Abstract
Background: Although ambient fine particulate matter (PM2.5; particulate matter ≤ 2.5 µm in aerodynamic diameter) has been linked to adverse human health effects, the chemical constituents that cause harm are unknown. To our knowledge, the health effects of PM2.5 constituents have not been reported for a developing country.
Objectives: We examined the short-term association between PM2.5 constituents and daily mortality in Xi’an, a heavily polluted Chinese city.
Methods: We obtained daily mortality data and daily concentrations of PM2.5, organic carbon (OC), elemental carbon (EC), and 10 water-soluble ions for 1 January 2004 through 31 December 2008. We also measured concentrations of fifteen elements 1 January 2006 through 31 December 2008. We analyzed the data using over-dispersed generalized linear Poisson models.
Results: During the study period, the mean daily average concentration of PM2.5 in Xi’an was 182.2 µg/m3. Major contributors to PM2.5 mass included OC, EC, sulfate, nitrate, and ammonium. After adjustment for PM2.5 mass, we found significant positive associations of total, cardiovascular, or respiratory mortality with OC, EC, ammonium, nitrate, chlorine ion, chlorine, and nickel for at least 1 lag day. Nitrate demonstrated stronger associations with total and cardiovascular mortality than PM2.5 mass. For a 1-day lag, interquartile range increases in PM2.5 mass and nitrate (114.9 and 15.4 µg/m3, respectively) were associated with 1.8% [95% confidence interval (CI): 0.8%, 2.8%] and 3.8% (95% CI: 1.7%, 5.9%) increases in total mortality.
Conclusions: Our findings suggest that PM2.5 constituents from the combustion of fossil fuel may have an appreciable influence on the health effects attributable to PM2.5 in Xi’an.
Keywords: air pollution, chemical constituents, fine particulate matter, mortality, time-series studies
Numerous epidemiological studies during the past 20 years have confirmed that short- and long-term exposure to outdoor air pollution contributes to increased cardiopulmonary mortality and morbidity (Brunekreef and Holgate 2002; Pope and Dockery 2006). Among various pollutants in the ambient mixture, fine particulate matter (PM2.5; particles ≤ 2.5 µm in aerodynamic diameter) shows the most consistent association with adverse health outcomes and therefore is of great public health concern (Ito et al. 2011; Ostro et al. 2007; Peng et al. 2009; Thurston et al. 2005; Zhou et al. 2011). However, the chemical components of PM2.5 responsible for these effects are still unknown. As the U.S. National Academy of Science pointed out, it is important to understand the contributions of specific components of ambient particulate matter (PM) to cardiopulmonary and other health effects (National Research Council 1998).
China has one of the highest PM2.5 levels in the world (van Donkelaar et al. 2010). However, PM2.5 is still not a criteria pollutant in China, and few studies in the country have investigated the adverse health effects of PM2.5 because of a lack of monitoring data. Currently, the Chinese government is reviewing its Air Quality Standards (AQS) and proposing to set the annual and daily average PM2.5 standards as 35 µg/m3 and 75 µg/m3, respectively (Chinese Ministry of Environmental Protection 2010). To our knowledge, only three published studies have estimated the effects of PM2.5 on daily mortality in China (Kan et al. 2007; Ma et al. 2011; Venners et al. 2003). Kan et al. (2007) and Ma et al. (2011) found significant associations between PM2.5 and daily mortality in Shanghai and Shenyang, China, whereas Venners et al. (2003) observed negative but statistically insignificant associations between PM2.5 and daily mortality in Chongqing. Obviously, more studies are needed to investigate the health effects of PM2.5 and its chemical constituents in China.
In the present study, we examined short-term associations between PM2.5 constituents and cardiopulmonary mortality in Xi’an, a heavily polluted Chinese city.
Methods
Data. Xi’an, with an area of 9,983 km2 and a resident population > 8.1 million in 2005, is the capital of Shanxi Province, China. Xi’an is the largest city in northwestern China, and it experiences some of the worst air pollution among China’s cities (Cao et al. 2005). Our study area was limited to the urban area of Xi’an, an area of 1,166 km2 with a resident population of > 2.7 million.
Mortality data. We obtained numbers of deaths among urban residents in Xi’an for each day for 1 January 2004 through 31 December 2008 from the Shanxi Provincial Center for Disease Control and Prevention (SPCDCP). In Xi’an, all deaths, regardless of whether they occur in a hospital or at home, must be reported to appropriate authorities before cremation of the remains. Hospital or community doctors must indicate the cause of death on a death certificate card that is sent to the SPCDCP. SPCDCP staff then classify the cause of death according to the International Classification of Diseases, 10th Revision [ICD-10; World Health Organization (WHO) 1992] as due to total nonaccidental causes (ICD-10 codes A00–R99), cardiovascular diseases (I00–I99), respiratory diseases (J00–J98), or injury (S00–T98). The Chinese government has mandated detailed quality assurance (QA) and quality control (QC) programs for the SPCDCP death registry.
Pollutant and meteorological data. For this study, we measured daily concentrations of PM2.5, organic carbon (OC), elemental carbon (EC), and 10 water-soluble ions [i.e., sodium ion (Na+), ammonium (NH4+), potassium ion (K+), magnesium ion (Mg2+) calcium ion (Ca2+), flouride (F–), choride (Cl–), nitrite (NO2–), sulfate (SO42–) and nitrate (NO3–)] for 1 January 2004 through 31 December 2008 (1,827 days). We also measured concentrations of 15 elements [i.e., sulfur (S), chlorine (Cl), potassium (K), calcium (Ca), titanium (Ti), chromium (Cr), manganese (Mn), iron (Fe), nickel (Ni), zinc (Zn), arsenic (As), boron (Br), molybdenum (Mo), cadmium (Cd), and lead (Pb)] for 1 January 2006 through 31 December 2008 (1,096 days).
