195.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121469109/-/DCSupplemental.
References
- 1.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 2.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 3.Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world's marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- 4.Estes JA, Williams TM, Doak D, DeMaster D, Brownell RL. Whales, Whaling and Ocean Ecosystems. Berkeley, CA: University of California Press; 2006. [Google Scholar]
- 5.Read AJ. The looming crisis: Interactions between marine mammals and fisheries. J Mammal. 2008;89:541–548. [Google Scholar]
- 6.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 7.Hilborn R, et al. State of the world's fisheries. Annu Rev Environ Resour. 2003;28:359–399. [Google Scholar]
- 8.Schiermeier Q. Ecologists fear Antarctic krill crisis. Nature. 2010;467:15. doi: 10.1038/467015a. [DOI] [PubMed] [Google Scholar]
- 9.Moore SE. Marine mammals as ecosystem sentinels. J Mammal. 2008;89:534–540. [Google Scholar]
- 10.Lewison RL, Crowder LB, Read AJ, Freeman SA. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol Evol. 2004;19:598–604. [Google Scholar]
- 11.Taylor BL, Martinez M, Gerrodette T, Barlow J, Hrovat YN. Lessons from monitoring trends in abundance of marine mammals. Mar Mamm Sci. 2007;23:157–175. [Google Scholar]
- 12.Pompa S, Ehrlich P, Ceballos G. Global distribution and conservation of marine mammals. Proc Natl Acad Sci USA. 2011;108:13600–13605. doi: 10.1073/pnas.1101525108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones MJ, Fielding A, Sullivan M. Analysing extinction risk in parrots using decision trees. Biodivers Conserv. 2006;15:1993–2007. [Google Scholar]
- 14.Cardillo M, et al. The predictability of extinction: Biological and external correlates of decline in mammals. Proc Biol Sci. 2008;275:1441–1448. doi: 10.1098/rspb.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Predicting extinction risk in declining species. Proc Biol Sci. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson AD, Hamilton MJ, Boyer AG, Brown JH, Ceballos G. Multiple ecological pathways to extinction in mammals. Proc Natl Acad Sci USA. 2009;106:10702–10705. doi: 10.1073/pnas.0901956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardillo M, Mace GM, Gittleman JL, Purvis A. Latent extinction risk and the future battlegrounds of mammal conservation. Proc Natl Acad Sci USA. 2006;103:4157–4161. doi: 10.1073/pnas.0510541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laidre KL, et al. Quantifying the sensitivity of Arctic marine mammals to climate-induced habitat change. Ecol Appl. 2008;18(2) Suppl:S97–S125. doi: 10.1890/06-0546.1. [DOI] [PubMed] [Google Scholar]
- 19.Oregon State University Ocean Productivity. 2006. Available at: http://www.science.oregonstate.edu/ocean.productivity/index.php.
- 20.Harvey PH, Clutton-Brock TH. Life history variation in primates. Evolution. 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 21.Bielby J, et al. The fast-slow continuum in mammalian life history: An empirical reevaluation. Am Nat. 2007;169:748–757. doi: 10.1086/516847. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton MJ, Davidson AD, Sibly RM, Brown JH. Universal scaling of production rates across mammalian lineages. Proc Biol Sci. 2011;278:560–566. doi: 10.1098/rspb.2010.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Union for the Conservation of Nature . 2008 IUCN Red List of Threatened Species. Gland, Switzerland: IUCN/SSC Red List Programme; 2008. Available at: http://www.redlist.org. [Google Scholar]
- 24.Cutler DR, et al. Random forests for classification in ecology. Ecology. 2007;88:2783–2792. doi: 10.1890/07-0539.1. [DOI] [PubMed] [Google Scholar]
- 25.Liaw A, Wiener M. Classification and regression by randomForest. R News. 2002;2:18–22. [Google Scholar]
- 26.Olden JD, Lawler JJ, Poff NL. Machine learning methods without tears: A primer for ecologists. Q Rev Biol. 2008;83:171–193. doi: 10.1086/587826. [DOI] [PubMed] [Google Scholar]
- 27.Prasad A, Iverson LR, Liaw A. Newer classification and regression tree techniques: Bagging and Random Forests for ecological prediction. Ecosystems. 2006;9:181–199. [Google Scholar]
- 28.Bielby J, Cardillo M, Cooper N, Purvis A. Modelling extinction risk in multispecies data sets: Phylogenetically independent contrasts versus decision trees. Biodivers Conserv. 2009;19:113–127. [Google Scholar]
- 29.Fowler CW. Systemic Management: Sustainable Human Interactions with Ecosystems and the Biosphere. Oxford: Oxford University Press; 2009. [Google Scholar]
- 30.Lotze HK, Coll M, Magera AM, Ward-Paige C, Airoldi L. Recovery of marine animal populations and ecosystems. Trends Ecol Evol. 2011;26:595–605. doi: 10.1016/j.tree.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Fowler CW. Population dynamics as related to rate of increase per generation. Evol Ecol. 1988;2:197–204. [Google Scholar]
