Abstract
Climate exerts a powerful influence on biological processes, but the effects of climate change on ecosystem nutrient flux and cycling are poorly resolved. Although rare, long-term records offer a unique opportunity to disentangle effects of climate from other anthropogenic influences. Here, we examine the longest and most complete record of watershed nutrient and climate dynamics available worldwide, which was collected at the Hubbard Brook Experimental Forest in the northeastern United States. We used empirical analyses and model calculations to distinguish between effects of climate change and past perturbations on the forest nitrogen (N) cycle. We find that climate alone cannot explain the occurrence of a dramatic >90% drop in watershed nitrate export over the past 46 y, despite longer growing seasons and higher soil temperatures. The strongest climate influence was an increase in soil temperature accompanied by a shift in paths of soil water flow within the watershed, but this effect explained, at best, only ∼40% of the nitrate decline. In contrast, at least 50–60% of the observed change in the N export could be explained by the long-lasting effect of forest cutting in the early 1900s on the N cycle of the soil and vegetation pools. Our analysis shows that historic events can obscure the influence of modern day stresses on the N cycle, even when analyses have the advantage of being informed by 0.5-century-long datasets. These findings raise fundamental questions about interpretations of long-term trends as a baseline for understanding how climate change influences complex ecosystems.
Keywords: forest ecosystems, long-term monitoring, streamwater chemistry, precipitation chemistry, nutrient cycles
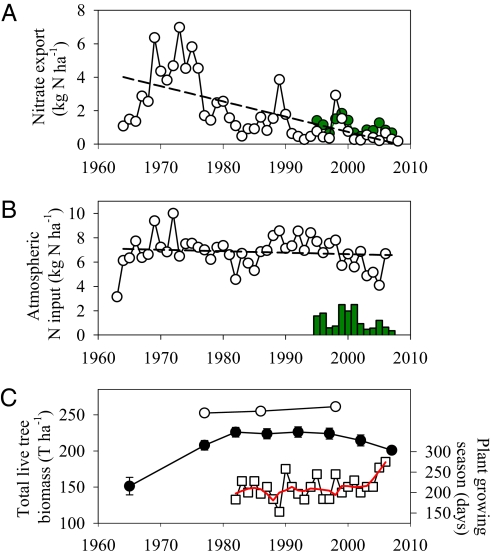
Our understanding of how climate change impacts complex ecological systems depends on our conception of a baseline against which change can be judged and knowledge of how this baseline has been shaped by historical conditions. At the Hubbard Brook Experimental Forest (HBEF) in New Hampshire, for example, we know that current concentrations of nitrate in watershed streams are the lowest in 46 y of measurement and that ecosystem nitrate losses have decreased by >90% over this time (Fig. 1A). If we were to take the early high nitrate period (1969–1976) as the historical reference, we would estimate that nitrate export has dropped by a total of ∼125 kg nitrogen (N) ha−1 during the 30 y of the decline (1977–2007) (Fig. 1A). Such a large drop in N export is ecologically relevant and constitutes a dramatic shift in the ecosystem N cycle: from a leaky cycle that retained only ∼30% of external inputs in the high stream water nitrate period to a highly retentive cycle that currently captures ∼90% of atmospheric inputs (Methods).
Fig. 1.
Historic N loads and forest dynamics at HBEF. (A) Annual hydrological export of nitrate (1964–2008; n = 45) and DON (since 1995; n = 13) from watershed 6 (W6; white and green circles, respectively). The linear trend of nitrate across years is shown with a dashed line (r2 = 0.38, slope ± SE = −0.08 ± 0.02 kg N ha−1 y−1, P < 0.001). Nitrate export declined from an average (± SE) of 5.13 ± 0.39 kg N ha−1 y−1 during the 1969–1976 high nitrate reference period to 0.42 ± 0.07 kg N ha−1 y−1 in 2000–2007. We quantified the nitrate decline (missing nitrate in the text) between 1977 and 2007 by subtracting the annual nitrate export observed each year from the average value of the high nitrate reference period (5.13 kg N ha−1 y−1; 1969–1976); this decline in export was equivalent to 125 ± 12 kg N ha−1 over 30 y. Similar quantities result from alternative calculations based on the trend in nitrate export determined from either linear or curvilinear fits to the long-term record (1969–2007). DON export showed no linear trend over time (P > 0.05). (B) Annual bulk deposition of inorganic N (nitrate + ammonium) in W6 (1964–2008; n = 45) did not display any significant linear trend over time (dashed line; P > 0.05). There was no significant linear trend in DON deposition since 1995 (green bars; n = 13, P > 0.05). (C) Total live tree biomass [≥10 cm diameter at breast height (DBH)] in W6 (1965–2007; n = 8) and the Bird Area (1981–2001; n = 3; black and white circles, respectively; error bars are as in ref. 14; error bars in the Bird Area are smaller than symbols). Length of the growing season from 1982 to 2006 is shown by squares (n = 25); locally weighted scatterplot smoothing is in red. Although there was no significant linear trend before 2002 (P > 0.05), the long-term trend became significantly positive after 2002 (r2 = 0.89, slope = 19.7 ± 4 d y−1, P < 0.02).
