Abstract
Inhibition of the ternary protein complex of the synaptic scaffolding protein postsynaptic density protein-95 (PSD-95), neuronal nitric oxide synthase (nNOS), and the N-methyl-d-aspartate (NMDA) receptor is a potential strategy for treating ischemic brain damage, but high-affinity inhibitors are lacking. Here we report the design and synthesis of a novel dimeric inhibitor, Tat-NPEG4(IETDV)2 (Tat-N-dimer), which binds the tandem PDZ1-2 domain of PSD-95 with an unprecedented high affinity of 4.6 nM, and displays extensive protease-resistance as evaluated in vitro by stability-measurements in human blood plasma. X-ray crystallography, NMR, and small-angle X-ray scattering (SAXS) deduced a true bivalent interaction between dimeric inhibitor and PDZ1-2, and also provided a dynamic model of the conformational changes of PDZ1-2 induced by the dimeric inhibitor. A single intravenous injection of Tat-N-dimer (3 nmol/g) to mice subjected to focal cerebral ischemia reduces infarct volume with 40% and restores motor functions. Thus, Tat-N-dimer is a highly efficacious neuroprotective agent with therapeutic potential in stroke.
Keywords: drug discovery, ischemic stroke, protein-protein interactions
Protein-protein interactions mediated by postsynaptic density protein-95 (PSD-95)/Discs-large/ZO-1 (PDZ) domains are important for intracellular signaling events, and several PDZ domains are potential drug targets for neuronal diseases and cancer (1, 2). The postsynaptic scaffolding protein PSD-95 simultaneously binds the N-methyl-d-aspartate (NMDA)-type of ionotropic glutamate receptors and the enzyme neuronal nitric oxide synthase (nNOS) through its PDZ1 and PDZ2 domains (3). Activation of the NMDA receptor causes influx of Ca2+, which activates nNOS thereby leading to nitric oxide generation (4), a key facilitator of glutamate-mediated excitotoxicity (5, 6). Ligands that bind to the first two PDZ domains of PSD-95 inhibit the formation of the ternary nNOS/PSD-95/NMDA receptor complex and uncouple the harmful production of nitric oxide from NMDA receptor activity (Fig. 1A). As PSD-95 inhibition does not affect ion-flux (7) or prosurvival signaling pathways (8) mediated by the NMDA receptor, it is believed that compounds targeting PDZ1 and PDZ2 of PSD-95 can provide an efficient and safe treatment of ischemic brain damage (9), where excitotoxicity is known to dominate in the acute poststroke period, as well as other NMDA receptor-related disorders such as chronic pain and Alzheimer’s disease (10–14).
Fig. 1.
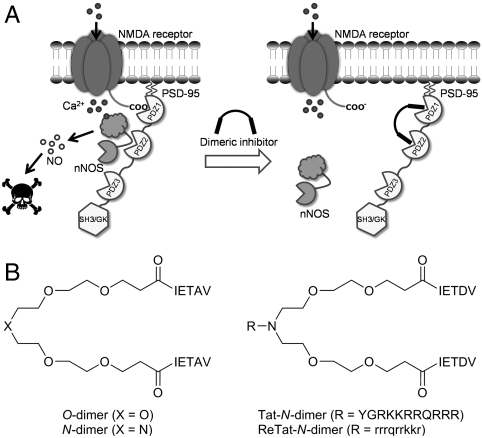
Dimeric PSD-95 inhibitors. (A) PSD-95 inhibitors targeting PDZ1-2 of PSD-95 block the formation of the ternary nNOS/PSD-95/NMDA receptor complex and uncouple the link between NMDA receptor activity and nitric oxide production, whereby neuroprotection against excitotoxicity is achieved. (B) Structures of O-dimer and N-dimer; and Tat-NPEG4(IETDV)2 and Retroinverso-d-Tat-NPEG4(IETDV)2, designated Tat-N-dimer and ReTat-N-dimer, respectively. Capital letters indicate l-amino acids, except for “N” (nitrogen), “O” (oxygen), “X” (N or O), and “R” (Tat or ReTat sequence), and lower case letters indicate d-amino acids.