The PM2.5 monitoring site was located on the rooftop of the Chinese Academy of Sciences’ Institute of Earth Environment building in an urban-scale zone of representation (Chow et al. 2002). The site was surrounded by a residential area where there were no major industrial activities nor local fugitive dust sources [see Supplemental Material, Figure 1 (http://dx.doi.org/10.1289/ehp.1103671). PM2.5 samples were obtained 10 m above the ground. Our previous studies suggest that the measured PM2.5 concentrations at this monitoring station are representative of the general status of PM2.5 pollution in Xi’an (Cao et al. 2005, 2007, 2009).
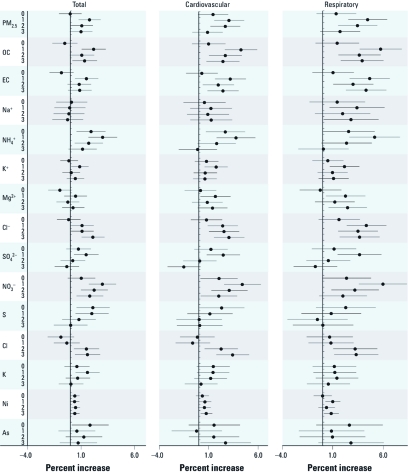
Figure 1.
Estimated percent increases [mean (95% CI)] in total, cardiovascular, and respiratory mortality per IQR increase in pollutant concentrations on the current day (lag 0) or the previous 1–3 days (lags 1, 2, and 3), adjusted for temporal trend, day of the week, temperature, relative humidity, and SO2 and NO2 concentrations.
Daily PM2.5 samples were collected using two battery-powered mini-volume samplers (MiniVol™ TAS; Airmetrics, Eugene, OR, USA) operating at a flow rate of 5 L/min (Cao et al. 2003). We used a relatively low flow rate due to high PM loading in Xi’an. PM2.5 samples were collected on 47-mm Whatman quartz microfiber filters that were pre-heated at 900°C for 3 hr before sampling. The quartz-fiber filters were analyzed gravimetrically for mass concentrations. We analyzed a 0.5-cm2 punch from each sample for OC and EC using a Desert Research Institute (DRI) model 2001 thermal/optical carbon analyzer (Atmoslytic Inc., Calabasas, CA, USA) for eight carbon fractions following the IMPROVE (Interagency Monitoring of Protected Visual Environments) thermal/optical reflectance (TOR) protocol (Chow et al. 2004). Levels of the five water-soluble cations (Na+, NH4+, K+, Mg2+ and Ca2+) and five water-soluble anions (F–, Cl–, NO2–, SO42– and NO3–) were determined in aqueous extracts of the sample filters using an ion chromatograph (Dionex 600; Dionex, Thermo Fisher Scientific, Inc., Cambridge, England, UK). Cation concentrations were determined using a CS12A column (Dionex), and anions were separated by an AS11-HC column (Dionex). The elemental concentrations of these samples were then determined by energy dispersive X-ray fluorescence (ED-XRF) spectrometry using the PANalytical Epsilon 5 XRF analyzer (PANalytical B.V., Almelo, the Netherlands). Detailed descriptions of the sample pretreatment, specific methods, detection limits, and QA/QC have been discussed previously (Cao et al. 2003, 2005; Shen et al. 2009a, 2009b).
To adjust for the effect of gaseous pollutants and weather on mortality, we obtained daily concentrations of sulfur dioxide (SO2) and nitrogen dioxide (NO2) from the Xi’an Environmental Monitoring Center, and daily mean temperature and humidity from the Xi’an Meteorological Bureau. The SO2 and NO2 concentrations were averaged from the available monitoring results across seven stations in our study area. According to the rules of the Chinese government, we assumed the monitoring data from these stations generally reflected the background urban air pollution of Xi’an rather than pollution from local sources.
Statistical methods. Due to different time periods for measuring PM2.5 constituents, we constructed two data sets to analyze the data: The first involved daily measurement of PM2.5, OC, EC, and ions for 1 January 2004 through 31 December 2008 and the second included daily concentrations of PM2.5 and constituent elements for 1 January 2006 through 31 December 2008.
Daily counts of deaths and air pollution levels were linked by date and analyzed with time–series analyses (Bell et al. 2004). Because daily counts of deaths approximate a Poisson distribution and the relationship between mortality and explanatory variables is mostly nonlinear, we used overdispersed generalized linear Poisson models (quasi-likelihood) with natural spline (ns) smoothers to analyze mortality, PM2.5 constituents, and covariate data.
In the basic model, we incorporated smoothed spline functions of time, accommodating both nonlinear and nonmonotonic relations between mortality and time and thus providing a flexible model to control for long-term and seasonal trends (Hastie and Tibshirani 1990). Day of the week (DOW) was included as a dummy variable (a variable that takes on the values 1 and 0; also called an indicator variable) in the basic models. Partial autocorrelation function (PACF) was used to guide the selection of degrees of freedom (df) for the time trend until the absolute values of the sum of PACF of the residuals for lag days of up to 30 reached a minimal value (Peng et al. 2006; Touloumi et al. 2004, 2006). We used residual plots and PACF plots to examine residuals of the basic model for discernable patterns and autocorrelation.
