Abstract
Insulin-like growth factor-binding protein 2 (IGFBP2) is increasingly recognized as a glioma oncogene, emerging as a target for therapeutic intervention. In this study, we used an integrative approach to characterizing the IGFBP2 network, combining transcriptional profiling of human glioma with validation in glial cells and the replication-competent ASLV long terminal repeat with a splice acceptor/tv-a glioma mouse system. We demonstrated that IGFBP2 expression is closely linked to genes in the integrin and integrin-linked kinase (ILK) pathways and that these genes are associated with prognosis. We further showed that IGFBP2 activates integrin β1 and downstream invasion pathways, requires ILK to induce cell motility, and activates NF-κB. Most significantly, the IGFBP2/integrin/ILK/NF-κB network functions as a physiologically active signaling pathway in vivo by driving glioma progression; interfering with any point in the pathway markedly inhibits progression. The results of this study reveal a signaling pathway that is both targetable and highly relevant to improving the survival of glioma patients.
Elevated insulin-like growth factor-binding protein 2 (IGFBP2) expression is found in many malignancies and can often serve as a prognostic factor (1). It is one of the most consistently elevated proteins in high-grade glioma, and high IGFBP2 expression is directly correlated with poor survival (2, 3). The finding that IGFBP2 is a driver of glioma development and progression in a spontaneous mouse model has provided the most convincing evidence of the significance of IGFBP2 in glioma (4). Given the clinical challenge of treating glioma and the lack of effective therapies, the elucidation of key protein-signaling networks that are essential to tumor growth and maintenance, such as IGFBP2, could provide new approaches to therapeutic intervention and significantly affect clinical outcome.
IGFBP2 is a member of the IGF system in which it binds and modulates IGF1 and IGF2 activity (5); however, IGFBP2 is better known for its IGF-independent roles in cancer, such as integrin binding through an arginine–glycine–aspartic acid (RGD) motif in the C terminus (6, 7). The interaction of IGFBP2 with integrin α5 has been reported to promote cellular de-adhesion and migration in Ewing's sarcoma (8), and we previously found that integrin α5 binding to IGFBP2 is responsible for the promigratory characteristics of glioma cells (7). Integrins transduce signaling through proteins such as integrin-linked kinase (ILK), which binds to the cytoplasmic domains of β1 and β3 integrins (9). ILK contributes to the oncogenic phenotype by stimulating invasion and migration, encouraging anchorage-independent growth, and inducing tumor angiogenesis (10). Although the function of ILK has been intimately associated with integrin function, no association with IGFBP2 has been reported.
IGFBP2 has been reported to influence multiple transcriptional factors, including the up-regulation of NF-κB (6), which activates the transcription of an array of cancer-promoting genes. NF-κB is constitutively active in many cancers, including glioma (11–13). Given that both integrin (14) and ILK (15, 16) signaling have been reported to activate NF-κB, we hypothesized that IGFBP2-induced glioma progression is driven by the integrin/ILK/NF-κB network. In this study, we confirmed its role as a physiologically active signaling pathway in driving glioma progression in a replication-competent ASLV long terminal repeat with a splice acceptor (RCAS)/Ntv-a glial-specific transgenic mouse model. The genetic inhibition of each network component blocked glioma progression; thus, we believe this newly identified network provides promising approaches to therapeutic intervention in glioma with elevated IGFBP2 expression. In this study, we provide evidence that the integrin/ILK/NF-κB network is functional in human glioma and has a crucial effect on patient survival.
Results
IGFBP2 Is Clinically Linked to the Integrin and ILK Pathways.
