Abstract
Background: Previous systematic reviews have indicated that pesticide exposure is possibly associated with Parkinson disease (PD). However, considerable heterogeneity has been observed in study results.
Objective: We aimed at providing an update of the literature published on PD and exposure to pesticides by performing a systematic review and meta-analysis. In addition, we investigated whether methodological differences between studies could explain the heterogeneity in study results.
Methods: We identified studies through a systematic literature search. We calculated summary risk ratios (sRRs) for pesticide exposure and subcategories using random effects meta-analyses and investigated sources of heterogeneity by meta-regression and stratified analyses.
Results: Thirty-nine case–control studies, four cohort studies, and three cross-sectional studies were identified. An sRR of 1.62 [95% confidence interval (CI): 1.40, 1.88] for pesticide exposure (ever vs. never) was found. Summary estimates for subclasses of pesticides indicated a positive association with herbicides and insecticides, but not with fungicides. Heterogeneity in individual study results was not related to study design, source of control population, adjustment of results for potential confounders, or geographical area. However, results were suggestive for heterogeneity related to differences in the exposure assessment. Job title–based exposure assignment resulted in a higher sRR (2.5; 95% CI: 1.5, 4.1) than did assignment based on self-reported exposure (e.g., for self-reported ever/never exposure, sRR = 1.5; 95% CI: 1.3, 1.8).
Conclusions: This review affirms the evidence that exposure to herbicides and insecticides increase the risk of PD. Future studies should focus on more objective and improved methods of pesticide exposure assessment.
Keywords: exposure assessment, fungicides, herbicides, insecticides, meta-analysis, Parkinson disease, pesticides, systematic review
Parkinson disease (PD) is an idiopathic degenerative disorder of the central nervous system that impairs motor skills, cognitive processes, and other functions. The etiology of PD is largely unknown, although some genetic factors have been identified (Bekris et al. 2010; Shulman et al. 2011). Based on published epidemiological and toxicological studies, pesticides may be involved in the etiology of PD (Brown et al. 2006). However, epidemiological evidence is far from conclusive, as considerable heterogeneity has been observed in study results (Brown et al. 2006; Li et al. 2005; Priyadarshi et al. 2000). Possible methodological causes of heterogeneity in study results have been suggested and include differences in study design, control selection, diagnosis of patients, and statistical analysis (Brown et al. 2006). Differences in exposure assessment methods could contribute to heterogeneity as well. Most previous studies relied almost exclusively on self-reported exposures, a process that is prone to recall bias, especially in case–control studies, and could lead to false-positive associations. Alternatively, one could speculate that PD patients might underreport pesticide exposure because of cognitive deficits, leading to false-negative associations. Furthermore, differences in the definition of exposure to pesticides (occupational vs. nonoccupational use, ever/never vs. regular use) could also result in heterogeneous study results. Lastly, the regions where the studies have been conducted could be of importance as regulation, types, and use of pesticides may differ from region to region.
Several recent studies have been published on pesticide exposure and PD risk, including some prospective (cohort) studies. In the present analysis, we aimed at providing an update of the literature published since the last systematic review on PD (Brown et al. 2006) and exposure to pesticides and pesticide subcategories by performing a systematic review and meta-analysis. We specifically set out to address the question of whether the previously described heterogeneity in study findings could be explained by differences in study design and exposure assessment methods.
Methods
Data source. We searched the databases Embase (http://www.Embase.com/), starting with 1974, and Medline (http://www.ncbi.nlm.nih.gov/pubmed/), starting with 1950, through November 2010 using the search term “Parkinson” in combination with “pesticide*,” “insecticide*,” “fungicide*,” “herbicide*,” “rodenticide*,” “organochlorine*,” “organophosphate*,” “carbamate*,” “glyphosate*,” “paraquat,” “maneb,” “lindane,” “dieldrin,” “rotenone,” “DDT,” or “environmental factors.” The search was limited to publications in English, French, German, or Dutch; to human studies; and to original publications. We also searched the reference lists of the retrieved publications.