After establishing the basic model, we introduced the PM2.5 constituents and covariates (including temperature, humidity, and SO2 and NO2 concentrations) in the model. Based on previous literature (Dominici et al. 2006), we used smoothed spline functions with 3 df (for the whole period of the study) to control for temperature and relative humidity. To examine the temporal relationship of PM2.5 constituents with mortality, we fitted the models with different lag structures from 0 lag days to 3 lag days because our previous work on PM2.5 and daily mortality in China showed little evidence of a significant association with a lag beyond 3 days (Kan et al. 2007; Ma et al. 2011). A lag of 0 days (lag 0) corresponds to the current-day PM2.5, and a lag of 1 day (lag 1) refers to the previous-day PM2.5. We used the smoothing spline, with 3 df for PM2.5, to graphically describe its relationships with mortality. We compared the linear and spline models by computing the difference between the deviances of the fitted two models (Dominici et al. 2002; Samoli et al. 2005). We estimated associations of PM2.5 constituents with mortality before and after adjustment for PM2.5 mass. Finally, to examine the robustness of our choice on the optimal values of df for time trend, we performed a sensitivity analysis to test the impact of df selection on the regression results.
All analyses were conducted in R version 2.10.1 (http://www.R-project.org) using the MGCV package. The results are presented as the percent change in daily mortality per interquartile range (IQR) increase of pollutant concentrations unless specified otherwise. Statistical significance was defined as p < 0.05.
Results
We identified 47,838 deaths that occured between 1 January 2004 and 31 December 2008 in our study population. On average, 26.2 nonaccidental deaths occurred per day, including 12.1 from cardiovascular diseases and 7.2 from respiratory diseases (Table 1). The mean daily average temperature and humidity in Xi’an were 13.4°C and 66.5%, respectively.
Table 1.
Distribution of daily data on mortality and weather conditions in Xi’an, China (2004–2008).
| Percentile | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Minimum | 25th | 50th | 75th | Maximum | |||||||
| Daily death counts | ||||||||||||
| Total nonaccidental | 26.2 ± 9.7 | 4.0 | 20.0 | 25.0 | 31.0 | 128.0 | ||||||
| Cardiovascular | 12.1 ± 5.7 | 0.0 | 8.0 | 11.0 | 15.0 | 39.0 | ||||||
| Respiratory | 7.2 ± 3.8 | 0.0 | 4.0 | 7.0 | 9.0 | 29.0 | ||||||
| Injury | 1.8 ± 1.7 | 0.0 | 1.0 | 1.0 | 3.0 | 19.0 | ||||||
| Weather conditions | ||||||||||||
| Temperature (°C) | 13.4 ± 9.8 | –8.0 | 5.0 | 14.0 | 22.0 | 32.0 | ||||||
| Relative humidity (%) | 66.5 ± 16.7 | 15.0 | 55.0 | 68.0 | 79.0 | 100.0 | ||||||
During 2004–2008, the Xi’an mean daily average concentration of PM2.5 was 182.2 µg/m3 (Table 2), which was much higher than the WHO Global Guidelines (annual average: 10 µg/m3; WHO 2006) and than the reported levels of PM2.5 for other Chinese cities such as Beijing (annual average: 122 µg/m3; Guo et al. 2009), Shanghai (annual average: 55 µg/m3; Kan et al. 2007), and Shenyang (annual average: 75 µg/m3; Ma et al. 2011). Meanwhile, the mean daily average concentrations of SO2 and NO2 were 48.4 and 38.2 µg/m3.
Table 2.
Descriptive statistics for air pollutants in Xi’an, China (2004–2008).
| Observation period | Pollutant | Observation (n) | Mean ± SD (µg/m3) | Minimum | Maximum | IQR (µg/m3) | PM2.5 mass (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 January 2004–31 December 2008 | |||||||||||||
| PM2.5 | 1,756 | 182.2 ± 110.1 | 16.4 | 768.6 | 114.9 | — | |||||||
| SO2 | 1,827 | 48.4 ± 28.9 | 8.0 | 260.0 | 30.0 | — | |||||||
| NO2 | 1,827 | 38.2 ± 15.0 | 6.4 | 110.0 | 21.0 | — | |||||||
| OC | 1,749 | 28.3 ± 18.3 | 5.1 | 142.3 | 19.3 | 15.5 | |||||||
| EC | 1,749 | 12.0 ± 8.3 | 0.2 | 84.2 | 8.8 | 6.6 | |||||||
| Na+ | 1,649 | 2.9 ± 1.4 | 0.0 | 12.7 | 1.9 | 1.6 | |||||||
| NH4+ | 1,538 | 8.8 ± 8.5 | 0.0 | 61.1 | 10.7 | 4.8 | |||||||
| K+ | 1,616 | 2.2 ± 2.3 | 0.0 | 35.3 | 1.9 | 1.2 | |||||||
| Mg2+ | 1,666 | 0.5 ± 0.3 | 0.0 | 3.7 | 0.3 | 0.3 | |||||||
| Ca2+ | 730 | 2.0 ± 2.4 | 0.0 | 22.4 | 1.9 | 1.1 | |||||||
| F– | 1,429 | 0.6 ± 0.3 | 0.0 | 3.4 | 0.5 | 0.3 | |||||||
| Cl– | 1,670 | 5.1 ± 3.5 | 0.3 | 32.6 | 3.6 | 2.8 | |||||||
| NO2– | 563 | 0.7 ± 0.4 | 0.0 | 3.0 | 0.4 | 0.4 | |||||||
| SO42– | 1,666 | 31.6 ± 24.4 | 0.8 | 198.2 | 27.8 | 17.4 | |||||||
| NO3– | 1,644 | 15.2 ± 12.7 | 0.0 | 85.5 | 15.4 | 8.4 | |||||||
| 1 January 2006–31 December 2008 | |||||||||||||
| S | 1,028 | 5.1 ± 3.5 | 0.1 | 24.8 | 4.3 | 2.8 | |||||||
