Abstract
The widespread occurrence of antibiotic resistance among human pathogens is a major public health problem. Conventional antibiotics typically target bacterial killing or growth inhibition, resulting in strong selection for the development of antibiotic resistance. Alternative therapeutic approaches targeting microbial pathogenicity without inhibiting growth might minimize selection for resistant organisms. Compounds inhibiting gene expression of streptokinase (SK), a critical group A streptococcal (GAS) virulence factor, were identified through a high-throughput, growth-based screen on a library of 55,000 small molecules. The lead compound [Center for Chemical Genomics 2979 (CCG-2979)] and an analog (CCG-102487) were confirmed to also inhibit the production of active SK protein. Microarray analysis of GAS grown in the presence of CCG-102487 showed down-regulation of a number of important virulence factors in addition to SK, suggesting disruption of a general virulence gene regulatory network. CCG-2979 and CCG-102487 both enhanced granulocyte phagocytosis and killing of GAS in an in vitro assay, and CCG-2979 also protected mice from GAS-induced mortality in vivo. These data suggest that the class of compounds represented by CCG-2979 may be of therapeutic value for the treatment of GAS and potentially other Gram-positive infections in humans.
Keywords: fibrinolysis, plasminogen, Streptococcus pyogenes
Current antibiotics generally act by interfering with critical biological processes in the pathogen, resulting in death or growth arrest. These mechanisms exert strong selective pressure that favors emergence of antibiotic-resistant strains (1). An alternative strategy is to suppress pathogen virulence without inhibiting growth, thereby attenuating the strong selection for resistance exerted by traditional bacteriostatic or bactericidal antibiotics. This approach has been applied to cholera, identifying a small molecule that blocks the production of two critical virulence factors, cholera toxin and the toxin coregulated pilus, by inhibiting the expression of their transcriptional regulator ToxT. This compound was then shown to protect infant mice from intestinal colonization by Vibrio cholerae (2).
Streptococcus pyogenes or group A Streptococcus (GAS) is an important human pathogen that is estimated to cause ∼700 million symptomatic infections per year worldwide (3). The clinical spectrum includes both mild conditions, such as pharyngitis, scarlet fever, and impetigo, as well as life-threatening disease, such as toxic shock-like syndrome and necrotizing fasciitis (4, 5). Streptococci are a diverse group of Gram-positive bacteria infecting humans and various other animals (6). S. pyogenes is highly specific to its human host, presumably because of the activity of species-specific virulence factors, including streptokinase (SK) (7). SK binds to the inactive zymogen plasminogen, resulting in the production of active plasmin, the central protease of the fibrinolytic system, through a coupling of conformational and proteolytic activation (8, 9).
The interaction of SK with plasminogen is highly species-specific, with the SK expressed by human GAS isolates active only against human plasminogen (10–13). Mice expressing human plasminogen exhibit markedly increased mortality after GAS infection, which is largely abrogated by deletion of the SK gene (ska) (7). These results and others show that SK is a key GAS virulence factor (14). We now report the identification of small-molecule inhibitors of GAS SK expression by high-throughput chemical screening and characterize the efficacy of these compounds both in in vitro and in vivo murine infection models.
Results
SK Expression Inhibitors Identified by High-Throughput Screening.
We previously reported that the species-specific interaction of SK with human plasminogen is critical to the pathogenicity of GAS in an in vivo murine infection model (7). These results suggested that inhibition of ska gene expression might provide an effective strategy for the treatment of GAS infection. To identify small molecules as candidates for this approach, a high-throughput screening assay was developed based on a kanamycin resistance gene under control of the ska promoter (strain SKKanGAS). Compounds were tested for the ability to inhibit SKKanGAS growth in the presence of kanamycin. A duplicate screen using the constitutively kanamycin-resistant strain UMAA2641 (15) served as control to detect compounds exhibiting nonspecific inhibition of GAS growth.
A total of 55,000 compounds at concentrations ranging from 5 to 10 μM were screened, leading to the identification of 95 compounds that exhibited ≥50% SKKanGAS inhibition of growth in the presence of kanamycin (i.e., inhibited SK promoter activity), with <10% inhibition of the UMAA2641 control strain. Dose response and IC50 analysis for these 95 compounds identified 20 with pIC50 values [−log(IC50)] of >4.5 for SKKanGAS growth inhibition in the presence of kanamycin and IC50 values for control growth inhibition that are at least 10-fold higher.