The shallow and elongated binding pocket of PDZ domains generally favor binding of peptides or peptide analogues and so far no drug-like small-molecule inhibitors of PDZ domains with affinities below 5 μM have been identified (15). Accordingly, the most advanced PSD-95 inhibitor is a 20-mer peptide, Tat-NR2B9c (7, 8, 16), composed of nine amino acids corresponding to the C-terminal of the GluN2B subunit of the NMDA receptor, fused to the HIV-1 Tat peptide (17). This peptide has shown promising effects against ischemic brain damage in rats (7, 8, 16), and is currently investigated in clinical trials (18). However, Tat-NR2B9c suffers from low affinity to PDZ1-2 of PSD-95 (19), which might limit its biological effectiveness and clinical potential. Here we present dimeric inhibitors that evade these shortcomings by exploiting that PSD-95 possesses two neighboring PDZ domains (Fig. 1A) and show up to 1,000 fold improved affinity compared to Tat-NR2B9c as well as improved blood plasma stability. Our dimeric inhibitors permeate the blood-brain barrier and demonstrate substantial neuroprotective properties in mice subjected to focal ischemic brain damage. Biophysical studies demonstrate that the dimeric design results in a bivalent binding mode, where the two ligand moieties bind PDZ1 and PDZ2 simultaneously, hence explaining the high affinity.
Results
Design and Activity of Novel Dimeric Inhibitors.
We have previously demonstrated that the five C-terminal amino acids of GluN2B are sufficient for maintaining wild-type binding affinity towards the individual PDZ1 (Ki = 14 μM) and PDZ2 (Ki = 3 μM) domains (19). Thus, by linking two pentapeptides (IETAV) with a monodisperse polyethylene glycol (PEG) linker of optimal length (PEG4), a high-affinity dimeric ligand, PEG4(IETAV)2 (O-dimer) (Fig. 1B), was obtained with Kd of 10 nM towards tandem PDZ1-2 of PSD-95 (20, 21). We here redesigned the PEG linker, and synthesized a unique PEG-based linker, termed NPEG (Scheme S1A), where the central oxygen of the PEG4 linker is replaced by nitrogen, which upon linkage with two IETAV moieties provides NPEG4(IETAV)2 (N-dimer) (Fig. 1B). In order to improve permeability across the blood-brain barrier, we attached the cell-penetrating peptide sequences, Tat and its inverse d-amino acid-containing version Retroinverso-d-Tat (22), to the linker nitrogen, and used IETDV as ligand moiety, which is more selective than IETAV towards PDZ1 and PDZ2 over PDZ3 (20). This resulted in dimeric ligands Tat-NPEG4(IETDV)2 (Tat-N-dimer) and Retroinverso-d-Tat-NPEG4(IETDV)2 (ReTat-N-dimer) (Fig. 1B, Scheme S1B, and Fig. S1).
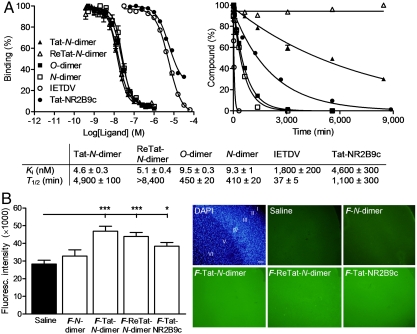
For in vitro affinity evaluation we developed a fluorescence polarization assay, where we prepared fluorescently (5-FAM, F) labeled N-dimer (F-N-dimer, Fig. S1) and used it as a high-affinity probe (Kd; mean ± SEM: 7.8 ± 0.1 nM). This allowed us to determine accurate Ki values for high-affinity ligands in a simple and fast format, as compared to previous studies based on isothermal titration calorimetry (20, 23). Tat-N-dimer and ReTat-N-dimer displayed affinities (Ki; mean ± SEM) of 4.6 ± 0.3 and 5.1 ± 0.4 nM, respectively, against PDZ1-2 of PSD-95, whereas N-dimer bound PDZ1-2 with an affinity (Ki; mean ± SEM) of 9.3 ± 1 nM (Fig. 2A). Tat-N-dimer is thereby by far the most potent PDZ domain inhibitor described (15, 24–26), and provides a 1,000-fold increase in affinity relative to monomeric Tat-NR2B9c (Ki; mean ± SEM: 4,600 ± 300 nM, Fig. 2A).
Fig. 2.