Study selection. We included studies that specifically investigated PD or parkinsonism. We included cohort studies, case–control studies, and cross-sectional studies. No reviews, case reports, or conference abstracts were included. We excluded studies that summarized results of pesticide exposure only within a broad category of “chemical exposure.” Exposure to pesticides was defined as use of pesticides by the subject, thus excluding environmental studies.
Data extraction. Two reviewers (M.M., M.B.) independently extracted reported risk estimates [i.e., odds ratios (ORs), risk ratios (RRs), or prevalence ratios], study designs, exposure assessment methods, and types of source population for the controls. We also evaluated subcategories of pesticides and extracted data about exposure–response relations and individual pesticides. Two other researchers (A.H., R.V.) acted as referees in cases of any differences. If authors reported adjustment for potential confounders, we preferred adjusted risk estimates over crude risk estimates. In cases where no risk estimate or 95% confidence interval (CI) was reported, we calculated crude risk estimates and 95% CIs with the reported numbers. Where risk estimates were reported separately for men and women, we pooled the risk estimates with a within-study meta-analysis (Vlaanderen et al. 2011). Of studies with more than one control group, the results of population controls were preferred above the results of hospital controls because population controls are generally considered to be a more representative comparison group than hospital controls.
Statistical analysis. Because of the observed heterogeneity in study results, we conducted a DerSimonian and Laird (1986) random effects meta-analysis to pool the results of the separate studies for risk for pesticide exposure and the subgroups of herbicides, insecticides, and fungicides. We also stratified by whether or not nonoccupational exposure (e.g., gardening) was included in the exposed group. This was because of potential differences between occupational and nonoccupational exposures in intensity and frequency of exposures. In one publication, results both for occupational and for occupational and/or nonoccupational exposure were reported (Frigerio et al. 2006). We chose to include risk estimates of the more inclusive exposure definition, although final results did not differ when we included the risk estimates based on only occupational exposure (data not shown).
Subsequently, we explored whether heterogeneity in observed risk estimates could be explained by study and exposure assessment characteristics. We did so by stratification and used meta-regression to explore statistical significance of these characteristics. Given the limited number of studies, we only explored one characteristic at a time. Characteristics explored were the type of exposure assessment (self-reported ever/never pesticide exposure, self-reported regular pesticide exposure, or exposure assessment based on reported job titles by expert judgment and/or applying a job-exposure matrix), source of control population [hospital, general population, or other (studies using family members or case acquaintances as controls, or studies that used a combination of different sources)], geographical area (North America, Europe, or other), and whether adjustments were made for potential confounders. The I2 measure was used to quantify the heterogeneity between studies; I2 can be interpreted as a measure of the percentage of the total variation that cannot be explained by chance (Higgins et al. 2003). p-Values for heterogeneity are based on the Q-statistic. Small study effects were tested with funnel plots and Egger’s test (Egger et al. 1997). All analyses were performed with Stata (version 10; StataCorp, College Station, TX, USA) with the metan, metareg, metafunnel, and metabias commands. All statistical tests were two sided, and a p-value of < 0.05 was considered statistically significant.
Results
The search in Embase and Medline yielded 883 publications, of which 52 publications met the inclusion criteria. We excluded 3 publications (Fong et al. 2005; Menegon et al. 1998; Smargiassi et al. 1998) where the study population had been included in subsequent publications (De Palma et al. 1998; Fong et al. 2007; McCann et al. 1998). Lastly, one study (Taylor et al. 1999) was excluded because the reported data showed risk per year of pesticide exposure, which was not comparable with reported risk ratios of other studies. Among the remaining 48 publications, there were two studies for which the relevant results were reported in two separate publications each (Firestone et al. 2005, 2010; Semchuk et al. 1992, 1993). Thus, results of a total of 46 studies were used in the meta-analysis.