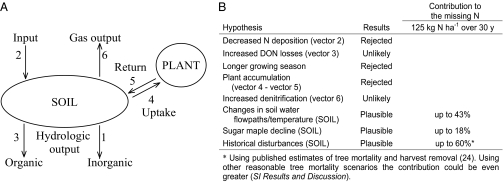
We adopt a watershed mass balance approach (1, 2) to examine the factor(s) responsible for this dramatic change in the forest N cycle (Fig. 2). Because climate is an overriding and powerful driver of biological process, we pay particular attention to whether the observed changes in ecosystem N dynamics were linked to climate change over the past five decades. Previous examinations of this trend have discounted effects of changing N deposition and forest maturation, and they have suggested that there may exist a previously unrecognized ecosystem sink for N (3, 4), possibly associated with the soil (5, 6). We evaluate these hypotheses and other competing hypotheses using an analysis that integrates several unique long-term records of biological, physical, and biogeochemical factors within the HBEF. From a mass balance perspective, we show in Fig. 2A how the decline in nitrate export (vector 1) could be caused by one or several mechanisms: (i) decreased N deposition (vector 2), (ii) increased export of dissolved organic N compounds (DON; vector 3), (iii) increased net accumulation of N in vegetation (sum of vectors 4 and 5), (iv) increased gaseous N loss by denitrification (vector 6), or (v) net increase in N stored in the soil pool (balance of vectors 1–6).
Fig. 2.
Schematic representation of N fluxes and storage pools for a typical temperate hardwood forest and synthesis of competing hypotheses related to the long-term declining trend in nitrate export. (A) Conceptual model of fluxes and storage pools of N in the northeastern US hardwood forests. Atmospheric deposition (vector 2) is the major input flux of nitrogen to the forest. Denitrification (vector 6) and leaching (vector 1 and 3) are the major output fluxes of nitrogen from the ecosystem. Nitrogen is taken up and stored by vegetation (vector 4), returning eventually to the soil pool as litterfall or root exudates (vector 5); also, it may be cycled by microbes and stored in the soil. (B) Synthesis of the competing hypotheses considered here and their potential contribution to declining nitrate export observed in W6 at the HBEF.
Results and Discussion
We first examine atmospheric N deposition (vector 2 in Fig. 2A), which has strong potential to influence trends in surface water nitrate (1, 7, 8). Neither the 46-y record of bulk deposition at HBEF (Fig. 1B) nor shorter records from nearby locations (SI Results and Discussion) can explain the observed drop in watershed nitrate export. Bulk N deposition at HBEF is today similar to values in the 1960s, and there has been no systematic decline large enough to explain the ∼125 kg N ha−1 reduction in nitrate export (Fig. 1 A vs. B). DON contributes <20% to bulk deposition and has declined only marginally since first measured routinely in 1995 (Fig. 1B). In addition, dry deposition contributes negligibly to atmospheric N inputs in this densely forested region [0.37 ± 0.02 (volume weighted average ± SE) kg N ha−1 y−1 or <6% of total N deposition] and does not show any clear trend since first measured routinely in 1989. We conclude that the long-term decline in nitrate export from HBEF watersheds cannot be explained by any coincident change in atmospheric deposition (Fig. 2B).
A second possibility is that watershed DON export (vector 3 in Fig. 2A) has increased since the 1970s to the extent of quantitatively offsetting the observed reduction in nitrate export. Our analyses show no increase in DON or evidence of such a shift in N forms since DON was first measured routinely in 1994 (Fig. 1A). Moreover, present day export (1 ± 0.1 kg N ha−1 y−1) is too low to balance N inputs (6.8 ± 0.2 kg N ha−1 y−1) or offset the historic nitrate decline. For example, a sustained linear increase of DON export from zero in the 1969–1976 reference period to 1 kg N ha−1 y−1 by 1995 (when it was first measured) (SI Results and Discussion) followed by export of 1 kg N ha−1 y−1 during 1995–2007 could explain <22 kg N ha−1 or <18% of the ∼125 kg ha−1 nitrate decline. We conclude that increased DON export cannot resolve the long-term nitrate decline in HBEF watersheds (Fig. 2B).