| Cl | 1,027 | 1.3 ± 1.6 | 0.0 | 11.8 | 1.5 | 0.7 | |||||||
| K | 1,007 | 1.8 ± 1.7 | 0.0 | 22.5 | 1.6 | 1.0 | |||||||
| Ca | 904 | 2.5 ± 3.3 | 0.0 | 30.6 | 2.3 | 1.4 | |||||||
| Ti | 1,026 | 0.14 ± 0.15 | 0.00 | 1.63 | 0.10 | 0.08 | |||||||
| Cr | 952 | 0.01 ± 0.01 | 0.00 | 0.10 | 0.01 | 0.01 | |||||||
| Mn | 1,026 | 0.11 ± 0.08 | 0.00 | 0.56 | 0.09 | 0.06 | |||||||
| Fe | 1,013 | 1.6 ± 1.7 | 0.0 | 20.0 | 1.3 | 0.87 | |||||||
| Ni | 836 | 0.01 ± 0.03 | 0.00 | 0.55 | 0.01 | 0.01 | |||||||
| Zn | 1,028 | 1.4 ± 1.1 | 0.0 | 8.6 | 1.2 | 0.79 | |||||||
| As | 676 | 0.04 ± 0.03 | 0.00 | 0.24 | 0.03 | 0.02 | |||||||
| Br | 962 | 0.04 ± 0.05 | 0.00 | 0.56 | 0.04 | 0.02 | |||||||
| Mo | 1,009 | 0.06 ± 0.05 | 0.00 | 0.37 | 0.03 | 0.03 | |||||||
| Cd | 990 | 0.03 ± 0.02 | 0.00 | 0.13 | 0.03 | 0.02 | |||||||
| Pb | 1,025 | 0.50 ± 0.38 | 0.00 | 3.13 | 0.41 | 0.27 | |||||||
Over the 5 years (1,827 days) of the study, we recorded 1,749 observations of OC and EC; the averaged concentrations were 28.3 µg/m3 for OC and 12.0 µg/m3 for EC, accounting for 15.5% and 6.6% of the total PM2.5 mass, respectively (Table 2). Besides OC and EC, the other largest contributors to PM2.5 were SO42– (17.4%), NO3– (8.4%), NH4+ (4.8%), and S (2.8%).
Generally, moderate to high correlations (r = 0.5–0.8) were observed for PM2.5 with OC, EC, S, Cl, K, Mg2+, Cl–, K+, SO42–, NO3–, and NH4+ levels [see Supplemental Material, Table 1 (http://dx.doi.org/10.1289/ehp.1103671)]. PM2.5 was modestly correlated with Na+ levels (r = 0.33). Consistent with previous studies (Ostro et al. 2007), Ni levels were weakly correlated with PM2.5 (r = 0.13) and other constituents.
Figure 1 summarizes the quantitative regression results for single-day lags 0–3 of PM2.5 mass and various constituents (before adjusting for PM2.5). We found significant associations of PM2.5 mass with daily mortality; an IQR increment in the 1-day lagged concentrations of PM2.5 (182.2 µg/m3) corresponded to a1.8% [95% confidence interval (CI): 0.8%, 2.8%], 3.1% (95% CI: 1.6%, 4.6%), and 4.5% (95% CI: 2.5%, 6.4%) increase of total, cardiovascular, and respiratory mortality, respectively. Consistent with previous studies (Ito et al. 2011; Ostro et al. 2007; Peng et al. 2009), the effect estimates of PM2.5 constituents varied by lag structures and mortality outcomes. OC, EC, NH4+, Cl–, NO3–, Cl, and Ni showed the strongest associations in that more than half of the associations assessed were positive and statistically significant. At least one positive significant association was found for Na+, K+, Mg2+, SO42–, S, K, and As. We did not observe positive significant associations for F–, Ca, Ti, Cr, Mn, Fe, Zn, Br, Mo, Cd, or Pb [see Supplemental Material, Figure 2 (http://dx.doi.org/10.1289/ehp.1103671)].
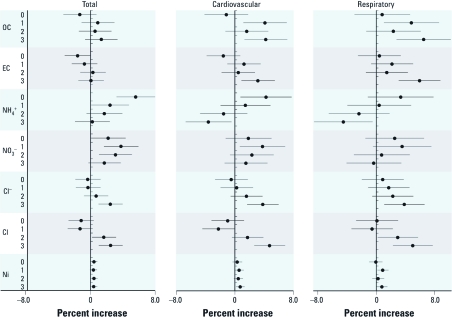
Figure 2.
Estimated percent increases [mean (95% CI)] in total, cardiovascular, and respiratory mortality per IQR increase in pollutant concentrations on the current day (lag 0) or the previous 1–3 days (lags 1, 2, and 3), adjusted for PM2.5 mass, temporal trend, day of the week, temperature, relative humidity, and SO2 and NO2 concentrations.
Figure 2 shows the effect estimates of PM2.5 constituents (OC, EC, NH4+, NO3–, Cl–, Cl, and Ni) that were significantly associated with at least one outcome and lag period after further adjustment for PM2.5 mass. OC and EC were positively associated with cardiovascular and respiratory mortality (for lags 1–3 and lag 3, respectively), but were not clearly associated with total mortality. NH4+ and NO3– were significantly associated with total and cardiovascular mortality, but not with respiratory mortality. Cl–, Cl, and Ni were significantly associated with all three mortality outcomes for at least one lagged exposure. It should be noted that NH4+ (lag 3) and Cl– (lag 1) were negatively and statistically significantly associated with cardiovascular or respiratory mortality. Na+, K+, Mg2+, SO42–, S, K, and As, after adjustment for PM2.5, were no longer positively and statistically significantly associated with any of the outcomes, and some of the adjusted associations even became negative and statistically significant [see Supplemental Material, Figure 3 (http://dx.doi.org/10.1289/ehp.1103671)]. Interestingly, after adjusting for PM2.5, associations with an IQR increase in NO3– were stronger than associations with an IQR increase in PM2.5 mass for total and cardiovascular mortality. For instance, for lag 1, an IQR increase in NO3– (15.2 µg/m3) was associated with 3.8% (95% CI: 1.7%, 5.9%) increase in total mortality, compared with 1.8% (95% CI: 0.8%, 2.8%) for an IQR increase (182.2 µg/m3) in PM2.5 mass.