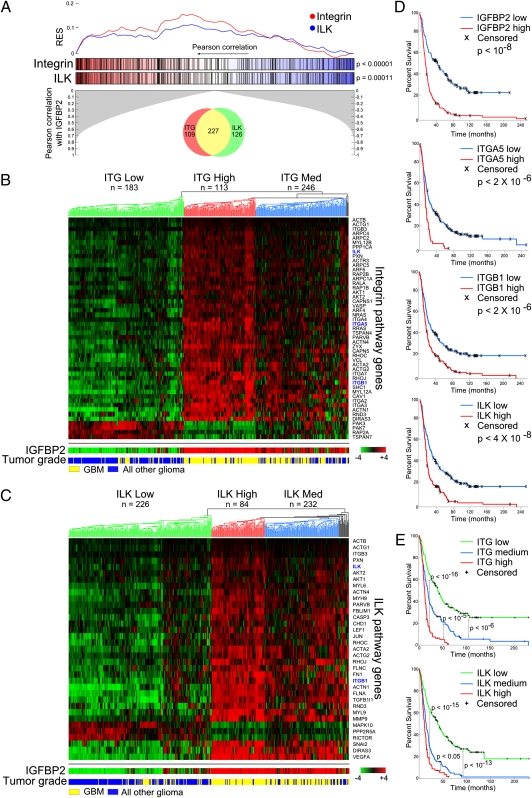
To obtain clinically oriented global information on the IGFBP2 pathway, we used the Repository for Molecular Brain Neoplasia Data (Rembrandt) to determine which genes were correlated with IGFBP2. We input this gene list into Ingenuity Pathway Analysis (IPA) software to determine which pathways were associated with IGFBP2 expression (Dataset S1). Many of the top pathways (6 of 25) were related to cellular migration and invasion. We focused on integrin and ILK signaling because IGFBP2 is known to bind integrin α5 and regulate cell motility through this interaction. To determine whether integrin and ILK pathway genes were enriched in samples with high IGFBP2 expression, we performed a gene set enrichment analysis (GSEA). Indeed, the genes in both pathways were significantly correlated with IGFBP2 (P < 0.001; Fig. 1A and Dataset S2).
Fig. 1.
IGFBP2 is associated with integrin and ILK pathways in human glioma. (A) GSEA for integrin and ILK pathway genes based on their correlation with IGFBP2 expression. Maximum running enrichment score (RES) for integrin and ILK pathway genes are 0.1602 and 0.1182, respectively. Venn diagram represents the number of pathway genes that are unique or shared between integrin and ILK. (B and C) Hierarchical clustering of integrin (B) and ILK (C) pathway genes that are correlated (more than 0.5 Pearson correlation) with IGFBP2. (D) Single-gene Kaplan–Meier survival curves of all glioma samples (n = 329). P values were calculated using the log-rank test. (E) Kaplan–Meier survival curves based on clusters in B and C. P values were calculated using the log-rank test.
To determine the expression patterns of integrin and ILK pathway genes in accordance with IGFBP2 expression and glioma grade, we performed unsupervised hierarchical clustering. Three major clusters formed, including low, intermediate, and high expression (Fig. 1 B and C). The clusters were related to IGFBP2 expression and tumor grade, with the high-expression cluster also containing the highest IGFBP2 expression and mostly glioblastoma, indicating a possible positive regulation of integrin and ILK pathway genes by IGFBP2. The pathway genes that were not correlated with IGFBP2 expression (Fig. 1 B and C) clearly demonstrated an opposing pattern with IGFBP2, suggesting negative regulation.
To determine the influence of integrin and ILK pathway gene expression on patient survival, we assessed individual genes, including IGFBP2, integrin α5, integrin β1, and ILK, among all tumor histologies (glioblastoma multiforme, astrocytoma, oligodendroglioma, and mixed). “High” and “low” groups were separated by the midpoint expression value. High expression of each gene was associated with significantly poorer survival (Fig. 1D). This survival trend remained upon individual evaluation of each glioma histology (Fig. S1). In addition, expression levels of integrin and ILK pathway genes (low-, intermediate-, and high-expression clusters) were significantly correlated with patient survival, highlighting the clinical significance of the integrin and ILK pathways in human glioma (Fig. 1E).
IGFBP2 Regulates Downstream Pathways via Integrin Activation.
To determine the significance of the downstream pathways affected by disruption of IGFBP2/integrin signaling, we performed a complementary DNA (cDNA) microarray analysis to compare two stably expressing cell lines originating from SNB19; two clones expressing a mutant form of IGFBP2 that cannot bind integrin (RGD → RGE point mutation; referred to as RGE mutant); and two clones expressing wild-type IGFBP2. Differentially expressed genes were subjected to IPA. Disruption of integrin binding with IGFBP2 (RGE mutant) led to many significantly altered pathways (Dataset S3). The integrin pathway was confirmed to be altered, and the third most-altered pathway was ILK, followed by other pathways involved in migration and invasion (Fig. 2A). GSEA was performed on the basis of absolute differential gene expression using six selected IPA pathways, and each was significantly enriched (Fig. 2B). The analysis showed that a statistically significant portion of the genes within each pathway were down-regulated (Fig. S2), indicating that disruption of IGFBP2 and integrin binding led to de-activation of cell motility pathways.