Affinity, stability, and blood-brain barrier permeability. (A) Affinity towards PDZ1-2 of PSD-95 for Tat-N-dimer, ReTat-N-dimer and Tat-NR2B9c as measured by fluorescence polarization (Left); and stability in human blood plasma in vitro at 37 °C (Right). Data are shown as mean ± SEM, N≥3. Mean affinity inhibition constants (Ki) and half-lives (T1/2) are shown in the table below. (B) Blood-brain barrier permeability of fluorescent analogues in nonmanipulated mice. The bar graph shows the mean fluorescence intensity of coronal brain sections of mice one hour after intravenous injection of F-N-dimer (p = 0.22), F-Tat-N-dimer (p = 0.0002), F-ReTat-N-dimer (p = 0.0008), F-Tat-NR2B9c (p = 0.011) compared to saline. Representative pictures of the cortical area of the coronal brain sections are shown. A nuclei-DAPI staining (blue) is included for anatomical reference showing layers I-VI of the frontal cortex. F: 5-FAM; Data are presented as mean ± SEM; ∗ / ∗∗ / ∗∗ ∗: p < 0.05/0.01/0.001 (nonparametric Mann-Whitney). Scale bar: 100 μm.
A general concern of peptide-based compounds is their susceptibility to enzymatic cleavage in biological fluids and tissues. Monomeric pentapeptide IETDV and Tat-NR2B9c showed half-lives (T1/2; mean ± SEM) of 37 ± 6 and 1,100 ± 300 min in human blood plasma in vitro, respectively, while Tat-N-dimer had a T1/2 (mean ± SEM) of 4,900 ± 100 min (Fig. 2A), thus having > 100-fold improved stability compared to IETDV. Hence, the combination of dimerization and attachment of cell-penetrating peptides has a strikingly positive effect on protease stability. Furthermore, no detectable degradation was observed for ReTat-N-dimer within the period of measurements, demonstrating the effect of incorporating d-amino acids into the cell-penetrating peptide sequence (Fig. 2A).
To test the effect of introducing cell-penetrating peptides on the permeability of the blood-brain barrier, the ligands were labeled with 5-FAM (F) and injected intravenously in mice (3 nmol/g) 1 h prior to termination of the animals. Fluorescence microscopy of coronal brain sections revealed that F-Tat-N-dimer, F-ReTat-N-dimer, and F-Tat-NR2B9c resulted in a strong uniform fluorescence throughout the brain including layer I-VI of the frontal cortex, as compared to sections from F-N-dimer and saline-treated mice (Fig. 2B). Thus blood-brain barrier permeability of the dimeric compounds is obtained by introduction of Tat or ReTat (7).
Dimeric Inhibitor Bivalently Interacts with PDZ1-2, Leading to a Flexible and Extended Conformation.
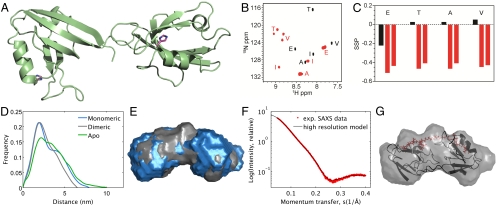
Previous kinetic and mutational studies have suggested that a dimeric ligand binds to PDZ1-2 in a two-step process, where initial binding to either of the two PDZ domains is followed by an intramolecular step leading to the bidentate complex (21). In order to provide insight into the structural and dynamic properties of the complex between PDZ1-2 and dimeric ligand, and to ascertain that both PDZ domains are occupied simultaneously by peptide moieties from the same dimeric ligand, we elucidated the molecular mode of action of our dimeric ligands. First, the domain orientation of the two PDZ domains in apo PDZ1-2 was determined by X-ray crystallography, which, however, showed that the two binding sites face approximately 180° away from each other in an extended conformation that cannot accommodate the dimeric ligands (Fig. 3A and Table S1). Therefore, we prepared a labeled dimeric ligand, [15N, 13C]-O-dimer, and subjected it to structural NMR studies. In a heteronuclear single quantum correlation (HSQC) spectrum of the dimeric, symmetrical ligand alone, five peaks were observed, whereas ten different peaks appeared when the ligand was incubated with PDZ1-2 (Fig. 3B). From secondary structural calculations based on the chemical shifts for the bound and unbound labeled dimeric ligand, we observed that the unbound ligand exhibits random coil character, while the bound ligand adopts a β-stranded structure (Fig. 3C). Thus, the two peptide moieties of the dimeric ligand face different protein environments (PDZ1 and PDZ2) and β-strand character of the peptide moieties is imposed by binding to PDZ1-2 similar to a canonical PDZ-peptide binding mode (27).
Fig. 3.