Figure 3.
Exposure–response relationships (smoothing plots) of PM2.5 against total (A), cardiovascular (B), and respiratory (C) mortality (df= 3) in Xi’an, adjusted for temporal trend, day of the week, temperature, relative humidity, and SO2 and NO2 concentrations. The x-axis is the PM2.5 concentrations (single day lag, L1); the y-axis is the estimated percent change in deaths; the solid blue lines indicate the estimated mean percent change in daily death numbers using the lowest PM2.5 concentration as the reference level; and the dashed lines represent the 95% CI.
Figure 3 shows the exposure–response relationships for PM2.5 mass (single day lag 1) with total, cardiovascular, and respiratory mortality between 2004 and 2008 in Xi’an. For all three mortality outcomes, we observed almost linear relationships, with no evidence of obvious threshold concentrations below which PM2.5 had no effect on mortality outcomes. The differences in the deviance between the linear and spline models did not indicate a significant improvement in the fit of the spline versus linear models. In the linear models, a 10-µg/m3 increment in the 1-day lagged PM2.5 was associated with 0.2% (95% CI: 0.1%, 0.3%), 0.3% (95% CI: 0.1%, 0.4%), and 0.4% (95% CI: 0.2%, 0.6%) increases in total, cardiovascular, and respiratory mortality, respectively.
As expected, deaths due to injury were not associated with PM2.5 constituents [there was only 1 significant association out of 92 comparisons when adjusted for PM2.5; see Supplemental Material, Table 2 (http://dx.doi.org/10.1289/ehp.1103671)]. Altering the df per year for time trend within a range of 3–10 df did not substantially change the regression results (data not shown).
Discussion
Evidence obtained in this time–series analysis showed that PM2.5 mass and several constituents were associated with total nonaccidental and cardiopulmonary disease-related mortality in Xi’an. The observed levels of PM2.5 and its constituents in our study were much higher than earlier health studies of PM2.5 constituents in developed countries. Several constituents that were associated with mortality (NH4+, NO3–, Cl–, OC, EC, Cl) are associated with the combustion of fossil fuels such as coal and heavy oil in Xi’an (Cao et al. 2005, 2009). We found stronger associations for NO3– with total and cardiovascular mortality than for PM2.5 mass. We did not find evidence of threshold concentrations below which PM2.5 was not associated with mortality in Xi’an. To our knowledge, this is the first study of its kind in a developing country to investigate the health effects of PM2.5 constituents.
The results of our study in Xi’an indicate considerable risk heterogeneity among the various PM2.5 constituents. Consistent with previous epidemiological studies on PM constituents (Ito et al. 2011; Laden et al. 2000; Ostro et al. 2007, 2008; Peng et al. 2009; Zhou et al. 2011), we found that PM2.5 constituents resulting from the combustion of fossil fuel (e.g., NH4+, NO3–, Cl–, OC, EC, Cl, Ni) maintained significant positive associations with mortality outcomes even after we adjusted for PM2.5. In contrast, we did not find significant associations between mortality and common crustal elements (e.g., Ca and K) in Xi’an, which is consistent with a previous study performed in six U.S. cities that showed PM2.5 crustal particles were not associated with daily mortality (Laden et al. 2000). It should be noted that we observed statistically significant associations for some, but not all, lag structures of PM2.5 constituents. Further research is needed to clarify relationships between the timing of exposures and their potential health effects.
Our analysis indicates positive associations of cardiopulmonary mortality with IQR increases in OC or EC during the previous 1–3 days even after adjusting for PM2.5 mass. This is consistent with the findings of a meta-analysis of short-term exposure time–series studies of EC and daily mortality that reported positive associations with cardiopulmonary mortality (Smith et al. 2009). The results of a recent cohort study in California suggest that long-term exposure to OC also increase cardiopulmonary mortality (Ostro et al. 2010). Additionally, several previous studies support the biological plausibility of a link between exposure to OC or EC and exacerbations of cardiopulmonary diseases (Gold et al. 2005; Henneberger et al. 2005; Jansen et al. 2005; Lanki et al. 2006; Lewne et al. 2007; Mar et al. 2005; Shih et al. 2008; von Klot et al. 2009). For example, one study in Germany examined weekly electrocardiograms of 56 men with a history of heart disease and found significant associations of OC or EC with changes in myocardial repolarization, which could increase the risk of sudden cardiac death (Henneberger et al. 2005). Gold et al. (2005) found associations of EC with ST-segment depression among a panel of 24 elderly Boston residents. Similarly, Lanki et al. (2006) examined the health effects of five PM2.5 components (Si, S, Ni, Cl, and EC), and found only EC had significant association with ST-segment depression in multipolluant models. Exposure to OC or EC was also associated with increased nitric oxide (NO) in exhaled breath, a marker of airway inflammation (Mar et al. 2005). Thus, exposures to both OC and EC are associated with a number of indicators that could contribute to cardiopulmonary mortality.
NO3– was positively associated with mortality in our study. To date, only a few epidemiological studies have examined the relationships of NO3– with mortality, and their findings were inconclusive. For example, Klemm et al. (2004) found a positive but insignificant association between NO3– and mortality in Atlanta (Georgia), whereas Ostro et al. (2007) found a significant association between NO3– and mortality in six California counties. More studies are needed to understand the health effects of NO3–. In our study, SO42– (mean level: 31.6 µg/m3) was not associated with mortality, which is consistent with toxicological studies showing little toxic evidence of SO42– effects on the cardiopulmonary system at typical environmental concentrations (Reiss et al. 2007). As Schlesinger and Cassee (2003) pointed out, the minimal effective concentration of SO42– to alter pulmonary mechanical function in normal humans following acute exposure is > 1,000 µg/m3.