Fig. 2.
IGFBP2 activates invasion-related pathways by activating integrin. (A) Ingenuity pathway analysis of data obtained from gene expression microarrays in RGE-mutant IGFBP2 or wild-type IGFBP2 SNB19 glioma cell lines. Pathways shown represent significantly altered pathways resulting from disruption of IGFBP2/integrin interaction. (B) GSEA plots of pathways shown in A. Genes are ranked according to differential expression of RGE-mutant to wild-type IGFBP2. (C) Immunofluorescence staining of 12G10, HUTS-21, and 4B7R in indicated cells after plating onto fibronectin-coated glass coverslips for 18 h, followed by cold methanol fixation. (Scale bar, 20 μm.) (D) Flow cytometric analysis of 12G10 and HUTS-21 (active conformation of β1) or 4B7R (total β1) in the indicated cells.
We next performed an immunofluorescence analysis and flow cytometry experiments to validate that IGFBP2 activates integrin β1. Levels of activated β1 were compared among SNB19 parental cells; cells stably expressing IGFBP2; and cells stably expressing RGE-mutant IGFBP2. Activated β1 was detected by HUTS-21 and 12G10, which are antibodies that bind to β1 only when it is in the active conformation (17, 18). Both parental and RGE-mutant cells exhibited a moderate level of activated β1 staining. Activated integrins were localized throughout the cytoplasm and on focal adhesions. In contrast, IGFBP2-expressing cells had high levels of staining, localized primarily along the leading edge of the cell (Fig. 2C). 4B7R, an antibody that recognizes all β1 conformations, served as the control. To further confirm that IGFBP2 activates integrin β1, we performed a flow cytometry analysis, the results of which confirmed the immunofluorescence data. The parental and RGE-mutant cells exhibited low levels of activated integrin, as measured with the HUTS-21 or 12G10 antibodies, whereas a higher proportion of cells was recognized by these antibodies in IGFBP2-overexpressing cells (Fig. 2D). 4B7R again served as the control, with similar levels of total β1 integrin among the cell lines, indicating that the difference in β1 activation was not merely due to increased expression.
ILK Is Required for IGFBP2-Induced Cell Mobility.
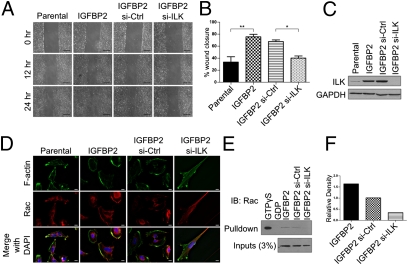
Because the ILK pathway was significantly enriched in IGFBP2-correlated genes and strongly affects patient prognosis, we assessed the functionality of ILK in the IGFBP2 network. Given that cell mobility is a major function of IGFBP2, we determined whether ILK was required by knocking down ILK in IGFBP2-expressing SNB19 cells and performing wound-healing assays. ILK knockdown resulted in a marked decrease in migration in IGFBP2-expressing cells compared with control siRNA or parental cells (Fig. 3A). IGFBP2-expressing SNB19 cells migrated into the wound in a significantly higher proportion than did parental cells (P < 0.01; Fig. 3B). In contrast, IGFBP2 cells treated with ILK siRNA exhibited a significant decrease in the wound-closure rate relative to IGFBP2 cells treated with control siRNA (P < 0.05; Fig. 3B). A Western blot analysis confirmed that ILK was sufficiently knocked down and further revealed that ILK protein levels were increased in IGFBP2-overexpressing cells (Fig. 3C).
Fig. 3.