Biophysical characterization of PDZ1-2 from PSD-95 and interaction with inhibitors. (A) X-ray crystal structure of apo PDZ1-2 (PDB 3ZRT). Two conserved histidine residues (His130 and His225) important for ligand binding and with a relative distance of 42 Å are highlighted. (B) 1H-15N correlation spectra of free [15N, 13C]-O-dimer (black contours) and [15N, 13C]-O-dimer in complex with PDZ1-2 from PSD-95 (red contours). Amino acid assignment is indicated with capital letters. (C) Secondary structure propensities (SSP) of unbound [15N, 13C]-O-dimer (black bars) and [15N, 13C]-O-dimer bound to PDZ1-2 (red bars) as calculated using the program SSP. A value of one indicates a fully formed α-helix and a value of minus one indicates a fully extended structure whereas a value around zero is indicative of a random coil. (D) Pair distance distribution functions derived from Fourier transformation of the SAXS data presented as the relative occurrence of distances (arbitrary scale) against distances in nm. Data from apo PDZ1-2 (green), PDZ1-2 with monomeric ligand (IETAV) (blue), and PDZ1-2 with dimeric ligand (O-dimer) (gray) are shown. The average radius of the two PDZ domains is visible as the first major peak (around 2 nm, PDZ1 and PDZ2 have similar radii), and the distance between the center of mass of PDZ1 and PDZ2 is seen as the second peak. (E) Surface representations of PDZ1-2 bound to IETAV (blue) or O-dimer (gray), showing that PDZ1-2 adopts a more compact conformation when bound to O-dimer compared to IETAV. (F) Comparison of the high-resolution O-dimer/PDZ1-2 model (black) and the experimental SAXS scattering data (red). X = 1.15. (G) High-resolution model of PDZ1-2 in complex with O-dimer.
The dynamic properties of PDZ1-2 upon ligand binding were revealed by calculating correlation times for molecular tumbling of PDZ1 and PDZ2 in PDZ1-2 in complex with O-dimer based on R1 and R2 relaxation rate measurements. This gave correlation times slightly larger and with less deviation (14.6 and 13.6 ns for PDZ1 and PDZ2, respectively, Table S2) than those reported for PDZ1-2 bound to monomeric ligand (14.4 and 12.5 ns) (28), but very different from values for unbound PDZ1-2 (18.3 and 18.2 ns, Table S2). Hence, these values suggest that the interdomain motion of PDZ1-2 is only slightly more restricted in complex with dimeric ligand than with a monomeric ligand, while unbound PDZ1-2 is essentially rigid.
To further examine the structural changes in PDZ1-2 upon ligand binding, we performed small-angle X-ray scattering (SAXS) studies of PDZ1-2. In apo PDZ1-2, a distinct distance of approximately 3.3 nm is observed (Fig. 3D), which likely represents the average distance between PDZ1 and PDZ2 when no ligand is bound to the protein. Interestingly, this distance increases upon binding of O-dimer (3.6 nm) and even more upon binding of monomeric ligand IETAV (3.9 nm) (Fig. 3D and Table S3). This demonstrates that the closed and compact apo PDZ1-2 becomes more extended when the dimeric ligand binds, and even further extended by the monomeric ligand, as visualized by low-resolution ab initio models of the O-dimer and IETAV PDZ1-2 complexes (Fig. 3E). The two PDZ domains can be located within the O-dimer/PDZ1-2 ab initio model, which differs from other reported PDZ1-2 conformations (Fig. S2), by energy minimization so that O-dimer bivalently binds to PDZ1-2, and the peptide moieties of O-dimer interact with each PDZ domain in a canonical manner (Fig. 3 F–G). In conclusion, the SAXS and NMR studies unambiguously demonstrate that the dimeric ligand spans the distance between individual domains, interacts with the PDZ domains in a canonical binding mode, and induces a conformation of PDZ1-2 that is more extended and flexible than unbound PDZ1-2, but more compact and restricted compared to PDZ1-2 in complex with monomeric ligand.
Tat-N-dimer Protects Against Ischemic Brain Damage in Mice.