In our analysis, an IQR increase of 0.01 µg/m3 in 1-day lagged Ni was associated with 0.4% (95% CI: 0.0%, 0.8%), 0.6% (95% CI: –0.1%, 1.2%) and 0.9% (95% CI: 0.2%, 1.7%) increases in total, cardiovascular, and respiratory mortality. As a transition metal, Ni may affect health by producing reactive oxygen species and increasing oxidative stress (Lippmann et al. 2006; Schlesinger et al. 2006). In fact, existing epidemiological studies provide evidence of adverse effects for several transition metals (Dominici et al. 2007; Huang et al. 2003; Lippmann et al. 2006; Ostro et al. 2007, 2008). For example, Huang et al. (2003) found that exposure to a factor including vanadium (V), Zn, and copper (Cu) from concentrated ambient particles was associated with increased blood fibrinogen levels. Using the National Morbidity, Mortality, and Air Pollution Study (NMMAPS) database, Lippmann et al. (2006) found that daily mortality rates in the 60 U.S. cities with speciation data were significantly associated with average levels of Ni and V, but not other measured species. In Xi’an, the major source of Ni in PM2.5 is fossil fuel combustion, especially heavy oil (Shen et al. 2009b). The role of Ni in PM2.5 health hazards should be investigated further.
In our analysis, a 10-µg/m3 increment in the 1-day lagged concentrations of PM2.5 was associated with 0.2% (95% CI: 0.1%, 0.3%), 0.3% (95% CI: 0.1%, 0.4%), and 0.4% (95% CI: 0.2%, 0.6%) increases in total, cardiovascular, and respiratory mortality, respectively. Compared with studies of PM2.5 and daily mortality in developed countries (Franklin et al. 2007; Ostro et al. 2006; Ueda et al. 2009; Zanobetti and Schwartz 2009), our estimations of the associations of PM2.5 with mortality were somewhat lower in magnitude per amount of PM2.5 mass. For example, a multicity analysis in 112 U.S. cities found that a 10-µg/m3 increase in PM2.5 was associated with a 1.0% increase in total mortality, a 0.9% increase in cardiovascular mortality, and a 1.7% increase in respiratory mortality (Zanobetti and Schwartz 2009), whereas our findings are in agreement with earlier evidence (Aunan and Pan 2004) suggesting weaker associations between health outcomes and unit increases in air pollution exposures in China than in developed countries. This may be explained by differences in the composition and toxicity of PM, as well as differences in local PM concentrations and population sensitivity to PM in addition to differences in age structure and other population characteristics. Lower risks of death per unit increases in pollutants when concentrations are high may reflect the selective attrition of vulnerable members of the population who die before concentrations reach the maximum level (Wong et al. 2008). Also, associations between mortality and PM exposures ranging from low (e.g., exposure levels associated with ambient air pollution) to high (e.g., exposure levels associated with cigarette smoking) concentrations suggest that the exposure–response curve of PM often tends to become flat at higher concentrations (Pope et al. 2009).
Accurate information on the shape of exposure–response relationships is crucial for public health assessment, and the demand for providing the relevant curves has been growing (Dominici et al. 2002). Dose–response relationships may vary by location depending on factors such as the air pollution mixture, climate, and overall health of the studied population (Samoli et al. 2005). In our study population, we did not observe evidence for a threshold concentration below which PM2.5 was not associated with mortality, suggesting that linear models without a threshold are appropriate for assessing the effect of PM2.5 on daily mortality for the high-exposure settings typical of developing countries.
Our study has limitations. First, we evaluated the associations of multiple constituents and lags with three different mortality outcomes; some significant associations, therefore, may have occurred by chance. Second, because of moderate-to-high collinearity among PM2.5 constituents, we could not adjust for multiple exposures, and some associations may reflect the effects of other correlated components. We did not measure several elements such as selenium (Se), V, and silicon (Si), although previous studies reported significant associations between these elements and adverse health outcomes (Laden et al. 2000; Ostro et al. 2007), and we could not evaluate ozone (O3) due to a lack of monitoring data in Xi’an. As in many previous time–series studies, we used PM2.5 monitoring results from a fixed station as a proxy measure for population exposures to air pollution. As a result, a number of issues may arise given that ambient monitoring results differ from personal exposure level to air pollutants (Sarnat et al. 2001, 2005). In addition, variation in the extent of exposure misclassification among individual constituents may influence associations. Finally, we did not conduct formal source apportionment of PM2.5 constituents, and therefore cannot identify the source components that contributed most to the associations between PM2.5 and mortality.
Conclusions
Our findings suggest that PM2.5 constituents from fossil fuel combustion may have an appreciable influence on the health effects attributable to PM2.5. Associations of PM2.5 with mortality in Xi’an are somewhat lower in magnitude per amount of PM2.5 mass compared with associations reported for populations in developed countries. Our findings add support to previously reported evidence of PM2.5-related health effects in China and suggest that combustion-associated pollutants are particularly important.
Supplemental Material
Footnotes
This study was supported by the National Basic Research Program (973 program) of China (2011CB503802), National Natural Science Foundation of China (40925009), and Gong-Yi Program of China Ministry of Environmental Protection (200809109, 200909016, and 201209008).
The authors declare they have no actual or potential competing financial interests.