ILK is required for IGFBP2-induced cell motility and Rac activation. (A) Wound-healing assay in SNB19 parental or IGFBP2-expressing cells transfected with negative control or ILK siRNA. Images were captured at the indicated times after the initial wound. (Scale bar, 500 μm.) (B) Graphical representation of wound healing ability after 24 h, as shown in A. P values represent ANOVA with Bonferroni's multiple comparison test. *P < 0.05, **P < 0.01. Error bars represent SEM. (C) Western blot analysis of ILK protein levels in SNB19 cell lines. (D) IGFBP2-expressing SNB19 cells transfected with negative control or ILK siRNA for 48 h, followed by cold methanol fixation and immunofluorescence staining of F-actin and Rac. (E and F) Rac activation assay in IGFBP2 cells that were untreated or transfected with scrambled or ILK siRNA. (F) Quantification of the amount of immunoprecipitated Rac1 normalized to the amount of Rac1 in the inputs. See Fig. S4 for the enlarged image.
We previously reported that IGFBP2/integrin binding leads to activation of Rac, an important protein in lamellipodia formation (7). Therefore, we determined whether ILK is also critical in this pathway. Immunofluorescence staining of F-actin and Rac demonstrated that F-actin was localized mostly to focal adhesions or throughout the cell membrane in parental cells but concentrated on the lamellipodia in IGFBP2 cells. Upon ILK knockdown, the cells’ morphologic characteristics became elongated, with fewer lamellipodia, and F-actin was present mainly in the focal adhesions. Rac was dispersed throughout the cytoplasm in parental cells but localized at the leading edge in IGFBP2 cells. Knockdown of ILK in IGFBP2 cells led to Rac's movement to the cell tail (Fig. 3D). To confirm Rac activation in these cells, we performed a Rac activation assay. IGFBP2 cells with ILK knockdown had significantly less activated Rac than did IGFBP2 cells that were untreated or treated with control siRNA (Fig. 3 E and F). These results indicate that ILK plays a direct role in IGFBP2/integrin α5-induced glioma cell migration.
IGFBP2 Regulates an Invasion-Related NF-κB Transcriptional Program.
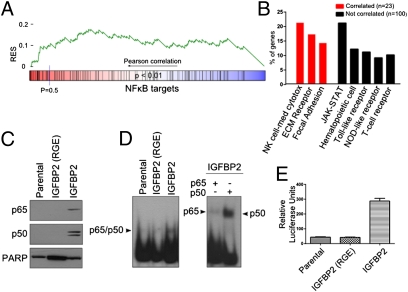
Changes in IGFBP2 expression via microarray analysis resulted in alterations in the expression of many genes, suggesting that IGFBP2 affects a transcriptional program via an unknown mechanism. Because NF-κB is a major transcriptional factor that controls a variety of cancer-related genes, we next determined whether it plays a role in the IGFBP2 pathway. We evaluated NF-κB target gene expression from both the SNB19 cell line microarray and the Rembrandt database. Enrichment of NF-κB target genes was observed in stably expressing IGFBP2 cell lines (Fig. S3). The Kolmogorov–Smirnov statistic was used to determine whether NF-κB target genes were significantly correlated with IGFBP2 expression in human glioma. NF-κB target genes were significantly enriched (P < 0.01; Fig. 4A and Table S1), although not as strongly as in the integrin and ILK pathways. NF-κB regulates many genes, resulting in pleiotropic effects ranging from immune regulation to cellular invasion. Therefore, we evaluated the NF-κB target genes that were positively correlated with IGFBP2. A Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis revealed that these genes were enriched in migration and invasion-related pathways, such as extracellular matrix-receptor interaction and focal adhesion. In contrast, target genes that were not correlated with IGFBP2 (Pearson correlation below 0.5) were not enriched in migration and invasion pathways, indicating that IGFBP2 likely activates an invasion-related NF-κB transcriptional program (Fig. 4B).
Fig. 4.
IGFPB2 activates an NF-κB transcriptional program. (A) GSEA plot from human glioma Rembrandt database. NF-κB target genes were ranked according to IGFBP2 correlation. (B) KEGG analysis of genes from A. Pathways shown are significantly enriched in NF-κB target genes that are correlated >0.5 (red bars) or <0.5 (black bars) with IGFBP2. Percentage of genes represents the number of NF-κB target genes in the pathway relative to the total number of correlated or noncorrelated genes. (C) Western blot analysis of p65 and p50 from nuclear extracts from the indicated cells. (D) Nuclear extracts from C were subjected to EMSA using an NF-κB consensus probe. Supershift assays were performed to validate the presence of p65 or p50. (E) NF-κB activation, as assessed by luciferase assay, in the indicated cells. *P < 0.0001, ANOVA with Tukey's multiple comparison test. Error bars represent SEM. See Fig. S5 for the enlarged image.