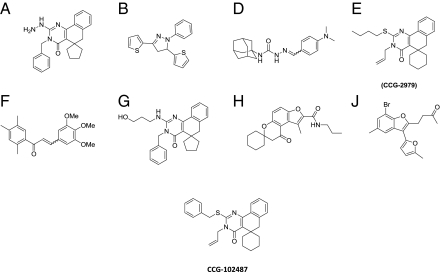
The 20 compounds were then prioritized for retesting based on lipophilicity [calculated log P (ClogP) < 6] and molecular mass (<500 Da). Compounds with these physical properties have the greatest likelihood of being bioavailable based on Lipinski's Rule of 5 (16), and they make better starting points for medicinal chemistry optimization, which often requires adding molecular mass and lipophilicity (17). Among those compounds passing these criteria, eight were available for purchase from commercial suppliers (Fig. 1, structures) and retested as fresh powders. Among this cohort, compounds A, G, H, and J all inhibited growth of the control GAS strain by more than 30% at a concentration of 50 μM and were eliminated from additional consideration. Of the remaining four compounds with reproducible inhibition of SK and little inhibition of growth of the control GAS strain (B, D, E, and F), compound B was predicted to be unstable to oxidation (either chemical or metabolic transformation of its thiophenes), and compound F was predicted to be the most susceptible to attack by cellular nucleophiles (e.g., glutathione) because of its highly electrophilic Michael acceptor enone functionality. Compounds D and F were also both noted to be active in ∼20% of previously unrelated high throughput screening (HTS) assays run by the University of Michigan Center for Chemical Genomics (CCG), suggesting a low level of target selectivity. By contrast, compound E (CCG-2979) inhibited expression of SK by ∼60% at 50 μM based on activity assay but inhibited GAS growth less than 15%. CCG-2979 was active in <10% of previous HTS assays (2 of 37), suggesting a higher level of target selectivity than the other candidates. For these reasons, CCG-2979 was selected for additional follow-up analysis.
Fig. 1.
Compound structures. Compounds (A–J) were identified in the original high-throughput screen. CCG-2979 was chosen as the lead compound (details in the text). CCG-102487 was identified as a commercially available analog of CCG-2979.
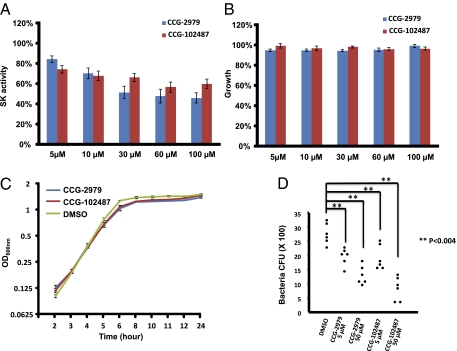
A search of commercial sources identified a CCG-2979 analog (CCG-102487) (Fig. 1) that also reduced SK activity with little inhibition of GAS growth. Fig. 2 summarizes the data for CCG-2979 and CCG-102487 purchased directly from a commercial supplier (ChemBridge Corporation) at different concentrations, confirming inhibition of ska gene expression (Fig. 2A) without a significant difference in efficacy between the two compounds. SK activity was decreased by 15.6 ± 3.2% (P < 0.001) compared with control by treatment with 5 μM CCG-2979 and 25.7 ± 3.9% (P < 0.001) compared with control by treatment with 5 μM CCG-102487. In contrast, 54.0 ± 5.0% inhibition was observed at 100 μM CCG-2979 (P < 0.001 between control and compound), and 40.2 ± 4.6% inhibition was observed at 100 μM CCG-102487 (P < 0.001 between control and compound). There was slight inhibition of growth of the control strain by CCG-2979, with maximum inhibition of 5.6 ± 0.1% observed at 30 μM (P < 0.001 between control and compound). The growth inhibition by CCG-102487 was only significant at 60 μM (P < 0.02 between control and compound), with maximum inhibition of 4.0 ± 0.1% (Fig. 2B). Growth curves for UMAA2616 in the presence of 50 μM for either compound showed only minor differences compared with control (Fig. 2C).
Fig. 2.
Effect of CCG-2979 and CCG-102487 on SK expression and GAS growth. (A) Effects of CCG-2979 and CCG-102487 on the production of SK activity. Normalized SK activity of GAS treated with CCG-2979 or CCG-102487 at concentrations from 5 to 100 μM (SK activity of culture media was divided by OD600 and then normalized to the value for DMSO-treated GAS, which was defined as 100%). The value was presented as mean ± SE for a total of 18 samples (pooled from six independent experiments with triplicates). (B) GAS growth as measured by OD600 of an overnight culture in the presence of CCG-2979 at concentrations of 5–100 μM normalized to the OD600 of control GAS grown in DMSO vehicle alone. The value was presented as mean ± SE for a total of 18 samples (pooled from six independent experiments with triplicates). (C) Growth curves for UMAA2616 in the presence of 0.1% DMSO, 50 μM CCG-2979, or 50 μM CCG-102487. Mean and SE for a total of six samples (two independent experiments with triplicates) are presented for each time point. (D) Decreased GAS resistance to phagocytosis in the presence of CCG-2979 or CCG-102487. Each point represents an individual experiment.
CCG-2979 and CCG-102487 Decrease GAS Resistance to Phagocytosis.
To test the effect of the SK-inhibiting compounds on other potential components of GAS virulence in vitro, the effect of the two compounds on GAS resistance to phagocytosis was studied. GAS strain UMAA2616 was incubated with whole-mouse blood in the presence or absence of CCG-2979 or CCG-102487 at final concentrations of 5 or 50 μM. Significant reduction in GAS survival was observed for both compounds (P < 0.004 and P < 0.001 for CCG-2979 at 5 and 50 μM vs. control, respectively; P < 0.003 and P < 0.001 for CCG-102487 at 5 and 50 μM vs. control, respectively). A dose-dependent killing of bacteria by host phagocytes was observed with both compounds (P < 0.03 for CCG-2979 and P < 0.001 for CCG-102487 between 5 and 50 μM) (Fig. 2D).