References
- Aunan K, Pan XC. Exposure–response functions for health effects of ambient air pollution applicable for China—a meta-analysis. Sci Total Environ. 2004;329(1–3):3–16. doi: 10.1016/j.scitotenv.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bell ML, Samet JM, Dominici F. Time-series studies of particulate matter. Annu Rev Public Health. 2004;25:247–280. doi: 10.1146/annurev.publhealth.25.102802.124329. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360(9341):1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Cao JJ, Chow J, Lee S, Li Y, Chen S, An Z, et al. Characterization and source apportionment of atmospheric organic and elemental carbon during fall and winter of 2003 in Xi’an, China. Atmos Chem Phys. 2005;5:3127–3137. [Google Scholar]
- Cao JJ, Lee SC, Chow JC, Watson JG, Ho KF, Zhang RJ, et al. 2007. Spatial and seasonal distributions of carbonaceous aerosols over China. J Geophys Res 112(D22S11); doi:10.1029/2006JD008205 [Online 10 November 2007].
- Cao JJ, Lee S, Ho K, Zhang X, Zou S, Fung K, et al. Characteristics of carbonaceous aerosol in Pearl River Delta Region, China during 2001 winter period. Atmos Environ. 2003;37(11):1451–1460. [Google Scholar]
- Cao JJ, Zhu CS, Chow JC, Watson JG, Han YM, Wang GH, et al. Black carbon relationships with emissions and meteorology in Xi’an, China. Atmos Res. 2009;94(2):194–202. [Google Scholar]
- Chinese Ministry of Environmental Protection. Proposed ambient air quality standards Beijing [in Chinese]. 2010. Available: http://www.zhb.gov.cn/gkml/hbb/bgth/201011/W020101130374443014849.pdf [accessed 24 December 2011]
- Chow JC, Engelbrecht JP, Watson JG, Wilson WE, Frank NH, Zhu T. Designing monitoring networks to represent outdoor human exposure. Chemosphere. 2002;49(9):961–978. doi: 10.1016/s0045-6535(02)00239-4. [DOI] [PubMed] [Google Scholar]
- Chow JC, Watson JG, Chen LW, Arnott WP, Moosmuller H, Fung K. Equivalence of elemental carbon by thermal/optical reflectance and transmittance with different temperature protocols. Environ Sci Technol. 2004;38(16):4414–4422. doi: 10.1021/es034936u. [DOI] [PubMed] [Google Scholar]
- Dominici F, Daniels M, Zeger SL, Samet JM. Air pollution and mortality: estimating regional and national dose–response relationships. J Am Stat Assoc. 2002;97(457):100–111. [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Ebisu K, Zeger SL, Samet JM, Bell ML. Does the effect of PM10 on mortality depend on PM nickel and vanadium content? A reanalysis of the NMMAPS data. Environ Health Perspect. 2007;115:1701–1703. doi: 10.1289/ehp.10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M, Zeka A, Schwartz J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J Expo Sci Environ Epidemiol. 2007;17(3):279–287. doi: 10.1038/sj.jes.7500530. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, MacCallum G, et al. Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect. 2005;113:883–887. doi: 10.1289/ehp.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Jia Y, Pan X, Liu L, Wichmann HE. The association between fine particulate air pollution and hospital emergency room visits for cardiovascular diseases in Beijing, China. Sci Total Environ. 2009;407(17):4826–4830. doi: 10.1016/j.scitotenv.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. London: Chapman & Hall; 1990. Generalized Additive Models. [Google Scholar]
- Henneberger A, Zareba W, Ibald-Mulli A, Ruckerl R, Cyrys J, Couderc JP, et al. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ Health Perspect. 2005;113:440–446. doi: 10.1289/ehp.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Ghio AJ, Stonehuerner J, McGee J, Carter JD, Grambow SC, et al. The role of soluble components in ambient fine particles-induced changes in human lungs and blood. Inhal Toxicol. 2003;15(4):327–342. doi: 10.1080/08958370304460. [DOI] [PubMed] [Google Scholar]
- Ito K, Mathes R, Ross Z, Nadas A, Thurston G, Matte T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ Health Perspect. 2011;119:467–473. doi: 10.1289/ehp.1002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen K, Larson T, Koenig J, Mar T, Fields C, Stewart J, et al. Associations between health effects and particulate matter and black carbon in subjects with respiratory disease. Environ Health Perspect. 2005;113:1741–1746. doi: 10.1289/ehp.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, London SJ, Chen G, Zhang Y, Song G, Zhao N, et al. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environ Int. 2007;33(3):376–384. doi: 10.1016/j.envint.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RJ, Lipfert FW, Wyzga RE, Gust C. Daily mortality and air pollution in Atlanta: two years of data from ARIES. Inhal Toxicol. 2004;16(suppl 1):131–141. doi: 10.1080/08958370490443213. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanki T, de Hartog JJ, Heinrich J, Hoek G, Janssen NA, Peters A, et al. Can we identify sources of fine particles responsible for exercise-induced ischemia on days with elevated air pollution? The ULTRA study. Environ Health Perspect. 2006;114:655–660. doi: 10.1289/ehp.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewne M, Plato N, Gustavsson P. Exposure to particles, elemental carbon and nitrogen dioxide in workers exposed to motor exhaust. Ann Occup Hyg. 2007;51(8):693–701. doi: 10.1093/annhyg/mem046. [DOI] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Chen R, Pan G, Xu X, Song W, Chen B, et al. 2011Fine particulate air pollution and daily mortality in Shenyang, China. Sci Total Environ, 4092473–2477. [DOI] [PubMed] [Google Scholar]
- Mar TF, Jansen K, Shepherd K, Lumley T, Larson TV, Koenig JQ. Exhaled nitric oxide in children with asthma and short-term PM2.5 exposure in Seattle. Environ Health Perspect. 2005;113:1791–1794. doi: 10.1289/ehp.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council 1998. Research Priorities for Airborne Particulate Matter. Washington DC:National Academy Press. [Google Scholar]