To validate that IGFBP2 activates NF-κB and to determine the role of integrin signaling in NF-κB activation, we used SNB19 parental, RGE-mutant, and wild-type IGFBP2 cells. A Western blot analysis of the extracts revealed high levels of p65 and p50 in wild-type IGFBP2 cells compared with low levels in parental and RGE-mutant cells (Fig. 4C). NF-κB/DNA binding was additionally assessed via an electrophoretic mobility shift assay (EMSA). Parental and RGE-mutant IGFBP2 cells exhibited low levels of DNA binding, whereas strong binding was observed in wild-type IGFBP2 extracts. Supershift assays confirmed that the binding was specific for p65 and p50 (Fig. 4D). Luciferase assays demonstrated that NF-κB transcriptional activity was significantly increased in cells expressing IGFBP2 versus parental or RGE-mutant cells (P < 0.0001; Fig. 4D), validating that IGFBP2 activates NF-κB through integrin signaling.
Integrin, ILK, and NF-κB Regulate IGFBP2-Induced Glioma Progression.
We have demonstrated that IGFBP2's relationship with integrin, ILK, and NF-κB is clinically significant and further validated that each is functional in vitro. We next determined whether these pathways play a critical role in glioma progression under physiologic conditions in a glioma mouse model that was initially tumor-free.
We used the RCAS/Ntv-a glial-specific transgenic mouse model to test our hypothesis in vivo. This system uses an avian retrovirus, RCAS, to infect cells expressing the avian tv-a receptor (19). Ntv-a mice have been engineered to express the tv-a receptor under the control of the nestin promoter (20). Nestin directs tv-a receptor expression to glial progenitor cells, leaving the remaining resident cells unaffected after RCAS infection. RCAS constructs containing the gene of interest are transfected into avian DF-1 fibroblast cells and subsequently injected intracranially into Ntv-a mice on postnatal day 1. If the gene or combination of genes is oncogenic, mice form gliomas that closely resemble human glioma in their pathogenic features, including perinuclear satellitosis, vascular proliferation, and pseudopalisading necrosis (21–23).
Using this mouse model, we previously demonstrated that intracranial injection of platelet-derived growth factor β (PDGFB) led to the development of low-grade diffuse glioma (LGDG) in most mice. Codelivery of IGFBP2 and PDGFB resulted in the development of high-grade diffuse glioma (HGDG) in nearly half the mice (4). We therefore used this glioma progression model to determine whether IGFBP2/integrin binding, ILK, and NF-κB are physiologically active pathways in vivo and determine their role in IGFBP2-mediated glioma progression.
To determine whether IGFBP2/integrin binding was required for progression, we injected RCAS-PDGFB alone or with RCAS vectors encoding the wild-type or RGE-mutant IGFBP2. PDGFB alone led to glioma formation in more than 83% (35/42) of mice; in 11% of these mice, the gliomas showed vascular proliferation or foci of necrosis, which qualified them as HGDG lesions. Codelivery of wild-type IGFBP2 and PDGFB produced gliomas in 72% (36/50) of mice, of which 44% were classified as HGDG; these findings were similar to those of our previous study (4). Codelivery of IGFBP2 (RGE) with PDGFB resulted in a 78% (25/32) glioma incidence, but strikingly, only 4% of these were classified as HGDG, indicating that the integrin-binding function of IGFBP2 is critical for its ability to drive glioma progression (Fig. 5 and Table S2).
Fig. 5.
IGFBP2 drives glioma progression by activating the integrin/ILK/NF-κB network. (A) The percentage of mice from each RCAS injection combination with LGDG or HGDG tumors. (B) Representative whole-brain sections from each injection combination. The dotted line indicates the tumor. (C) Representative H&E sections from tumors generated from each RCAS injection combination. (Scale bar, 50 μm.) (D) Model of IGFBP2-induced glioma progression. IGFBP2 lies at the top of a signaling cascade that requires integrin binding, ILK, and p65/p50 to induce glioma progression (HGDG). Disruption of integrin binding to IGFBP2, inhibition of ILK kinase activity, or inhibition of p65/p50 blocks IGFBP2's ability to induce glioma progression (LGDG tumors are produced).