We next examined a mechanism that is highly sensitive to climate change and can exert strong influence on the forest N cycle: accelerated plant growth (vectors 4 and 5 in Fig. 2A). More favorable climate, increased atmospheric CO2, and longer growing seasons can, in theory, trigger growth and increase N stored in plant biomass (9, 10). We used satellite observations of normalized difference vegetation index (NDVI) to quantify any secular trend in the HBEF growing season (11) (SI Results and Discussion). Our analysis revealed an increase in growing season length by several weeks (Fig. 1C) since satellite imagery first became available in 1982. Closer inspection shows that the trend was caused by increasingly benign autumn conditions since 2002, a period during which nitrate export did not change (Fig. 1 A vs. C). There was no consistent trend in growing season length before 2002. Although limited to conditions after 1982, this analysis fails to establish any clear link between changes in the plant growing season and nitrate export at HBEF.
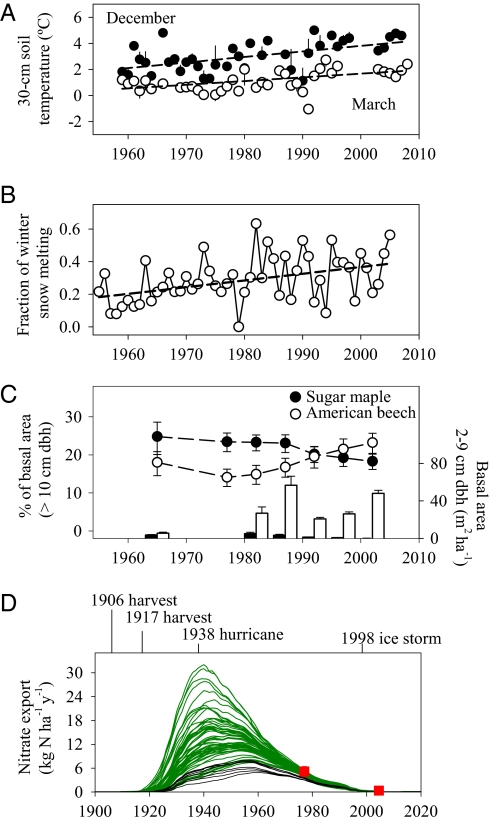
We also evaluated the possibility of a climate–ecosystem link by examining direct measures of soil temperatures, which in contrast to NDVI, are available for the long-term record at HBEF. We examined temperatures in deeper soil layers, because these temperatures are less influenced by day to day weather deviations (Methods and SI Results and Discussion). We found that temperatures have consistently increased since 1961 at 30-cm soil depth, with the steepest change occurring from December to March (Fig. 3A and SI Results and Discussion). Air temperatures and records of lake ice cover also indicate a warming trend over the same period in the Hubbard Brook Valley (12). These findings show that HBEF forests have experienced substantial warming in late fall to early spring over the past five decades, which in turn, may have caused increased plant growth.
Fig. 3.
Historic soil temperature and hydrological data, changes in forest species composition, and simulated long-term trend of nitrate export with the LM3V model. (A) Mean (error bars are ± SE) soil temperature at 30-cm depth in December and March (1959–2007). The linear fit (dashed lines) was significant in both cases (P < 0.001; December: r2 = 0.28, slope = 0.046 ± 0.011 °C y−1, n = 39; March: r2 = 0.27, slope = 0.028 ± 0.007 °C y−1, n = 38). There was no significant trend in late spring and summer (P > 0.05 for April to September). (B) Fraction of intermittent warming events that caused winter snowmelt (1955–2007). The dashed line is the linear fit (r2 = 0.18, slope = 0.004 ± 0.001 y−1, P < 0.01, n = 51 y). (C) Relative contribution to the total basal area (≥10 cm DBH; circles, n = 7) and basal area for the saplings (2–9 cm DBH; bars, n = 6) of sugar maple and American beech in the mid- and low-elevation areas in the W6 forest (1965–2002). Error bars are 95% confidence intervals of the mean are based on plot-scale variation. (D) Nitrate export at HBEF simulated with the LM3V model. The lines represent a subset of 500 simulations, where different intensities of tree mortality have been prescribed (SI Results and Discussion). Red squares are volume weighted average nitrate export for the high N export period (1969–1976) and the low N export period (2000–2007) measured at HBEF. Green lines represent simulations that reproduce peak nitrate export as observed during the high N period. The range in tree mortality for this subset of runs was 0–55% and 45–95% for the 1906 and 1917 events, respectively (SI Results and Discussion) Black lines denote simulations with tree mortality from the literature (24) (20% ± 10% and 60% ± 10% for the 1906 and 1917 events, respectively). In all cases, tree mortality was set to 20% and 30% for the 1938 hurricane and the 1998 ice storm (25, 26).