CCG-102487 Alters the GAS Gene Expression Program.
Because of the trend to less GAS growth inhibition and increased phagocytosis, CCG-102487 was used to investigate the effect of this class of compounds on GAS gene expression by global microarray transcriptional analysis using strain MGAS2221. The latter strain has been used previously for similar experiments, and its full genome sequence has been previously determined (18, 19). The doubling time of MGAS2221 was ∼53 and ∼56 min in the absence and presence of CCG-102487 (20 μg/mL per 46.7 μM), respectively, with nearly identical growth curves (Fig. 3 A and B).
Fig. 3.
The effect of CCG-102487 on the GAS gene expression program. Growth curves for MGAS2221 in the presence of CCG-102487 (46.7 μM) or DMSO vehicle alone as determined by OD (A) or cfu (B). (C) Graphic summary of gene expression changes. Genes down-regulated on treatment with CCG-102487 are indicated in green, and genes up-regulated on treatment with CCG-102487 are indicated in red. Genes of known homology or function were included. Complete data for gene expression changes are summarized in Table S1.
The microarray data showed high reproducibility and separation of clusters driven by growth phase and treatment (Fig. S1). The mRNA levels for ∼29% of all GAS transcripts were significantly altered by the presence of CCG-102487 (Table 1). Most of the observed changes were in the range of 1.5- to 2.0-fold (Table 2), with decreases in transcript level more frequent than increases and the greatest difference seen in stationary phase.
Table 1.
Summary of expression microarray data: total transcripts detected by the microarray analysis and altered by CCG-102487 treatment
| Number of transcripts | |
| All nonredundant probes on the chip | 1,872 |
| Transcripts detected during at least one experimental condition | 1,702 |
| Transcript significantly changed during at least one time point as a result of treatment with CCG-102487 (ratios above or below 1.5 and P values ≤ 0.05) | 536 |
| Changed transcript excluding phage genes | 490 |
Table 2.
Summary of expression microarray data: dynamics of transcript changes during different bacterial growth phases
| Down-regulated |
Up-regulated |
||||||
| Growth phase | ↓ ≥2 | ↓ 1.5–1.9 | Total down | ↑ ≤2 | ↑ 1.5–1.9 | Total up | All changes |
| ML | 38 | 95 | 133 | 14 | 19 | 33 | 166 |
| LL | 56 | 78 | 134 | 10 | 22 | 32 | 166 |
| S | 101 | 113 | 214 | 21 | 41 | 62 | 276 |
ML, midlogarithmic growth phase; LL, transition from logarithmic to stationary phase; S, stationary phase.
Gene ontology analyses identified clustered changes in genes involved in energy production, metabolism, and virulence (Fig. 3C and Table S1). Of particular note, CCG-102487 treatment altered the transcript level for key GAS virulence genes (Fig. 3C and Table S1), including multiple adhesins (fibronectin binding proteins, collagen-like surface protein, laminin-binding surface protein, and M protein), cytolytic toxins (streptolysins O and S), secreted extracellular proteases (Mac, C5a peptidase, and SpeB), streptodornase Sda1 (DNase), complement inhibitor protein, and streptokinase (Table S1). Many of these factors, including Sda1, SIC, M protein, and C5a peptidase (ScpA) are involved in GAS resistance to host phagocytosis and immunity (20). One of the most dramatic down-regulations of expression (3.8-fold) was observed for the ska gene encoding SK. Spd3, a streptodornase, was most dramatically down-regulated at midlogarithmic growth phase (7.3-fold). In addition, changes in expression of sortases and signal peptidase involved in virulence factor secretion were also observed.
Although only a minority of genes was up-regulated in the presence of CCG-102487, this group includes three presumed operons of unknown function encoding putative protease of the CAAX family. The first operon, Spy1384-1386, encodes a transcriptional regulator and hypothetical membrane protein in addition to the putative CAAX family protease. The second operon, Spy1714-1717, encodes elements of a putative copper exporting system, and the third operon, Spy2172-2174, encodes a regulator and two putative membrane-associated proteins (Fig. 3C and Table S1).
CCG-2979 Protects Mice from GAS Infection Mortality.