We next determined whether ILK produced an effect similar to that of IGFBP2 and whether kinase-dead ILK (ILK-KD) was capable of blocking IGFBP2-mediated progression. Wild-type ILK or ILK-KD (S343A) was injected with PDGFB. Wild-type ILK led to a significant increase in HGDG incidence compared with PDGFB alone (from 11 to 49%, P = 0.0007), with a similar proportion of anaplastic progression as the PDGFB and IGFBP2 combination. In contrast, ILK-KD combined with PDGFB did not produce a significant increase in HGDG incidence (14 vs. 11%, P = 0.100). When ILK-KD was coinjected with IGFBP2 and PDGFB, an 18% HGDG incidence was observed, which also was not statistically significantly different from that for PDGFB alone, indicating that ILK is a critical downstream effector of the IGFBP2 pathway (Fig. 5 and Table S2).
We determined the role of NF-κB in IGFBP2-induced glioma progression by coinjecting a mutant form of IκBα (IκBαM; S32,36A) with PDGFB and IGFBP2 or PDGFB and ILK. IκBαM inhibited NF-κB activation by retaining it in the cytoplasm. Strikingly, inhibiting NF-κB in the IGFBP2 combination produced LGDG tumors in all of the mice, suggesting that IGFBP2 requires NF-κB to induce progression. Similarly, inhibiting NF-κB in the ILK combination also prevented progression (22% grade 3 incidence; P = 0.42 vs. PDGFB alone).
Thus, IGFBP2 drives glioma progression through integrin signaling, ILK, and NF-κB; genetically blocking any step of the pathway robustly prevents glioma progression, highlighting the potential therapeutic value of these findings (Fig. 5D).
Discussion
Elucidating complex oncogenic signaling pathways is necessary to identify potential therapeutic targets that can be translated into clinical treatments. Although IGFBP2 is now recognized as an important oncogene in glioma progression, its signaling pathway and mechanism of action had been under-characterized. In the current study, we combined transcriptional profiling of human glioma, biochemical analysis, and perturbation of IGFBP2-associated pathways and a spontaneous glioma mouse model to determine the clinical significance of IGFBP2 in relation to the integrin and ILK pathways and to NF-κB transcriptional regulation of invasion-related target genes. We found that blocking any constituent of the integrin/ILK/NF-κB network prevented IGFBP2-driven glioma progression in vivo.
The major function of IGFBP2 in glioma is regulating cellular migration and invasion. In our previous study, we found that tumors that arose from Ink4a-Arf loss and PDGFB overexpression had elevated endogenous IGFBP2 levels that were localized at the invasive front (24), providing compelling evidence that IGFBP2 plays a dominant role in tumor invasion. Although IGFBP2 has been shown to promote cell migration through an integrin interaction, little else was known about the functional significance of this interaction. From the genomics analysis in which IGFBP2/integrin binding was disrupted in glioma cell lines, we demonstrated that multiple pathways were de-activated, most notably the cell motility and invasion pathways. These data closely mirrored findings in human glioma, in which IGFBP2 expression is not only intimately associated with integrin and ILK pathway activation but also is significantly correlated with prognosis.
In this study, we demonstrated that IGFBP2 activates integrin β1 and downstream pathways, requires ILK for cell migration, and activates NF-κB. The most decisive evidence of these findings is functional validation that these relationships represent a true physiologically active signaling pathway in vivo. Cell culture experiments and in vivo analysis in xenografts answer key questions, but better systems are required to ultimately test clinical applicability and significance. The RCAS/Ntv-a spontaneous glioma mouse model has enabled us to closely recapitulate human glioma by transforming normal murine glial cells with combinations of clinically relevant oncogenes (25). This study further uses this model to dissect an entire signaling pathway. We validated that IGFBP2 drives glioma progression through an integrin/ILK/NF-κB network and, most importantly, showed that genetically blocking each point prevented progression.
The diffusely infiltrative nature of glioma cells makes complete surgical resection difficult and is a major contributor to rapid tumor recurrence. Therefore, targeted disruption of the IGFBP2/integrin/ILK/NF-κB pathway and subsequent inhibition of migration may decrease the likelihood of tumor recurrence and invasion into surrounding parenchyma, preventing the destruction of normal brain architecture. No IGFBP2 inhibitors are available; however, integrin (26), ILK (27, 28), and NF-κB (29) are druggable targets that have been used in preclinical studies and clinical trials. These drugs could be used to target glioma with high IGFBP2 expression either in combination or with current standard therapy. In summary, IGFBP2 is a strong, clinically relevant oncogene that exploits an integrin/ILK/NF-κB network to create the major driving force behind glioma progression, providing a convincing argument that inhibition of this pathway should be investigated clinically. We are hopeful that IGFBP2 pathway inhibition will result in tumor regression and a subsequent survival benefit, not only for glioma patients but also for patients with a broad range of other cancers.