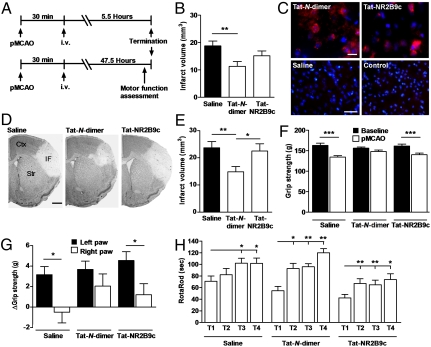
Encouraged by the remarkably high affinity of Tat-N-dimer towards PSD-95 and its stability in blood plasma, we examined Tat-N-dimer as a potential in vivo active neuroprotectant. We tested Tat-N-dimer as well as Tat-NR2B9c and O-dimer in the permanent middle cerebral artery occlusion (pMCAO) model of focal ischemia in adult mice (29). Compounds were injected (3 nmol/g) once, intravenously, 30 min after the pMCAO insult, followed by a 6-h postsurgical survival period (Fig. 4A). Animals receiving Tat-N-dimer showed a 40% reduction in ischemic tissue damage compared to saline-treated animals (p = 0.0081) (Fig. 4B), whereas treatment with O-dimer gave no neuroprotective effect (p = 0.62, N = 19), indicating the necessity for introducing cell-penetrating peptides into the dimeric ligands to obtain effects in vivo. Treatment with Tat-NR2B9c did not provide a statistically significant reduction in infarct volumes (p = 0.22) (Fig. 4B), although we verified by immunofluorescence using an anti-Tat antibody that both Tat-N-dimer and Tat-NR2B9c were located inside neuron-like cells (30) 6 h after pMCAO (Fig. 4C).
Fig. 4.
Neuroprotection of Tat-N-dimer, 6 and 48 h after pMCAO in mice. (A) Compounds were administered 30 min postsurgery, followed by a survival period of 5.5 or 47.5 h. (B) Mean infarct volumes 6 h after pMCAO of mice treated with Tat-N-dimer (N = 17), Saline (N = 16), or Tat-NR2B9c (N = 18). (C) Immunofluorescent staining of cells containing Tat-based compounds, Tat-N-dimer and Tat-NR2B9c, 6 h after pMCAO, colabelled with nuclei-DAPI staining (blue). Sections from both saline-injected mice, and Tat-N-dimer-treated mice where the antibody was substituted with isotype control, were devoid of signal. Scale bar: 20 μm (Upper), 40 μm (Lower). (D) Toluidine blue staining showing the ischemic brain damage 48 h after pMCAO. IF: Infarct; Ctx: Cortex; Str: Striatum. Scale bar: 1 mm. (E) Mean infarct volumes 48 h after pMCAO of mice treated with Tat-N-dimer (N = 17), Saline (N = 16), or Tat-NR2B9c (N = 17). (F-H) Motor function assessment of mice with 48 h postsurgical survival. (F) Mean total grip strength of both front paws before (baseline) and after pMCAO. P-values: < 0.0001 (Saline), 0.13 (Tat-N-dimer), 0.0011 (Tat-NR2B9c). (G) Ischemia-induced grip strength asymmetry in the front paws. P-values: 0.014 (Saline), 0.11 (Tat-N-dimer), 0.013 (Tat-NR2B9c). (H) Rotarod performance test over four trials (T1-T4). A learning component is observed along the trials in all groups, but treatment with Tat-N-dimer gave a more pronounced improvement (cf. T2) compared to saline, and increased endurance compared to Tat-NR2B9c (see text). (B, E–H) Data are shown as mean ± SEM; ∗ / ∗∗ / ∗∗ ∗: p < 0.05/0.01/0.001; (B and E) Nonparametric Mann-Whitney test; (F–G) Paired Student’s t test; (H) Wilcoxon matched pairs test.
To evaluate prolonged effects of Tat-N-dimer, we performed the pMCAO experiment with a 48 h postsurgical survival period (Fig. 4A) and observed a 37% reduction in infarct size compared to saline-treated mice (p = 0.0042) (Fig. 4 D–E). Again, Tat-NR2B9c gave no statistically significant infarct reductions (p = 0.89) (Fig. 4D-E), but treating animals with ReTat-N-dimer under similar conditions led to a 34% reduction in infarct volumes (p = 0.0094) (Fig. S3). Body temperature, weight, and blood gas parameters were monitored during the pMCAO experiment and no differences were observed among the treated groups (Fig. S4 and Table S4).
Focal cerebral ischemia induced by pMCAO in mice affects cortical brain areas controlling the contralateral front- and hind-limb including the paws (31). Thus, we included behavioral tests that challenged the motor function of mice subjected to pMCAO. In accordance with reduced infarct volumes, the total grip strength of both paws was unaffected for animals treated with Tat-N-dimer, but reduced for those given saline or Tat-NR2B9c (Fig. 4F). Similarly, there was no difference in grip strength between the right and left front paw for Tat-N-dimer-treated animals in contrast to treatment with saline or Tat-NR2B9c (Fig. 4G). Finally, in the rotarod performance test we observed that Tat-N-dimer treatment resulted in a more pronounced short-term learning skill improvement compared to mice treated with saline (Fig. 4H), and the total time spent on the rod was significantly longer (83.5 ± 4.1 s; average of trial 1–4 ± SEM) than for mice treated with Tat-NR2B9c (65.7 ± 3.7 s; average of trial 1-4 ± SEM) (P < 0.001). The effects on motor control of ReTat-N-dimer were similar to those observed for Tat-N-dimer (Fig. S3).