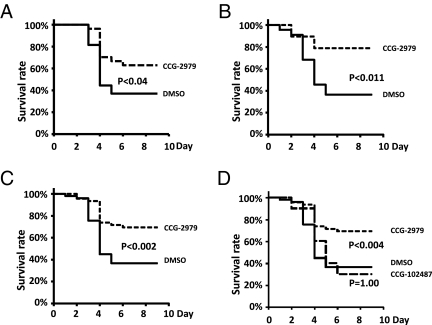
In vivo efficacy was evaluated in a model of GAS infection in which expression of a human plasminogen transgene renders mice markedly susceptible to GAS infection, presumably because of the species-specific interaction between SK and human plasminogen (7). Human PLG Tg+ mice were inoculated s.c. with UMAA2616 and then treated by i.p. delivery with daily doses of CCG-2979 (5 or 40 μg) starting 24 h after infection. Improved survival was observed for both the 5- (P < 0.038) (Fig. 4A) and 40-μg (P < 0.011) (Fig. 4B) doses of CCG-2979 (P < 0.002 for the pooled data) (Fig. 4C). Although a statistically significant improvement in survival was not observed with compound CCG-102487, analysis of the pooled data from all experiments by a pairwise multiple comparison procedure to correct for multiple observations (Bonferroni method) showed statistically significant protection for compound CCG-2979 treatment vs. control (P < 0.004) (Fig. 4D).
Fig. 4.
Treatment with CCG-2979 improves survival in an in vivo mouse model for GAS infection. (A) Effect on survival of CCG-2979 [12.7 nmol (5 μg) per mouse] injected daily for 5 d beginning 24 h after infection of PLGTg+ mice with UMAA2616. Data were pooled from four independent experiments (with GAS inoculation of 8.3, 2.8, 3.6, or 4.0 × 105 cfu/mouse, respectively). A total of 27 mice were represented in the control group treated with vehicle alone (solid line), and 27 mice were represented in the CCG-2979–treated group (dashed line). (B) The same model as in A except that CCG-2979 was injected at a dose of 101.4 nmol (40 μg) per mouse per day for 4 d. Data were pooled from three independent experiments (with GAS inoculation of 4.22, 3.16, or 1.24 × 106 cfu/mouse, respectively). A total of 22 mice were represented in the control group (solid line), and 19 mice were represented in the CCG-2979–treated group (dashed line). (C) Pooled data from A and B, including all data for CCG-2979. (D) Pooled data for both compounds. CCG-102487 [93.3 nmol (40 μg) per mouse] was injected daily for 4 d beginning 24 h after infection of PLGTg+ mice with UMAA2616. Data for CCG-102487 (dotted line) are from one experiment (total of 10 mice with GAS inoculation of 4.22 × 106 cfu/mouse). P values were calculated with all pairwise multiple comparison procedures (Bonferroni method).
Discussion
Antibiotic resistance is an increasing problem for the treatment of most human pathogens. Although GAS resistance to penicillin has not yet been reported, macrolide resistance is increasing worldwide, with frequencies as high as 48% in some populations (21–23). Using high-throughput molecular screening, we identified a class of compounds that inhibits ska expression without perturbing bacterial growth, an approach that might be less likely to select for antibiotic resistance.
Of note, we also showed reduced mortality in mice treated with one of these compounds (CCG-2979) in an in vivo infection model. It was significant that we could observe protective effect of CCG-2979 even without extensive effort to optimize the dosing and delivery regimen, underscoring the great potential of this chemical series of compounds in treating GAS infection. CCG-102487 failed to show in vivo efficacy, although it showed inhibitory effects on expression of a number of virulence genes and decreased GAS resistance to phagocytosis. The different in vivo efficacies of the two compounds are likely caused by differences in pharmacokinetic properties rather than intrinsic potency. Analysis of GAS exposed to an analog of CCG-2979 (CCG-102487) identified expression changes in a number of genes, including SK and other known virulence factors, suggesting that these compounds may interact with a gene regulator(s) that controls gene expression for a broad spectrum of genes.
Although CCG-2979 and CCG-102487 exhibit their effects at relatively high concentrations (30–50 μM), these compounds have not been optimized for potency. CCG-2979 is a screening hit, and CCG-102487 is one of the first commercially available analogs selected. It is clear that this chemical series will require substantial medicinal chemistry optimization to maximize potency at inhibiting SK expression without introducing bacterial growth suppression or toxicity in mammalian cells. Nonetheless, the in vivo activity observed with CCG-2979 without obvious toxicity suggests that development of such improved compounds should be feasible.
Although reduction of SK expression alone could account for the observed in vivo protection from GAS-induced mortality (7), alterations in the expression of other genes by this class of compounds might also contribute. One of the genes down-regulated by CCG-102487, fasA, belongs to the two-component system fibronectin/fibrinogen binding/hemolytic activity/streptokinase regulator (FasBCAX). FasBCAX and control of virulence (CovRS) genes are major two-component systems that are both known to regulate ska expression (24–26). CovRS inhibits (24), whereas FasBCAX induces ska expression (25). Other targets in addition to ska were also down-regulated by CCG-102487, including streptolysin S and fibronectin-binding proteins (25), suggesting that some or all of these changes could be secondary to the inhibition of FasBCAX. Streptolysin S is a powerful hemolysin that can impede host neutrophil recruitment by inhibiting the production of chemotactic signals (27).