The existence of regular censuses of above- and belowground live biomass since 1965 allows us to examine directly whether the observed warming trend has caused increased plant growth and storage of plant N in HBEF forests—either through temperature alone or the combined effect of temperature plus increasing CO2. Although tree biomass increased between 1965 and 1982 as expected for an aggrading forest (13), net biomass accumulation declined to approximately zero after 1982 and decreased by ∼10% between 1997 and 2007 (Fig. 1C) (14, 15) (SI Results and Discussion). Such deceleration of growth is not only inconsistent with conditions of improved climate and increased CO2 but also with the observed decline in nitrate export (3). Growth deceleration acts to reduce plant N demand, which in turn, should cause increased rather than decreased export of N at the watershed scale (16–18). That we observe a large drop in nitrate as biomass accumulation decelerates indicates that factors other than plant biomass govern the long-term N dynamics of this forest. This rather counterintuitive result raises fundamental questions about the role of climate and plant growth in shaping the forest N cycle over decades. The results do not, however, support the idea that increased plant growth (vectors 4 and 5 in Fig. 2) can resolve the observed nitrate decline.
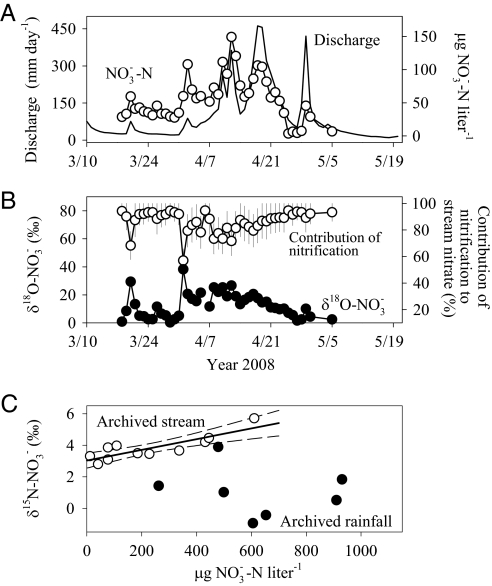
We next evaluated whether increased microbial denitrification (vector 6 in Fig. 2A) could explain the drop in nitrate (NO3−-N) export. This mechanism might seem unlikely on theoretical grounds, because denitrification generally is high in nitrate-rich (i.e., early HBEF record) but low in nitrate-poor conditions (late HBEF record) (19, 20). We used a stable isotope approach that takes advantage of the substantial isotopic discrimination of δ15N-NO3− that occurs during microbial denitrification (21). We expected a negative correlation between δ15N-NO3− and nitrate concentration if denitrification was the primary determinant of the stream water nitrate trend. We analyzed archived samples of stream water and bulk deposition since 1990 and fresh samples from 2008 (Methods and SI Results and Discussion). The δ15N-NO3− signature in archived bulk precipitation [0.47 ± 0.78‰ (average ± SE), n = 8] did not differ from the precipitation in 2008 samples (−1.07 ± 0.44‰, n = 6; Wilcoxon rank sum test, z = −1.48, P > 0.05) and showed no trend with nitrate concentration (Fig. 4C). In contrast, δ15N-NO3− in archived stream water (4.1 ± 0.29‰, n = 11) was strongly but positively correlated with stream water nitrate concentration (Fig. 4C) (Spearman's ρ = 0.74, P < 0.01). This positive correlation does not support the idea that denitrification was the primary determinant of stream water nitrate. Rather, the pattern supports the theoretical expectation that denitrification is high when nitrate also is high. We conclude that stream nitrate does not bear the identifying signature of denitrification acting as the major determinant of the nitrate trend (vector 6 in Fig. 2).
Fig. 4.
Stream discharge and stream water nitrate concentration during the winter and spring of 2008, and isotopic natural abundance of nitrate for actual and archived water samples. (A) Discharge (n = 79) and stream nitrate concentration (n = 46) from March 18, 2008 to May 5, 2008 in W6. (B) The δ18O-NO3− (‰) in stream water (black circles; n = 45) and the relative contribution of internal sources (nitrification) to stream nitrate (white circles) during the winter and spring of 2008 in W6. We assumed δδ18O-NO3− in stream water results from two end members: nitrate in atmospheric deposition (84.2 ± 1.8‰; our measures) vs. nitrate produced by microbial nitrification in soils. Bars represent the range in the relative contribution of nitrification after sensitivity analyses for microbially produced nitrate ranging from −10‰ to +15‰ (29). (C) Relationship between stream nitrate concentrations and the δ15N-NO3− (‰) signal for archived bulk deposition (black circles; n = 8) and stream (white circles; n = 11) samples for the period of 1990–2007. The linear fit was significant only for the archived stream water samples (black line; r2 = 0.72; P < 0.001). Dashed lines show the ±95% confidence interval.