Discussion
PSD-95 is a potential target for protection against ischemic brain damage (9), and recent studies demonstrate promising effects of PSD-95 inhibitors in animal models of pain (10–12) and Alzheimer’s disease (13). Despite this intriguing role of PSD-95, there is a lack of inhibitors against PSD-95, and PDZ domains in general, with reasonably affinity and in vivo activity, which impede the possibilities for exploiting PDZ domains as drug targets. Here we have advanced the concept of dimeric inhibitors against the tandem PDZ1-2 domain of PSD-95 in order to develop potent in vivo active ligands. Previously, two decapeptides targeting PDZ1 and PDZ2 have been linked via disulphide bonds from N-terminal cysteine residues, which resulted in a 5–20-fold increase in affinity relative to the monomeric ligand (24), and a related design with peptide sequences of 15 amino acids resulted in a similar 8–25-fold increase in affinity (25). In the present work, the dimeric ligands provide a remarkable 400-fold increase in affinity compared to the monomeric peptide. This has been achieved by optimizing peptide length and sequence as well as the linker length, and in particular, by the essential modification of changing the PEG linker into NPEG, which allows cell-penetrating peptides to be attached to the dimeric structure without compromising binding affinities. Tat-N-dimer displays an unprecedented high affinity for any PDZ domain-mediated interaction with a Ki value of 4.6 nM, which is a 1,000-fold improvement compared to Tat-NR2B9c. In addition, our design provides a solution to the inherent problem of peptides being degraded in biological fluids, as Tat-N-dimer, and in particular ReTat-N-dimer, demonstrates greatly enhanced stability in blood plasma. Moreover, both compounds cross the blood-brain barrier in mice and demonstrate significant in vivo neuroprotective properties, hence Tat-N-dimer reduces ischemic stroke damage in mice with 40% and significantly improves motor functions.
We observe that the high-affinity compounds, Tat-N-dimer and ReTat-N-dimer, are more efficient in vivo neuroprotectants in the mouse pMCAO model compared to the low-affinity monomeric inhibitor Tat-NR2B9c when these compounds are tested in parallel and under the same conditions and dosages. Tat-NR2B9c has previously shown robust neuroprotective effects in both transient and permanent focal ischemic stroke models in rats (7, 8, 16), but these studies cannot be directly compared to the current mouse study due to experimental differences (32). Therefore, whether our results represent generally improved neuroprotective properties across species and types of ischemic stroke models of our compounds relative to Tat-NR2B9c needs confirmation by future studies. However, the permanent MCAO model induces a smaller ischemic penumbra than the transient MCAO model in the acute phase after stroke (< 4–6 h after arterial occlusion) where neuroprotection is believed to be achievable (33, 34). As a result of this, large percentages of rescued tissues are harder to obtain in the permanent model. Hence, a 40% infarct reduction in a permanent model as a result of a single poststroke administration of Tat-N-dimer is highly promising, and its relevance is underlined by the concomitant improvement in motor functions and persistency after 48 h (32).
To elucidate the mode of action at the molecular level of the dimeric ligands we applied a combination of X-ray crystallography, NMR, and SAXS. Previous NMR studies suggest that apo PDZ1-2 of PSD-95 adopts a closed and rigid conformation (24), in agreement with the C-shaped arrangement of full-length PSD-95 observed by electron microscopy (35), and that the interdomain mobility of PDZ1 and PDZ2 is increased upon monomeric peptide binding, leading to a flexible and more extended peptide-bound conformation (28). Based on these observations, it was suggested that this increased conformational freedom of PDZ1-2 upon monomeric ligand binding provides extra conformational entropy, which facilitates ligand binding (28). This intriguing model initially seemed contradictory to the fact that our dimeric ligands display such a large affinity-increase compared to monomeric compounds, as one would expect dimeric ligands to rigidify PDZ1-2 and hence lead to a large entropy penalty. However, our NMR and SAXS studies provide unambiguous evidence for apo PDZ1-2 to be compact and rigid compared to when PDZ1-2 is bound to monomeric compound where it is more extended and flexible. Moreover, these studies demonstrate that dimeric ligand binding, although causing a more compact PDZ1-2 structure relative to monomeric ligand binding, still facilitates interdomain flexibility of PDZ1-2 to about the same extent as monomeric ligand, thus potentially allowing the conformational entropy of PDZ1-2 to be increased. This result could also explain the pronounced difference in affinity of the different types of dimeric inhibitors of PSD-95. We have used very flexible NPEG or PEG-based linkers to dimerize the peptide ligands, whereas other dimeric ligands are less flexible (24, 25) and might therefore be paying a higher entropic penalty, leading to decreased affinity, due to rigidifying PDZ1-2.