Other changes in gene expression induced by CCG-102487 that could contribute to the protective effect of this class of compounds in vivo include alterations in genes central to carbohydrate use (e.g., amyA, amyB, malC, malD, malE, malM, and malX) (28–31) and genes regulating nucleoside transport and metabolism (e.g., purN, purD, pyrF, and pyrE) (32, 33). The inhibition of GAS resistance to phagocytosis by CCG-2979 and CCG-102487 could be attributed to the observed down-regulation of one or more virulence factors known to be important for this process, including Sda1, SIC, M protein, or C5a peptidase (scpA) (20, 34–38). SpeB, a cysteine protease that has been reported to cleave and inactivate Sda1, was modestly up-regulated by CCG-102487, which would be predicted to decrease the ability of GAS to spread systemically (37, 39).
Taken together, these data suggest that the class of compounds represented by CCG-2979 and CCG-102487 target a network of virulence factors including SK, resulting in significant attenuation of GAS pathogenicity and accounting for the protection from GAS-induced mortality observed in vivo in the mouse. The complex changes in gene expression program after compound exposure could be a direct effect of alterations in a single transcriptional pathway or reflect interactions with multiple gene regulators. Future identification of the specific target(s) of compound action within GAS should provide new insight into the regulation of GAS virulence.
The SK and fasA genes are conserved among several pathogenic streptococci that are closely related to GAS, including S. dysgalactiae and S. equi (11, 26, 40–42). Of note, S. equi is the cause of strangles, a serious and often epidemic disease of horses (43). Fas also shares homology with the com and blp operons in S. pneumoniae and the agr operon in S. aureus (25, 44), suggesting that derivatives of CCG-2979 and/or related compounds might also be of potential value in the treatment of other important human and veterinarian pathogens. Finally, in addition to the advantage of milder evolutionary pressure for developing resistance, this class of antimicrobials, acting through an independent mechanism, also offers the potential for synergy with conventional antibiotics (45).
Materials and Methods
Bacterial Strains.
UMAA2616 was derived from the GAS M type 1 strain MGAS166 (46). UMAA2616 (originally designated as UMAA2392) contains mutations in the CovR and CovS gene, leading to enhanced expression of SK and other virulence factors (47). SKKanGAS was generated from UMAA2616 by insertion of a plasmid containing a kanamycin resistance gene (encoding aminoglycoside phosphotransferase aphA-3) driven by the SK gene (ska) promoter. Briefly, the kanamycin resistance gene (generated by PCR using primers A and B) (Table S2) was linked to a 1,071-bp GAS genomic sequence upstream of the ska coding sequence (generated by PCR using primers C and D) (Table S2) and a 1,004-bp sequence downstream of the ska coding sequence (generated by primer E and F) (Table S2), with the final construct verified by direct DNA sequencing. This DNA fragment was then inserted into the multiple cloning sites of the PLZ12(spec) plasmid (36), which replicates and is maintained in Gram-positive bacteria by spectromycin selection. This plasmid was electroporated into the UMAA2616 strain. This newly engineered GAS strain (SKKanGAS) was shown to be kanamycin-resistant, indicating that the ska promoter is constitutively active under standard culture conditions. The UMAA2641 strain, generated previously and reported by Khil et al. (15), carries a Ωkm-2 cassette constitutively expressing a kanamycin resistance gene (aminoglycoside phosphotransferase aphA-3) (48), and it was used as a control for high-throughput screening.
The UMAA2616 strain was cultured in Todd–Hewitt broth containing 0.2% yeast extract (THY) (Difco) supplemented with 100 μg/mL streptomycin (7). SKKanGAS was grown in the same media also supplemented with 40 μg/mL kanamycin. Strain MGAS2221 (serotype M1) (49) was grown in THY medium without antibiotics.
High-Throughput, Small-Compound Screen.
A diversity-based library of 55,000 small molecules was obtained from the University of Michigan CCG, a collection consisting largely of commercial libraries from ChemBridge, Maybridge, and ChemDiv (www.lsi.umich.edu/ccg/chemical-diversity). Compounds were placed in single wells of a 384-well microtiter plate (#3701; Corning) using the Biomek FX HDR pin tool (Beckman Coulter) at a final concentration of 5–10 μM in 30 μL THY medium containing 40 μg/mL kanamycin. Each compound was screened in duplicate against SKKanGAS and UMAA2641. GAS cultures grown to OD600 = 0.7–0.8 were diluted 12.5- (SKKanGAS) or 25-fold (UMAA2641) into 40 μg/mL kanamycin THY medium; and 10 μL this mixture were added into each well using Multidrop (Thermo Labsystems). The difference in GAS dilution compensated for the slower growth in kanamycin of SKKanGAS vs. UMAA2641, probably because of differences in strength between the two promoters driving kanamycin resistance. Positive control wells containing 5 μg/mL tetracycline, which should completely inhibit GAS growth, were included on each plate. Plates were cultured at 37 °C for 16–20 h, and bacteria growth in each well was measured by absorbance at 600 nm.
Selected active compounds from the high-throughput screen were serially diluted (1:2) into 40 μg/mL kanamycin THY media followed by addition of SKKanGAS or UMAA2641 with the protocol described above to a final DMSO concentration of 1% and final compound concentrations ranging from 3 nM to 100 μM. Plates were incubated at 37 °C for 15–20 h (humidity of 50%), and absorbance was measured at 600 nm in a PHERAstar (BMG Labtech) to estimate the pIC50 [−log(IC50)] of bacterial growth inhibition for each compound with kanamycin.