- Ostro B, Broadwin R, Green S, Feng WY, Lipsett M. Fine particulate air pollution and mortality in nine California counties: results from CALFINE. Environ Health Perspect. 2006;114:29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environ Health Perspect. 2007;115:13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Malig BJ, Green RS, Lipsett MJ. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occup Environ Med. 2008;65(11):750–756. doi: 10.1136/oem.2007.036673. [DOI] [PubMed] [Google Scholar]
- Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California teachers study. Environ Health Perspect. 2010;118:363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Bell ML, Geyh AS, McDermott A, Zeger SL, Samet JM, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2009;117:957–963. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Dominici F, Louis TA. Model choice in time series studies of air pollution and mortality. Journal of the Royal Statistical Society, Series A. 2006;169(2):179–203. [Google Scholar]
- Pope CA, III, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure–response relationship. Circulation. 2009;120(11):941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manage Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Reiss R, Anderson EL, Cross CE, Hidy G, Hoel D, McClellan R, et al. Evidence of health impacts of sulfate- and nitrate-containing particles in ambient air. Inhal Toxicol. 2007;19(5):419–449. doi: 10.1080/08958370601174941. [DOI] [PubMed] [Google Scholar]
- Samoli E, Analitis A, Touloumi G, Schwartz J, Anderson HR, Sunyer J, et al. Estimating the exposure–response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect. 2005;113:88–95. doi: 10.1289/ehp.7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures—implications for studying the health effects of particles. Epidemiology. 2005;16(3):385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- Sarnat JA, Schwartz J, Catalano PJ, Suh HH. Gaseous pollutants in particulate matter epidemiology: confounders or surrogates? Environ Health Perspect. 2001;109:1053–1061. doi: 10.1289/ehp.011091053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger RB, Cassee F. Atmospheric secondary inorganic particulate matter: the toxicological perspective as a basis for health effects risk assessment. Inhal Toxicol. 2003;15(3):197–235. doi: 10.1080/08958370304503. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB, Kunzli N, Hidy GM, Gotschi T, Jerrett M. The health relevance of ambient particulate matter characteristics: coherence of toxicological and epidemiological inferences. Inhal Toxicol. 2006;18(2):95–125. doi: 10.1080/08958370500306016. [DOI] [PubMed] [Google Scholar]
- Shen Z, Cao J, Arimoto R, Han Z, Zhang R, Han Y, et al. Ionic composition of TSP and PM2.5 during dust storms and air pollution episodes at Xi’an, China. Atmos Environ. 2009a;43(18):2911–2918. [Google Scholar]
- Shen Z, Cao J, Tong Z, Liu S, Reddy L, Han Y, et al. Chemical characteristics of submicron particles in winter in Xi’an. Aerosol Air Qual Res. 2009b;9(1):80–93. [Google Scholar]
- Shih TS, Lai CH, Hung HF, Ku SY, Tsai PJ, Yang T, et al. Elemental and organic carbon exposure in highway tollbooths: a study of Taiwanese toll station workers. Sci Total Environ. 2008;402(2-3):163–170. doi: 10.1016/j.scitotenv.2008.04.051. [DOI] [PubMed] [Google Scholar]
- Smith KR, Jerrett M, Anderson HR, Burnett RT, Stone V, Derwent R, et al. Public health benefits of strategies to reduce greenhouse-gas emissions: health implications of short-lived greenhouse pollutants. Lancet. 2009;374(9707):2091–2103. doi: 10.1016/S0140-6736(09)61716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston GD, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, et al. Workgroup report: workshop on source apportionment of particulate matter health effects—intercomparison of results and implications. Environ Health Perspect. 2005;113:1768–1774. doi: 10.1289/ehp.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touloumi G, Atkinson R, Tertre AL, Samoli E, Schwartz J, Schindler C, et al. Analysis of health outcome time series data in epidemiological studies. Environmetrics. 2004;15(2):101–117. [Google Scholar]
- Touloumi G, Samoli E, Pipikou M, Le Tertre A, Atkinson R, Katsouyanni K. Seasonal confounding in air pollution and health time-series studies: effect on air pollution effect estimates. Stat Med. 2006;25(24):4164–4178. doi: 10.1002/sim.2681. [DOI] [PubMed] [Google Scholar]
- Ueda K, Nitta H, Ono M, Takeuchi A. Estimating mortality effects of fine particulate matter in Japan: a comparison of time-series and case-crossover analyses. J Air Waste Manag Assoc. 2009;59(10):1212–1218. doi: 10.3155/1047-3289.59.10.1212. [DOI] [PubMed] [Google Scholar]
- van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, et al. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environ Health Perspect. 2010;118:847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venners SA, Wang B, Peng Z, Xu Y, Wang L, Xu X. Particulate matter, sulfur dioxide, and daily mortality in Chongqing, China. Environ Health Perspect. 2003;111:562–567. doi: 10.1289/ehp.5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Klot S, Gryparis A, Tonne C, Yanosky J, Coull BA, Goldberg RJ, et al. Elemental carbon exposure at residence and survival after acute myocardial infarction. Epidemiology. 2009;20(4):547–554. doi: 10.1097/EDE.0b013e31819d9501. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Geneva. World Health Organization. 1992. Available: http://apps.who.int/classifications/icd10/browse/2010/en [accessed 21 January 2012]
- WHO (World Health Organization) Geneva, Switzerland: World Health Organization. Available: whqlibdoc.who.int/hq/2006/WHO_SDE_PHE_OEH_06.02_eng.pdf [accessed 21 January 2012]; 2006. WHO Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide. Global Update 2005. [Google Scholar]
- Wong CM, Vichit-Vadakan N, Kan H, Qian Z. Public health and air pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect. 2008;116:1195–1202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Ito K, Lall R, Lippmann M, Thurston G. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ Health Perspect. 2011;119:461–466. doi: 10.1289/ehp.1002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.