We next considered two mechanisms related to increased N storage in soils (SOIL in Fig. 2A): (i) enhanced N immobilization caused by changing paths of soil water flow and (ii) increased N accumulation caused by historic disturbance events. Both mechanisms are difficult to detect by direct soil sampling, because the change in N needed to resolve the observed nitrate decline (∼125 kg N ha−1) is <2% of total soil N—an amount too small to detect given uncertainties and errors inherent in quantifying the soil N pool (22).
We considered whether climate-induced changes in watershed hydrology might have increased the immobilization of nitrate by soils. Several lines of evidence imply the existence of climate-mediated effects of hydrology on nitrate in the HBEF record, especially during spring snowmelt, which is a period of peak export of both nitrate and water (68% and 54% of annual total, respectively) (1). First, stream nitrate declined most strongly during December to April when soil warming was most rapid, with the steepest drop in March and April when snowmelt volumes are greatest (1) (SI Results and Discussion). Second, we found substantial long-term changes in the dynamics of snow cover and snowmelt. Although annual rain and snowfall amounts have increased slightly (5), the period of snow cover has shortened by ∼6 d decade−1, whereas maximum snow depth has declined by ∼6 cm decade−1 between 1956 and 2006 (SI Results and Discussion). Moreover, the snowfall volume that melted in intermittent warming events (rather than as a single spring melt pulse) has more than doubled from <20% in the 1950s to >40% today (Fig. 3B) (Methods and SI Results and Discussion). The frequency of short-term soil freezing events has increased in response to these intermittent warming periods (5). These results point to dramatic changes in watershed hydrology at HBEF, with potentially strong links to warming soils and periods of changing nitrate export.
We next evaluated whether these observed hydrological changes might trigger differences in soil nitrate immobilization. Microbial immobilization and root uptake of N depend on the contact time between dissolved nitrate and the soil complex, such that nitrate can escape to watershed streams when water flows on top of frozen soil or in macropores during large snow melt events (23). It is, therefore, plausible that the observed long-term decline of large snowmelt events would have caused increased contact time between nitrate and soils, thus increasing microbial and plant immobilization and decreasing nitrate export. In addition, warmer early spring conditions could also act to enhance microbial/plant immobilization.
To test this hypothesis directly, we monitored watershed discharge, dissolved nitrate, and natural abundance isotopes of oxygen in nitrate (δ18O-NO3−) in bulk deposition, accumulated snow, and stream water during the 2008 snowmelt period (Methods and SI Results and Discussion). Stream nitrate and δ18O-NO3− values were both low during base flow, but they increased substantially during large snowmelt events (Fig. 4 A and B). An isotopic mixing model indicated that >95% of stream nitrate originated from soil nitrifying bacteria during base flow but that up to 40% derived directly from snowmelt during large melt events (Fig. 4B). We conclude that large melt events can short circuit water flow paths and allow nitrate to bypass the soil immobilization trap. This finding raises the question of whether the HBEF nitrate decline could be explained by changes in snowmelt dynamics: from few and large melt events early to frequent and small events later in the long-term record.
We can place an upper limit to this hydrologic mechanism by assuming that snowmelt bypassed soils during the high nitrate period of 1969–1976 but was fully intercepted and immobilized by microbes and fine roots since 1977. This calculation indicates a maximum soil sink of 54 kg N ha−1, which can explain up to 43% of the long-term decline in N export (Methods and SI Results and Discussion). As discussed earlier, however, there is no evidence that such an induced root sink for N has translated into faster plant growth or higher aboveground biomass (Fig. 1C). We conclude that changes in hydrological flow paths and soil temperature could influence nitrate retention by soils but that the effect is not large enough to cause a detectable increase in plant growth or explain the entire decline in nitrate export (Fig. 2B).