SK Activity Assay.
UMAA2616 was inoculated into THY medium, grown at 37 °C for 15–20 h, diluted 1:1,000 into fresh THY medium containing varying concentrations of test compounds in a final concentration of 0.1% DMSO, and grown in triplicates at 37 °C to an OD600 = 1.0; 20 μL culture supernatant were collected after centrifugation at 11,000 × g for 8 min, mixed with 100 μL PBS, 10 μL human plasma (Innovative Research), and10 μL S2403 (1 mg/mL; Diapharma Group Inc.), and incubated at 37 °C for 2 h. SK activity was determined by measurement of cleaved S2403 at OD405 and calculated as the percentage of SK activity in a control UMAA2616 culture grown under the same conditions (minus the test compound). Each experiment was performed in triplicate, with six independent experiments for each compound.
Bacterial Growth Assay.
UMAA2616 was inoculated into THY medium, grown at 37 °C for 15–20 h, diluted 1:100 into fresh THY medium, and cultured with 50 μM CCG-2979, 50 μM CCG-102487, or DMSO vehicle alone. OD600 was measured at 2, 3, 4, 6, 8, 10, 11, 12, and 24 h to monitor growth. For strain MGAS2221, an overnight culture was diluted 1:100 in prewarmed 50-mL aliquots of THY medium and grown at 37 °C in a 5% CO2 atmosphere. CCG-102487 solubilized in DMSO (final concentration = 20 μg/mL; 46.7 μM), or an equivalent volume of DMSO as control was added; bacterial growth was monitored by measuring OD600 and viable cell count using the serial dilution method.
Phagocytosis Assay.
A stationary phase culture of UMAA2616 was diluted 1:100 into fresh THY medium and grown at 37 °C to an OD600 of 0.5; 1 mL culture was subject to centrifugation at 10,000 × g for 10 min and washed three times in 1 mL sterile PBS. The bacterial pellet was resuspended in 1 mL PBS and then diluted 1:104 in PBS; 30 μL GAS suspension were mixed with 90 μL whole blood collected in 0.2 mg/mL heparin from 6- to 8-wk-old C57BL/6J mice, and test compounds were added to a final concentration of 5 or 50 μM. Control samples were treated with vehicle (DMSO) alone. After incubation at 37 °C with slow rotation for 3 h, 10 μL were removed and plated on THY plates to determine total cfu. Data were analyzed by one-way ANOVA (Turkey method) using the SigmaStat program (Systat Software).
Microarray Analysis.
Microarray analysis was performed using strain MGAS2221 (serotype M1) for comparison with published GAS expression studies as previously described (49). An overnight culture of strain MGAS2221 in THY medium was diluted 1:100 in six prewarmed 50-mL aliquots of THY medium. Three independent cultures were grown in the presence of CCG-102487 (final concentration = 20 μg/mL; 46.7 μM), and three cultures were grown in an equivalent volume of vehicle (DMSO) alone; 5-mL samples were collected by centrifugation at OD600∼0.45 (corresponding to midlogarithmic growth phase), 0.85 (transition from logarithmic to stationary phase), and 3 h after entering stationary phase, and they were stored at −80 °C. These 18 samples (three biological replicates × three growth phases × two treatments) were processed simultaneously to minimize experimental variance. RNA was isolated, and targets were prepared as described previously (50). Target labeling, hybridization, and data collection were performed according to instructions provided by the array manufacturer (Affymetrix). Samples were hybridized with a custom GAS array designed for The Methodist Hospital and manufactured by Affymetrix (51).
Chip hybridization data were collected with Affymetrix GeneChip Operating Software (GCOS 1.4). The collected hybridization values were normalized to total hybridization intensity (individual intensities of probes were divided by the sum of all intensities of hybridizing probes). Data derived from three biological replicates were used to calculate mean values. The average values were used to calculate the inhibitor to control transcript ratios. Only genes with ratios above or below 1.5 and P values ≤ 0.05 were included in the results. PartekPro (Partek) and Array Assist (Stratagene) software was used to assess chip quality and chip to chip variability as well as for data mining and visualization.
Murine GAS Infection Model.
Six- to eight-week-old human PLG transgenic mice on a C57BL/6J background (backcrossed greater than 10 generations) were injected s.c. with GAS as previously described (7). Briefly, an overnight culture from a single UMAA2616 GAS colony was diluted 1:50 into fresh THY medium and grown to late log phase (OD600 = 0.75), and the specific innoculum dose was administered in a 100-μL volume (diluted in THY medium). The same cultures were plated in parallel on THY plates to determine the exact inoculation titer. After inoculation, mice were injected i.p. daily for 5 d starting 1 d after infection with 100 μL of 50 μg/mL test compound in 1.5% DMSO/PBS or vehicle alone. In separate experiments, the test compound dose was increased to 400 μg/mL delivered daily for 4 d starting 1 d after infection. Mice were monitored for 9 d for morbidity and mortality. Data were analyzed by the Kaplan–Meier method. Survival in different groups was compared using the log-rank test, and all pairwise multiple comparison procedures (Bonferroni method) used the SigmaStat program (Systat Software).