- 32.Costa DP, Weise MJ, Arnould JPY. Potential influences of whaling on the status and trends of pinniped populations. In: Estes JA, Williams TM, Doak D, DeMaster D, editors. Whales, Whaling and Ocean Ecosystems. Berkeley, CA: University of California Press; 2006. pp. 342–357. [Google Scholar]
- 33.Alter SE, Rynes E, Palumbi SR. DNA evidence for historic population size and past ecosystem impacts of gray whales. Proc Natl Acad Sci USA. 2007;104:15162–15167. doi: 10.1073/pnas.0706056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardillo M, et al. The predictability of extinction: Biological and external correlates of decline in mammals. Proc Biol. Sci. 2008;275:1441–1448. doi: 10.1098/rspb.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodhi NS, et al. Measuring the meltdown: Drivers of global amphibian extinction and decline. PloS ONE. 2008;3(2):e1636. doi: 10.1371/journal.pone.0001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee TM, Jetz W. Unravelling the structure of species extinction risk for predictive conservation science. Proc Biol Sci. 2011;278:1329–1328. doi: 10.1098/rspb.2010.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laliberte AS, Ripple WJ. Range contractions of North American carnivores and ungulates. Bioscience. 2004;54:123–138. [Google Scholar]
- 38.Moore SE, Huntington HP. Arctic marine mammals and climate change: Impacts and resilience. Ecol Appl. 2008;18(2) Suppl:s157–s165. doi: 10.1890/06-0571.1. [DOI] [PubMed] [Google Scholar]
- 39.Cox TM, et al. Understanding the impacts of anthropogenic sound on beaked whales. J Cetacean Res Manage. 2006;7:177–187. [Google Scholar]
- 40.International Union for the Conservation of Nature and United Nations Environment Programme-World Conservation Monitoring Centre . The World Database on Protected Areas (WDPA): Annual Release. Cambridge, UK: UNEP-WCMC; 2010. Available at: http://www.wdpa-marine.org/Default.aspx#/countries/about. [Google Scholar]
- 41.Block BA, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475:86–90. doi: 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- 42.Worm B, Tittensor DP. Range contraction in large pelagic predators. Proc Natl Acad Sci USA. 2011;108:11942–11947. doi: 10.1073/pnas.1102353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Shea TJ, Odell DK. Large-scale marine ecosystem change and the conservation of marine mammals. J Mammal. 2008;89:529–533. [Google Scholar]
- 44.Dalton R. Endangered-porpoise numbers fall to just 250. Nature. 2010;465:674–675. doi: 10.1038/465674b. [DOI] [PubMed] [Google Scholar]
- 45.Clapham PJ, Young SB, Brownell RL. Baleen whales: Conservation issues and the status of the most endangered populations. Mammal Rev. 1999;29:35–60. [Google Scholar]
- 46.Ceballos G, Pompa S, Espinoza E, Garcia A. Extralimital distribution of Galapagos (Zalophus wollebaeki) and Northern (Eumetopias jubatus) sea lions in Mexico. Aquat Mamm. 2010;36:188–194. [Google Scholar]
- 47.Convention on Biological Diversity . Strategic Plan for Biodiversity 2011–2020. Nagoya, Japan: United Nations Environment Program; 2011. pp. 1–31. [Google Scholar]
- 48.Kaschner K, Tittensor DP, Ready J, Gerrodette T, Worm B. Current and future patterns of global marine mammal biodiversity. PLoS ONE. 2011;6:e19653. doi: 10.1371/journal.pone.0019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tittensor DP, et al. Global patterns and predictors of marine biodiversity across taxa. Nature. 2010;466:1098–1101. doi: 10.1038/nature09329. [DOI] [PubMed] [Google Scholar]
- 50.Schipper J, et al. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science. 2008;322:225–230. doi: 10.1126/science.1165115. [DOI] [PubMed] [Google Scholar]
- 51.Costa DP, et al. Approaches to studying climatic change and its role on the habitat selection of Antarctic pinnipeds. Integr Comp Biol. 2010;50:1018–1030. doi: 10.1093/icb/icq054. [DOI] [PubMed] [Google Scholar]
- 52.Reeves R, Stewart B, Clapham P, Powell J. National Audubon Society Guide to Marine Mammals of the World. New York: Alfred A. Knopf; 2002. pp. 1–527. [Google Scholar]
- 53.Breiman L, Cutler A. Random Forests. 2011. Available at: http://www.stat.berkeley.edu/∼breiman/RandomForests/cc_home.htm.
- 54.Ceballos G, Ehrlich PR. Global mammal distributions, biodiversity hotspots, and conservation. Proc Natl Acad Sci USA. 2006;103:19374–19379. doi: 10.1073/pnas.0609334103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Orme CDL, et al. Global patterns of geographic range size in birds. PLoS Biol. 2006;4:e208. doi: 10.1371/journal.pbio.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.