PDZ domains generally work as structural and functional modules in neuronal scaffolding and adaptor proteins, and frequently appear as tandem supramodular domains, similar to PDZ1-2 of PSD-95 (36). The dimeric design presented here is in principle applicable to any protein containing a tandem PDZ domain. Thus, by linking appropriate peptide ligands using the NPEG linker and attachment of cell-penetrating peptides, the methodology demonstrated here is a versatile and straightforward way of generating in vivo active tool compounds and potential therapeutics for proteins containing tandem PDZ domains and even for other bimodular targets.
In summary, we have designed and synthesized dimeric ligands that are highly efficient inhibitors of the tandem PDZ1-2 domain of the scaffolding protein PSD-95. Tat-N-dimer binds PDZ1-2 with unprecedented high affinity, displays extensive stability in blood plasma, crosses the blood-brain barrier, reduces ischemic stroke damage in mice with 40%, and improves postischemic motor functions. Using biophysical methods, we have provided unequivocal evidence for a bivalent binding mechanism and characterized the conformational changes upon ligand binding. Tat-N-dimer is at present the most efficient PSD-95 inhibitor described, and could become a valuable pharmacological tool for studying PSD-95-related biology and for paving the way for dimeric PSD-95 inhibitors as drug candidates.
Experimental Procedures
Complete descriptions of ligand synthesis and characterization, protein purification, affinity and stability in vitro assays, X-ray crystallography, NMR, SAXS, modeling, and in vivo experiments are found in SI Experimental Procedures.
Supplementary Material
Acknowledgments.
We thank G. F. Pang for initial help in generating PDZ1-2 crystals, and Professor M. Zhang and Dr. W. Wang (The Hong Kong University of Science and Technology) for the chemical shifts of their NMR based PDZ1-2 model. MAX-lab, Lund, Sweden and ESRF, Grenoble, France are thanked for providing beam time. This work was supported by the Danish Council for Independent Research (Technology and Production Sciences, A.B.; Medical Sciences, K.S., B.V.), the Lundbeck Foundation (K.S., K.L.L.), GluTarget (K.S., A.S.K.), the Swedish Research Council (Grant 2009-5659, P.J.; Grant 2008-4285, P.L.), the Novo Nordisk Foundation (K.L.L.), the Carlsberg Foundation (K.L.L.), the Danish Council for Strategic Research, Programme Commission on Nanoscience, Nanotechnology, Biotechnology and Information and Communication Technology (NABIIT, reference 09-060780, M.M), and Danish Centre for the use of Synchrotron X-ray and Neutron facilities (DANSCATT) (P.L.S., J.S.K., M.G.).
Footnotes
The authors declare no conflict of interest.
Data deposition: Coordinates and structure factors for the PDZ1-2 crystal structure have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3ZRT).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113761109/-/DCSupplemental.