Finally, we explored whether historical vegetation disturbances could explain the observed decline in N export through changes in the soil N pool (SOIL in Fig. 2). The long-term influence of such disturbances on the N cycle is often elusive; long-term records are scarce, and it is difficult to disentangle past and present perturbations in real world datasets. Model calculations can help resolve such complex historical interactions, and we used a modeling approach to examine two influences: (i) major events of tree mortality (>20% of total live biomass) caused by harvests in 1906 and 1917, the 1938 hurricane, and the 1998 ice storm at HBEF (24–26), and (ii) shifts in tree species caused by the onset of sugar maple (Acer saccharum) decline (27).
We applied a dynamic terrestrial vegetation model (28) designed to explicitly resolve C–N interactions and feedbacks, including N limitation of plant productivity and N dependence on organic matter decomposition (SI Results and Discussion). The model simulates the effects of historical disturbances and tree mortality on soil N dynamics and nitrate leaching. We considered scenarios of tree mortality caused by the major historic events reported at HBEF as outlined in SI Results and Discussion. Across all these scenarios, our model showed a distinct postdisturbance pattern of a pulse increase in nitrate losses followed by a sustained decline from high to low nitrate export over the past five decades (Fig. 3D and SI Results and Discussion). In all scenarios, the nitrate decline was caused by net accumulation of soil N over decades, because the forest progressively recovered from abrupt losses of internal ecosystem N pools (28) (SI Results and Discussion).
We explored the sensitivity of our result to different assumptions about the severity of individual disturbances on vegetation N pools (SI Results and Discussion). A wide range of values recreated the overall pattern observed in the HBEF long-term record: high nitrate export in the 1970s followed by a strong decline over the next 30 y (Fig. 3D, green lines). Although historical values of tree mortality are poorly known, our use of published estimates for the HBEF (24–26) generated a nitrate decline of 48–60% of the missing N (Fig. 3D, black lines and SI Results and Discussion). These results indicate that watershed nitrate export is highly sensitive to historical legacies of forest disturbance and their influence on soil N pools. In the case of HBEF, the observed decline in nitrate export is broadly consistent with the series of disturbances that these forests have experienced since the 1906 harvest (Fig. 2B).
We also evaluated whether sugar maple decline could influence nitrate export from HBEF watersheds (Methods and SI Results and Discussion). Sugar maple litter promotes nitrate production in soils, which in turn, could promote watershed nitrate export. Historical HBEF census records show a 26% decline in basal area of adult sugar maples and a 12-fold loss of sapling basal area since 1977 (Fig. 3C). We estimated the effect of this decline using known decomposition and nitrification rates for sugar maple and American beech (Betula allegheniensis), the two most common hardwood species at HBEF (Methods and SI Results and Discussion). These calculations indicate that decline in sugar maples would cause only small changes in the soil N pool (3 kg N ha−1 25 y−1) (SI Results and Discussion). Even our extreme case scenario, in which we assumed that all nitrogen mineralized by sugar maple is exported as nitrate, implied a decrease in nitrate export of only 19 kg N ha−1 25 y−1. We conclude that sugar maple decline can explain only a minor fraction (<16%) of the observed decline in N export at HBEF (Fig. 2B).
Our analysis of the unique long-term record of N flux and cycling at HBEF offers several unexpected findings, which we summarize in the form of competing mechanisms in Fig. 2B. Most startling is perhaps the lack of any evidence for direct effects of climate change on net vegetation growth and plant N demand, despite substantial increases in air and soil temperatures and growing season length. We could identify only one potential climate effect: a shift in snowmelt hydrology induced by the progressive warming of soils with limited influence on nitrate export. In contrast, our analyses indicated that historical disturbances of vegetation could have very large and lasting influences on soil N pools and in turn, patterns of nitrate export over many decades.
Our results raise the critical issue of what constitutes the baseline against which climate change and other anthropogenic impacts should be evaluated in the HBEF record. Our finding of long-term influences of forest disturbances, for instance, suggests that the 1969–1976 high nitrate period may be a transient response to earlier disturbances rather than a baseline that characterizes the historic condition of the N cycle at HBEF. We conclude that historic disturbances can obscure the effects of climate change on the forest N cycle, even when analyses are advantaged by the availability of half-century long datasets. Resolution of how present day impacts interact with historical trajectories of ecosystem function represents one of the greatest challenges in understanding natural ecosystems today.
Methods
Historical Data Analysis.