Materials and Methods
Animal Care, RCAS Injection, and Tumor Pathologic Analysis.
DF-1 cells encoding RCAS viral particles were collected, mixed, and suspended in 1× PBS, and 2 μL of the cell suspension was injected into the right hemisphere of postnatal day 1 Ntv-a mice using a Hamilton syringe. Mice were euthanized upon evidence of brain tumor or after 13 wk. The brains were collected as described previously (4) and graded by a neuropathologist. All animal experiments were performed in accordance with the University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee guidelines.
Bioinformatic Analysis.
We performed a human glioma analysis of gene expression microarray data from the National Cancer Institute Rembrandt public data repository (30), which is composed of 329 tumors: 59 oligodendrogliomas, 102 astrocytomas, and 178 glioblastomas. Single-gene survival analysis of human glioma was separated by midpoint expression value. Gene sets for all pathways were obtained from IPA software. The NF-κB target gene set was compiled from http://bioinfo.lifl.fr/NF-KB/ and TRANSFAC and composed of validated human genes. See SI Materials and Methods for KEGG. For GSEA, gene sets were ranked according to Pearson correlation with IGFBP2, and an enrichment score (ES) was calculated on the basis of the Komogorov–Smirnov statistic. P values were calculated by comparing ES to 1,000 random permutations. Hierarchical clustering was performed on genes in integrin and ILK pathways that were correlated more than 0.5 (positive or negative). The distance metric was L1, using the unweighted mean distance as the linkage criterion. Groups were highlighted manually. See SI Materials and Methods for details on microarray preparation. Genes, with their expression values, were uploaded to IPA v8.6–3003 (http://www.ingenuity.com) to perform pathway analysis. The differentially expressed genes were identified in IPA by selecting genes with expression values below a twofold cutoff. The Ingenuity knowledge base (genes only) was used as a reference set.
Statistical Analysis.
Wound healing and luciferase assay results were analyzed by ANOVA, followed by Bonferroni's multiple comparison test. The log-rank test was used to obtain a P value for the significance of Kaplan–Meier curves’ divergence. The statistical significance of the mouse tumor data were determined using a two-ends Fisher's exact test. A significance level was set at P < 0.05 for all tests.
Supplementary Material
Acknowledgments
We thank Brittany C. Parker (MD Anderson Cancer Center) and Ann Sutton (Scientific Editor, Department of Scientific Publications, MD Anderson Cancer Center) for editorial assistance and Hua Wang (MD Anderson Cancer Center) for technical assistance. This work was partially supported by the Goldhirsh Foundation (W.Z. and G.N.F.); National Institutes of Health/National Cancer Institute R01 grants (to W.Z. and G.N.F.); a National Institutes of Health Roadmap Pharmacoinformatics Training Grant (to K.M.H.); an American Cancer Society Postdoctoral Fellowship (to L.M.M.); a National Institutes of Health Pharmacoinformatics Training Fellowship and an American Legion Fellowship (to S.M.D.); and the Finnish Funding Agency for Technology and Innovation Finland Distinguished Professor Programme (M.N.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE35467).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120375109/-/DCSupplemental.