References
- 1.Blazer LL, Neubig RR. Small molecule protein-protein interaction inhibitors as CNS therapeutic agents: Current progress and future hurdles. Neuropsychopharmacology. 2009;34:126–141. doi: 10.1038/npp.2008.151. [DOI] [PubMed] [Google Scholar]
- 2.Dev KK. Making protein interactions druggable:Targeting PDZ domains. Nat Rev Drug Discov. 2004;3:1047–1056. doi: 10.1038/nrd1578. [DOI] [PubMed] [Google Scholar]
- 3.Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-d-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 4.Sattler R, et al. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 5.Dawson VL, et al. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z, et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 7.Aarts M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 8.Soriano FX, et al. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. J Neurosci. 2008;28:10696–10710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tymianski M. Emerging mechanisms of disrupted cellular signaling in brain ischemia. Nat Neurosci. 2011;14:1369–1373. doi: 10.1038/nn.2951. [DOI] [PubMed] [Google Scholar]
- 10.Tao F, Su Q, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther. 2008;16:1776–1782. doi: 10.1038/mt.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeBlanc BW, et al. A cyclic peptide targeted against PSD-95 blocks central sensitization and attenuates thermal hyperalgesia. Neuroscience. 2010;167:490–500. doi: 10.1016/j.neuroscience.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Mello R, et al. Perturbing PSD-95 interactions with NR2B-subtype receptors attenuates spinal nociceptive plasticity and neuropathic pain. Mol Ther. 2011;19:1780–1792. doi: 10.1038/mt.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ittner LM, et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 14.Gardoni F. MAGUK proteins: New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol. 2008;585:147–152. doi: 10.1016/j.ejphar.2008.01.048. [DOI] [PubMed] [Google Scholar]
- 15.Bach A, et al. Cell-permeable and plasma-stable peptidomimetic inhibitors of the postsynaptic density-95/N-methyl-d-aspartate receptor interaction. J Med Chem. 2011;54:1333–1346. doi: 10.1021/jm1013924. [DOI] [PubMed] [Google Scholar]
- 16.Sun HS, et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39:2544–2553. doi: 10.1161/STROKEAHA.107.506048. [DOI] [PubMed] [Google Scholar]
- 17.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 18. Tat-NR2B9c is found under the name NA-1 at www.clinicaltrials.gov.
- 19.Bach A, et al. Modified peptides as potent inhibitors of the postsynaptic density-95/N-methyl-d-aspartate receptor interaction. J Med Chem. 2008;51:6450–6459. doi: 10.1021/jm800836w. [DOI] [PubMed] [Google Scholar]
- 20.Bach A, et al. Design and synthesis of highly potent and plasma-stable dimeric inhibitors of the PSD-95–NMDA receptor interaction. Angew Chem Int Ed. 2009;48:9685–9689. doi: 10.1002/anie.200904741. [DOI] [PubMed] [Google Scholar]
- 21.Chi CN, et al. Deciphering the kinetic binding mechanism of dimeric ligands using a potent plasma-stable dimeric inhibitor of postsynaptic density protein-95 as an example. J Biol Chem. 2010;285:28252–28260. doi: 10.1074/jbc.M110.124040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wender PA, et al. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc Natl Acad Sci USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X. Fluorescence polarization competition assay: The range of resolvable inhibitor potency is limited by the affinity of the fluorescent ligand. J Biomol Screen. 2003;8:34–38. doi: 10.1177/1087057102239666. [DOI] [PubMed] [Google Scholar]
- 24.Long JF, et al. Supramodular structure and synergistic target binding of the N-terminal tandem PDZ domains of PSD-95. J Mol Biol. 2003;327:203–214. doi: 10.1016/s0022-2836(03)00113-x. [DOI] [PubMed] [Google Scholar]
- 25.Sainlos M, et al. Biomimetic divalent ligands for the acute disruption of synaptic AMPAR stabilization. Nat Chem Biol. 2011;7:81–91. doi: 10.1038/nchembio.498. [DOI] [PubMed] [Google Scholar]
- 26.Paduch M, et al. Bivalent peptides as models for multimeric targets of PDZ domains. Chembiochem. 2007;8:443–452. doi: 10.1002/cbic.200600389. [DOI] [PubMed] [Google Scholar]
- 27.Doyle DA, et al. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, et al. Creating conformational entropy by increasing interdomain mobility in ligand binding regulation: A revisit to N-terminal tandem PDZ domains of PSD-95. J Am Chem Soc. 2009;131:787–796. doi: 10.1021/ja8076022. [DOI] [PubMed] [Google Scholar]
- 29.Lambertsen KL, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29:1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyck L, Krøigård T, Finsen B. Unbiased cell quantification reveals a continued increase in the number of neocortical neurones during early post-natal development in mice. Eur J Neurosci. 2007;26:1749–1764. doi: 10.1111/j.1460-9568.2007.05763.x. [DOI] [PubMed] [Google Scholar]
- 31.Pronichev I, Lenkov D. Functional mapping of the motor cortex of the white mouse by a microstimulation method. Neurosci Behav Physiol. 1998;28:80–85. doi: 10.1007/BF02461916. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hata R, et al. Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:306–315. doi: 10.1097/00004647-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Hata R, et al. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000;20:937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa T, et al. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by l27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Feng W, Zhang M. Organization and dynamics of PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci. 2009;10:87–99. doi: 10.1038/nrn2540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.