We analyzed historical hydrometeorological and chemistry data and forest inventories from the HBEF for the biogeochemical reference watershed [watershed 6 (W6); 43°56′ N, 71°45′ W]. For annual budgets, we considered the first of June as the start of the water year (1). The hydrological retention of nitrate for each water year was estimated as the difference between bulk inorganic N deposition (Ninput) and stream water nitrate load (Nexport) in relative terms 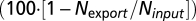 . We analyzed weekly 30-cm-depth soil temperature data recorded by the US Forest Service from 1961 to 2003. We also analyzed δ15N-NO3− and δ18O-NO3− in archived samples of stream water and bulk precipitation from 1990 to 2007. We used weekly measures of snow depth recorded by the US Forest Service at the W6 snow course from 1956 to 2005 to quantify the fraction of winter precipitation melted in individual intermittent events. We estimate monthly inputs of N as snow using historic records of monthly volume weighted average inorganic N concentration in bulk deposition at HBEF rain gauge 6. Data sources and calculation details are in SI Methods.
. We analyzed weekly 30-cm-depth soil temperature data recorded by the US Forest Service from 1961 to 2003. We also analyzed δ15N-NO3− and δ18O-NO3− in archived samples of stream water and bulk precipitation from 1990 to 2007. We used weekly measures of snow depth recorded by the US Forest Service at the W6 snow course from 1956 to 2005 to quantify the fraction of winter precipitation melted in individual intermittent events. We estimate monthly inputs of N as snow using historic records of monthly volume weighted average inorganic N concentration in bulk deposition at HBEF rain gauge 6. Data sources and calculation details are in SI Methods.
Spring Sample Collection.
Stream samples were collected in W6 before, during, and after the 2008 spring pulse of snowmelt discharge (n = 50). Bulk deposition (n = 5) and snow samples (n = 3) were collected at approximately monthly intervals. Samples were filtered through prewashed GF/F filters before laboratory analysis. All samples were analyzed for NO3−, δ15N-NO3−, and δ18O-NO3− (details for chemical analysis are in SI Methods).
Forest Floor N Model.
We first calculated whether N retention caused by the slower decomposition rate of American beech litter could increase HBEF soil N pools such that it could explain the long-term nitrate decline. In subsequent scenarios, we tested whether reduction in sugar maple abundance could cause lower nitrification and thus, reduced losses of nitrate from the forest floor pool (model structure and parameters used are in SI Methods).
Supplementary Material
Acknowledgments
We thank Cliff Dahm and David Tilman for constructive comments on a previous version of the manuscript; C. Driscoll and C. Fuss for field assistance during collection of 2008 snowmelt samples; A. Bailey for US Forest Service data on soil temperature; C. Ferguson for normalized difference vegetation index data; the D. Sigman Laboratory for assistance with isotopic analysis; and L. Shevliakova for comments on the LM3V model application. The work by S.B. was supported by a Fulbright Postdoctoral Scholarship from the Spanish Ministry of Science and Innovation. S.B., S.G., and L.O.H. were supported by grants from the National Oceanic and Atmospheric Administration, the National Science Foundation (DEB-0614116), and the A. W. Mellon Foundation. The Hubbard Brook Experimental Forest is operated and maintained by the US Forest Service, Northeast Forest Research Station, Newtown Square, PA. Financial support for the long-term data is provided by the National Science Foundation (including the Long-Term Ecological Research and Long-Term Research in Environmental Biology programs), the A. W. Mellon Foundation, and the US Forest Service.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121448109/-/DCSupplemental.
References
- 1.Likens GE, Bormann FH. Biogeochemistry of a Forested Ecosystem. 2nd Ed. Berlin: Springer-Verlag; 1995. [Google Scholar]
- 2.Bormann FH, Likens GE. Nutrient cycling. Science. 1967;155:424–429. doi: 10.1126/science.155.3761.424. [DOI] [PubMed] [Google Scholar]
- 3.Goodale CL, Aber JD, Vitousek PM. An unexpected nitrate decline in New Hampshire streams. Ecosystems. 2003;6:75–86. [Google Scholar]
- 4.Bernhardt ES, et al. Can't see the forest for the stream? In-stream processing and terrestrial nitrogen exports. Bioscience. 2005;55:219–230. [Google Scholar]