An overview of the study characteristics of the included studies can be found in Table 1. There were 39 case–control studies, 4 cohort studies, and 3 cross-sectional studies; 40 publications reported on pesticides, 15 on herbicides, 15 on insecticides, and 9 on fungicides. Three studies included all parkinsonism (Duzcan et al. 2003; Engel et al. 2001a; Tanner et al. 2009); the rest studied idiopathic PD. Four studies showed only results in men (Engel et al. 2001a; Fall et al. 1999; Petersen et al. 2008; Petrovitch et al. 2002). One study included only cases with a disease diagnosis before 51 years of age (Butterfield et al. 1993), which is much lower than the average age of disease onset in all other studies (generally ~ 60 years of age). Information about participation rates was provided for only 13 of the 39 case–control studies. Studies that reported participation rates had rates between 69% and 100% for cases and between 41% and 100% for controls.
Table 1.
Overview of the studies included in the meta-analyses.
| Study | Study design | Location | Cases | Controls | Exposure assessment | Adjustments | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ho et al. 1989 | CCo | Hong Kong | 35 PD patients Age range, 65–87 years | 105 age/sex matched | SR-E/N Occ/Non-Occ P | — | — | |||||||
| Koller et al. 1990 | CCh | USA | 150 PD patients Age range, 39–87 years Mean age, 66 years | 150 age/sex matched | SR-E/N Occ only P | — | OR calculated from reported numbers | |||||||
| Golbe et al. 1990 | CCo | USA | 106 PD patients No age information | 106 spouses | SR-R Occ/Non-Occ P | — | OR calculated from reported numbers | |||||||
| Zayed et al. 1990 | CCp | Canada | 42 PD patients No age information | 84 age/sex matched | SR-R Occ/Non-Occ P | Age, sex | — | |||||||
| Wong et al. 1991 | CCh | USA | 38 PD patients Mean age, 70 years | 38 age/sex matched | SR-E/N Occ/Non-Occ P | — | OR calculated from reported numbers | |||||||
| Stern et al. 1991 | CCo | USA | 80 PD patients, diagnosed after 60 years of age No age information | 80 age/sex/race/ participating center matched | SR-E/N Non-Occ only H, I | — | — | |||||||
| Jiménez-Jiménez et al. 1992 | CCh | Spain | 128 PD patients Mean age, 66.8 years | 256 age/sex matched | SR-R Occ/Non-Occ P | — | OR calculated from reported numbers | |||||||
| Semchuk et al. 1992, 1993 | CCp | Canada | 130 PD patients Age range, 36–97 years Mean age, 68.5 years Participation, 88% | 260 age/sex matched Participation, 76% | SR-E/N Occ only P, H, I, F | — | Herbicides OR adjusted for PD family history and head trauma | |||||||
| Hubble et al. 1993 | CCo | USA | 63 PD patients Mean age: urban patients, 69.3 years; rural patients, 69.0 years | 75 with similar mean age | SR-R Occ/Non-Occ P | Age < 65 years; male; lifestyle; ethnicity; family history; fresh produce consumption; history of head trauma, depression or CNS infection | — | |||||||
| Butterfield et al. 1993 | CCo | USA | 63 PD patients, diagnosed before 51 years of age Age range, 35–72 years Mean age, 49 years Participation, 69% | 68 age/sex/diagnosis year frequency matched Participation, 41% | SR-R Occ/Non-Occ H, I, F | Age, sex, race, age at diagnosis, education, family history | 95% CIs calculated from ORs and p-values F-OR is not adjusted | |||||||
| Morano et al. 1994 | CCh | Spain | 74 PD patients Mean age, 68.4 years | 148 age/sex matched | SR-R Occ/Non-Occ P | — | OR calculated from reported numbers | |||||||
| Hertzman et al. 1994 | CCp | Canada | 142 PD patients Mean age, 70.4 years | 124 controls 45–80 years of age Participation, 61% | SR-E/N Occ only P, H, I, F | — | Reported results were pooled for men and women A second control group consisting of hospital controls was not used in this meta-analysis | |||||||
| continued next page | ||||||||||||||