Supplementary Material
Acknowledgments
We thank Drs. Mark Walker and Colin Kretz for their insightful comments. This work was supported by National Institutes of Health Grants 1R21AI076675-01 (to H.S.), 1R03MH084152-01 (to H.S.), and P01HL573461 (to H.S. and D.G.). S.D.L. and J.M.M. received additional National Institutes of Health funding. D.G. is a Howard Hughes Medical Institute investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1201031109/-/DCSupplemental.
References
- 1.Högberg LD, Heddini A, Cars O. The global need for effective antibiotics: Challenges and recent advances. Trends Pharmacol Sci. 2010;31:509–515. doi: 10.1016/j.tips.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science. 2005;310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 3.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 4.Bisno AL, Stevens DL. Streptococcal infections of skin and soft tissues. N Engl J Med. 1996;334:240–245. doi: 10.1056/NEJM199601253340407. [DOI] [PubMed] [Google Scholar]
- 5.Cole JN, Barnett TC, Nizet V, Walker MJ. Molecular insight into invasive group A streptococcal disease. Nat Rev Microbiol. 2011;9:724–736. doi: 10.1038/nrmicro2648. [DOI] [PubMed] [Google Scholar]
- 6.Köhler W. The present state of species within the genera Streptococcus and Enterococcus. Int J Med Microbiol. 2007;297:133–150. doi: 10.1016/j.ijmm.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Sun H, et al. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- 8.Boxrud PD, Verhamme IM, Bock PE. Resolution of conformational activation in the kinetic mechanism of plasminogen activation by streptokinase. J Biol Chem. 2004;279:36633–36641. doi: 10.1074/jbc.M405264200. [DOI] [PubMed] [Google Scholar]
- 9.Boxrud PD, Bock PE. Coupling of conformational and proteolytic activation in the kinetic mechanism of plasminogen activation by streptokinase. J Biol Chem. 2004;279:36642–36649. doi: 10.1074/jbc.M405265200. [DOI] [PubMed] [Google Scholar]
- 10.Marcum JA, Kline DL. Species specificity of streptokinase. Comp Biochem Physiol B. 1983;75:389–394. doi: 10.1016/0305-0491(83)90345-0. [DOI] [PubMed] [Google Scholar]
- 11.McCoy HE, Broder CC, Lottenberg R. Streptokinases produced by pathogenic group C streptococci demonstrate species-specific plasminogen activation. J Infect Dis. 1991;164:515–521. doi: 10.1093/infdis/164.3.515. [DOI] [PubMed] [Google Scholar]
- 12.Nowicki ST, Minning-Wenz D, Johnston KH, Lottenberg R. Characterization of a novel streptokinase produced by Streptococcus equisimilis of non-human origin. Thromb Haemost. 1994;72:595–603. [PubMed] [Google Scholar]
- 13.Schroeder B, Boyle MD, Sheerin BR, Asbury AC, Lottenberg R. Species specificity of plasminogen activation and acquisition of surface-associated proteolytic activity by group C streptococci grown in plasma. Infect Immun. 1999;67:6487–6495. doi: 10.1128/iai.67.12.6487-6495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol. 2009;11:1–12. doi: 10.1111/j.1462-5822.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 15.Khil J, et al. Plasminogen enhances virulence of group A streptococci by streptokinase-dependent and streptokinase-independent mechanisms. J Infect Dis. 2003;188:497–505. doi: 10.1086/377100. [DOI] [PubMed] [Google Scholar]
- 16.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 17.Rishton GM. Nonleadlikeness and leadlikeness in biochemical screening. Drug Discov Today. 2003;8:86–96. doi: 10.1016/s1359644602025722. [DOI] [PubMed] [Google Scholar]
- 18.Perez N, et al. A genome-wide analysis of small regulatory RNAs in the human pathogen group A Streptococcus. PLoS One. 2009;4:e7668. doi: 10.1371/journal.pone.0007668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumby P, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bessen DE. Population biology of the human restricted pathogen, Streptococcus pyogenes. Infect Genet Evol. 2009;9:581–593. doi: 10.1016/j.meegid.2009.03.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green M, Martin JM, Barbadora KA, Beall B, Wald ER. Reemergence of macrolide resistance in pharyngeal isolates of group a streptococci in southwestern Pennsylvania. Antimicrob Agents Chemother. 2004;48:473–476. doi: 10.1128/AAC.48.2.473-476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green MD, et al. Multicentre surveillance of the prevalence and molecular epidemiology of macrolide resistance among pharyngeal isolates of group A streptococci in the USA. J Antimicrob Chemother. 2006;57:1240–1243. doi: 10.1093/jac/dkl101. [DOI] [PubMed] [Google Scholar]
- 24.Federle MJ, McIver KS, Scott JR. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J Bacteriol. 1999;181:3649–3657. doi: 10.1128/jb.181.12.3649-3657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreikemeyer B, Boyle MD, Buttaro BA, Heinemann M, Podbielski A. Group A streptococcal growth phase-associated virulence factor regulation by a novel operon (Fas) with homologies to two-component-type regulators requires a small RNA molecule. Mol Microbiol. 2001;39:392–406. doi: 10.1046/j.1365-2958.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 26.Malke H, Steiner K. Control of streptokinase gene expression in group A & C streptococci by two-component regulators. Indian J Med Res. 2004;119(Suppl):48–56. [PubMed] [Google Scholar]