- 5.Judd KE, Likens GE, Buso DC, Bailey AC. Minimal response in watershed nitrate export to severe soil frost raises questions about nutrient dynamics in the Hubbard Brook experimental forest. Biogeochemistry. 2011;106:443–459. [Google Scholar]
- 6.Huntington TG. Can nitrogen sequestration explain the unexpected nitrate decline in New Hampshire streams? Ecosystems. 2005;8:331–333. [Google Scholar]
- 7.Aber J, et al. Nitrogen saturation in temperate forest ecosystems: Hypotheses revisited. Bioscience. 1998;48:921–934. [Google Scholar]
- 8.Dise NB, Wright RF. Nitrogen leaching from European forests in relation to nitrogen deposition. Forest Ecol Manag. 1995;71:153–161. [Google Scholar]
- 9.Euskirchen ES, McGuire AD, Chapin FS, 3rd, Yi S, Thompson CC. Changes in vegetation in northern Alaska under scenarios of climate change, 2003–2100: Implications for climate feedbacks. Ecol Appl. 2009;19:1022–1043. doi: 10.1890/08-0806.1. [DOI] [PubMed] [Google Scholar]
- 10.Chapin FS, 3rd, et al. Role of land-surface changes in arctic summer warming. Science. 2005;310:657–660. doi: 10.1126/science.1117368. [DOI] [PubMed] [Google Scholar]
- 11.Pettorelli N, et al. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol Evol. 2005;20:503–510. doi: 10.1016/j.tree.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JL, et al. Long-Term Trends from Ecosystem Research at the Hubbard Brook Experimental Forest. General Technical Report NRS-17. US Department of Agriculture, Forest Service, Northeastern Research Station, Newtown Square, PA; 2007. [Google Scholar]
- 13.Bormann FH, Likens GE. Pattern and Process in a Forested Ecosystem. Berlin: Springer-Verlag; 1979. [Google Scholar]
- 14.Siccama TG, et al. Population and biomass dynamics of trees in a northern hardwood forest at Hubbard Brook. Can J Forest Res. 2007;37:737–749. [Google Scholar]
- 15.Lindenmayer DB, Likens GE. Effective Ecological Monitoring. CSIRO Publishing, Collingwood, VIC and Earthscan, London; 2010. p. 123. [Google Scholar]
- 16.Vitousek PM, Reiners WA. Ecosystem succession and nutrient retention: A Hypothesis. Bioscience. 1975;25:376–381. [Google Scholar]
- 17.Hedin LO, Armesto JJ, Johnson AH. Patterns of nutrient loss from unpolluted, old-growth temperate forests: Evaluation of biogeochemical theory. Ecology. 1995;76:493–509. [Google Scholar]
- 18.Menge DNL, Pacala SW, Hedin LO. Emergence and maintenance of nutrient limitation over multiple timescales in terrestrial ecosystems. Am Nat. 2009;173:164–175. doi: 10.1086/595749. [DOI] [PubMed] [Google Scholar]
- 19.Hall SJ, Matson PA. Nitrogen oxide emissions after nitrogen additions in tropical forests. Nature. 1999;400:152–155. [Google Scholar]
- 20.Hedin LO, Vitousek PM, Matson PA. Nutrient losses over four million years of tropical forest development. Ecology. 2003;84:2231–2255. [Google Scholar]
- 21.Houlton BZ, Sigman DM, Hedin LO. Isotopic evidence for large gaseous nitrogen losses from tropical rainforests. Proc Natl Acad Sci USA. 2006;103:8745–8750. doi: 10.1073/pnas.0510185103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huntington TG, Ryan DF, Hamburg SP. Estimating soil nitrogen and carbon pools in a northern hardwood forest ecosystem. Soil Sci Soc Am J. 1988;52:1162–1167. [Google Scholar]
- 23.Sebestyen SD, et al. Sources, transformation, and hydrological processes that control stream nitrate and dissolved organic matter concentrations during snowmelt in an upland forest. Water Resour Res. 2008;44:W12410. 10.1029/2008WR006983. [Google Scholar]
- 24.Aber JD, et al. Inorganic nitrogen losses from a forested ecosystem in response to physical, chemical, biotic and climatic perturbations. Ecosystems. 2002;5:648–658. [Google Scholar]
- 25.Peart DR, Cogbill CV, Palmiotto PA. Effects of logging history and hurricane damage on canopy structure in a northern hardwoods forest. Bull Torrey Bot Club. 1992;119:29–38. [Google Scholar]
- 26.Houlton BZ, et al. Nitrogen dynamics in ice storm-damaged forest ecosystems: Implications for nitrogen limitation theory. Ecosystems. 2003;6:431–443. [Google Scholar]
- 27.Lovett GM, Mitchell MJ. Sugar maple and nitrogen cycling in the forests of eastern North America. Front Ecol Environ. 2004;2:81–88. [Google Scholar]
- 28.Gerber S, Hedin LO, Oppenheimer M, Pacala SW, Shevliakova E. Nitrogen cycling and feedbacks in a global dynamic land model. Global Biogeochem Cycles. 2010;24:GB1001. 10.1029/2008GB003336. [Google Scholar]
- 29.Kendall C. Tracing nitrogen sources and cycles in catchments. In: Kendall C, McDonnell JJ, editors. Isotope Tracers in Catchment Hydrology. Amsterdam: Elsevier Science; 1998. pp. 519–576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.