References
- 1.Fuller GN, et al. Reactivation of insulin-like growth factor binding protein 2 expression in glioblastoma multiforme: A revelation by parallel gene expression profiling. Cancer Res. 1999;59:4228–4232. [PubMed] [Google Scholar]
- 2.Sallinen SL, et al. Identification of differentially expressed genes in human gliomas by DNA microarray and tissue chip techniques. Cancer Res. 2000;60:6617–6622. [PubMed] [Google Scholar]
- 3.Zhang W, Wang H, Song SW, Fuller GN. Insulin-like growth factor binding protein 2: Gene expression microarrays and the hypothesis-generation paradigm. Brain Pathol. 2002;12(1):87–94. doi: 10.1111/j.1750-3639.2002.tb00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlap SM, et al. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Natl Acad Sci USA. 2007;104:11736–11741. doi: 10.1073/pnas.0703145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranke MB, Elmlinger M. Functional role of insulin-like growth factor binding proteins. Horm Res. 1997;48(Suppl 4):9–15. doi: 10.1159/000191304. [DOI] [PubMed] [Google Scholar]
- 6.Frommer KW, et al. IGF-independent effects of IGFBP-2 on the human breast cancer cell line Hs578T. J Mol Endocrinol. 2006;37(1):13–23. doi: 10.1677/jme.1.01955. [DOI] [PubMed] [Google Scholar]
- 7.Wang GK, Hu L, Fuller GN, Zhang W. An interaction between insulin-like growth factor-binding protein 2 (IGFBP2) and integrin alpha5 is essential for IGFBP2-induced cell mobility. J Biol Chem. 2006;281:14085–14091. doi: 10.1074/jbc.M513686200. [DOI] [PubMed] [Google Scholar]
- 8.Schütt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32:859–868. doi: 10.1677/jme.0.0320859. [DOI] [PubMed] [Google Scholar]
- 9.Hannigan GE, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379(6560):91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 10.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: A cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5(1):51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, et al. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab Invest. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 12.Smith D, Shimamura T, Barbera S, Bejcek BE. NF-kappaB controls growth of glioblastomas/astrocytomas. Mol Cell Biochem. 2008;307(1-2):141–147. doi: 10.1007/s11010-007-9593-4. [DOI] [PubMed] [Google Scholar]
- 13.Xie TX, et al. Aberrant NF-kappaB activity is critical in focal necrosis formation of human glioblastoma by regulation of the expression of tissue factor. Int J Oncol. 2008;33(1):5–15. [PubMed] [Google Scholar]
- 14.Bhoopathi P, et al. Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 2008;283:1545–1552. doi: 10.1074/jbc.M707931200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Tan C, Mui A, Dedhar S. Integrin-linked kinase regulates inducible nitric oxide synthase and cyclooxygenase-2 expression in an NF-kappa B-dependent manner. J Biol Chem. 2002;277:3109–3116. doi: 10.1074/jbc.M108673200. [DOI] [PubMed] [Google Scholar]
- 16.Wani AA, Jafarnejad SM, Zhou J, Li G. Integrin-linked kinase regulates melanoma angiogenesis by activating NF-κB/interleukin-6 signaling pathway. Oncogene. 2011;30:2778–2788. doi: 10.1038/onc.2010.644. [DOI] [PubMed] [Google Scholar]
- 17.Luque A, et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 18.Mould AP, Garratt AN, Askari JA, Akiyama SK, Humphries MJ. Identification of a novel anti-integrin monoclonal antibody that recognises a ligand-induced binding site epitope on the beta 1 subunit. FEBS Lett. 1995;363(1–2):118–122. doi: 10.1016/0014-5793(95)00301-o. [DOI] [PubMed] [Google Scholar]
- 19.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci USA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai C, et al. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holland EC. A mouse model for glioma: Biology, pathology, and therapeutic opportunities. Toxicol Pathol. 2000;28(1):171–177. doi: 10.1177/019262330002800122. [DOI] [PubMed] [Google Scholar]
- 23.Moore LM, Holmes KM, Fuller GN, Zhang W. Oncogene interactions are required for glioma development and progression as revealed by a tissue specific transgenic mouse model. Chin J Cancer. 2011;30(3):163–172. doi: 10.5732/cjc.010.10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore LM, et al. IGFBP2 is a candidate biomarker for Ink4a-Arf status and a therapeutic target for high-grade gliomas. Proc Natl Acad Sci USA. 2009;106:16675–16679. doi: 10.1073/pnas.0900807106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Moore L, Ji P. Mouse models for cancer research. Chin J Cancer. 2011;30(3):149–152. doi: 10.5732/cjc.011.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: Lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 27.Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: Not only figurants in the cancer story. Nat Rev Cancer. 2010;10:858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- 28.Kalra J, et al. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model. Breast Cancer Res. 2009;11:R25. doi: 10.1186/bcr2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8(1):33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute 2005. REMBRANDT. http://rembrandt.nci.nih.gov. Accessed October 11, 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.