- 27.Lin A, Loughman JA, Zinselmeyer BH, Miller MJ, Caparon MG. Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection. Infect Immun. 2009;77:5190–5201. doi: 10.1128/IAI.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hytönen J, Haataja S, Finne J. Streptococcus pyogenes glycoprotein-binding strepadhesin activity is mediated by a surface-associated carbohydrate-degrading enzyme, pullulanase. Infect Immun. 2003;71:784–793. doi: 10.1128/IAI.71.2.784-793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shelburne SA, 3rd, et al. Regulation of polysaccharide utilization contributes to the persistence of group a streptococcus in the oropharynx. Infect Immun. 2007;75:2981–2990. doi: 10.1128/IAI.00081-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shelburne SA, 3rd, et al. Maltodextrin utilization plays a key role in the ability of group A Streptococcus to colonize the oropharynx. Infect Immun. 2006;74:4605–4614. doi: 10.1128/IAI.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Bueren AL, Higgins M, Wang D, Burke RD, Boraston AB. Identification and structural basis of binding to host lung glycogen by streptococcal virulence factors. Nat Struct Mol Biol. 2007;14:76–84. doi: 10.1038/nsmb1187. [DOI] [PubMed] [Google Scholar]
- 32.Alcantara RB, Read RD, Valderas MW, Brown TD, Roop RM., 2nd Intact purine biosynthesis pathways are required for wild-type virulence of Brucella abortus 2308 in the BALB/c mouse model. Infect Immun. 2004;72:4911–4917. doi: 10.1128/IAI.72.8.4911-4917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samant S, et al. Nucleotide biosynthesis is critical for growth of bacteria in human blood. PLoS Pathog. 2008;4:e37. doi: 10.1371/journal.ppat.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akesson P, et al. Streptococcal inhibitor of complement-mediated lysis (SIC): An anti-inflammatory virulence determinant. Microbiology. 2010;156:3660–3668. doi: 10.1099/mic.0.039578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji Y, McLandsborough L, Kondagunta A, Cleary PP. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringdahl U, et al. A role for the fibrinogen-binding regions of streptococcal M proteins in phagocytosis resistance. Mol Microbiol. 2000;37:1318–1326. doi: 10.1046/j.1365-2958.2000.02062.x. [DOI] [PubMed] [Google Scholar]
- 37.Walker MJ, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13:981–985. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- 38.Wexler DE, Chenoweth DE, Cleary PP. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci USA. 1985;82:8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchanan JT, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Holden MT, et al. Genomic evidence for the evolution of Streptococcus equi: Host restriction, increased virulence, and genetic exchange with human pathogens. PLoS Pathog. 2009;5:e1000346. doi: 10.1371/journal.ppat.1000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timoney JF. The pathogenic equine streptococci. Vet Res. 2004;35:397–409. doi: 10.1051/vetres:2004025. [DOI] [PubMed] [Google Scholar]
- 42.Beres SB, et al. Genome sequence of a Lancefield group C Streptococcus zooepidemicus strain causing epidemic nephritis: New information about an old disease. PLoS One. 2008;3:e3026. doi: 10.1371/journal.pone.0003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrington DJ, Sutcliffe IC, Chanter N. The molecular basis of Streptococcus equi infection and disease. Microbes Infect. 2002;4:501–510. doi: 10.1016/s1286-4579(02)01565-4. [DOI] [PubMed] [Google Scholar]
- 44.Martin B, Quentin Y, Fichant G, Claverys JP. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 2006;14:339–345. doi: 10.1016/j.tim.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 46.Musser JM, et al. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: Clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engleberg NC, Heath A, Miller A, Rivera C, DiRita VJ. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J Infect Dis. 2001;183:1043–1054. doi: 10.1086/319291. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Casal J, Caparon MG, Scott JR. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumby P, Whitney AR, Graviss EA, DeLeo FR, Musser JM. Genome-wide analysis of group a streptococci reveals a mutation that modulates global phenotype and disease specificity. PLoS Pathog. 2006;2:e5. doi: 10.1371/journal.ppat.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sitkiewicz I, Musser JM. Expression microarray and mouse virulence analysis of four conserved two-component gene regulatory systems in group a streptococcus. Infect Immun. 2006;74:1339–1351. doi: 10.1128/IAI.74.2.1339-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shelburne SA, 3rd, et al. A direct link between carbohydrate utilization and virulence in the major human pathogen group A Streptococcus. Proc Natl Acad Sci USA. 2008;105:1698–1703. doi: 10.1073/pnas.0711767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.