| Table 1. continued | ||||||||||||||
| Study | Study design | Location | Cases | Controls | Exposure assessment | Adjustments | Remarks | |||||||
| Chaturvedi et al. 1995 | CS | Canada | 87 PD patients No age information | 2,070 controls from cross-sectional study among elderly | SR-R Non-Occ only P | — | — | |||||||
| Seidler et al. 1996 | CCp | Germany | 379 PD patients < 66 years of age Mean age, 56.2 years Participation, 71% | 379 age/sex matched | SR-E/N Occ/Non-Occ H, I | Smoking, education | The reported results for exposure categories were pooled A second control group consisting of neighborhood was not used in this meta-analysis | |||||||
| Liou et al. 1997 | CCh | Taiwan | 120 PD patients Age range, 37–91 years Mean age, 63.1 years | 240 age/sex matched | SR-R Occ/Non-Occ P | — | — | |||||||
| De Palma et al. 1998 | CCh | Italy | 100 PD patients Mean age, 66.6 years | 200 controls, similar in age and sex | JT Occ/Non-Occ P | — | Substantial leisure activities were also classified for exposure | |||||||
| Chan et al. 1998 | CCh | Hong Kong | 215 PD patients Age < 60 years, 13.5% Age 60–69 years, 33.5% Age 70–79 years, 33.5% Age ≥ 80 years, 19.5% | 313 age/sex/hospital matched | SR-E/N Occ only P | Smoking, family history, rural living, well-water drinking, farming, consumption of tea, fruit vegetables and vitamins/liver oil supplements | Substantial difference between OR from unadjusted and adjusted analysis. Unadjusted OR = 1.80 (95% CI: 0.90, 3.58) | |||||||
| McCann et al. 1998 | CCo | Australia | 224 PD patients Mean age, 70.3 years | 310 age/sex/ ethnicity/residential area/site of collection matched | SR-R Occ only P | — | — | |||||||
| Gorell et al. 1998 | CCp | USA | 144 PD patients 50 years or older Age 50–59 years, 9.0% Age 60–69 years, 30.6% Age 70–79 years, 46.5% Age ≥ 80 years, 13.9% Participation, 81% | 464 ages/sex/race frequency matched Participation, 65% | SR-E/N Occ only, and Non-Occ only H, I, F | Age, sex, race, smoking | — | |||||||
| Werneck and Alvarenga 1999 | CCh | Brasil | 92 PD patients Age range, 55–78 years Mean age, 70.6 years | 110 age/sex matched | SR-R Occ/Non-Occ P | — | — | |||||||
| Fall et al. 1999 | CCp | Sweden | 113 PD patients Age range, 40–75 years Mean age, 63.9 years Participation, 90% | 263 from same age category Participation, 82% | SR-E/N Occ only P, I | Smoking, alcohol, coffee, and fried/ broiled meat consumption, carpenters, cabinetmakers | Only results for men are shown I-OR is not adjusted | |||||||
| Kuopio et al. 1999 | CCp | Finland | 123 PD patients Mean age, 69.3 years | 246 age/sex/ municipality matched Participation, 68% | SR-E/N Occ only H | — | The reported results for “pesticides” do not contain herbicides and are not included in this review | |||||||
| Preux et al. 2000 | CCh | France | 140 PD patients Mean age, 71.1 years | 280 age/sex matched | SR-E/N Occ/Non-Occ P | — | OR calculated from reported numbers | |||||||
| Herishanu et al. 2001 | CCh | Israel | 93 PD patients No age information | 93 age/sex matched | SR-E/N Occ/Non-Occ P | Smoking, birth country, peptic disease, work in construction or in mechanical factory | — | |||||||
| Engel et al. 2001a | CS | USA | 65 parkinsonism patients No age information | 310 of original 1,300 men who previously participated in a cohort study | SR-E/N Occ only P, H, I, F | Age, smoking | The study was among men only | |||||||
| Behari et al. 2001 | CCh | India | 377 PD patients Age range, 24–86 years Mean age, 56.8 years Participation, 100% | 377 age matched Participation, 100% | SR-E/N Occ/Non-Occ H, I | — | ORs calculated from reported numbers | |||||||
| Zorzon et al. 2002 | CCh | Italy | 136 PD patients Mean age, 70.0 years | 272 age/sex matched | SR-E/N Occ/Non-Occ P | Smoking | ||||||||
| Petrovitch et al. 2002 | Co | Hawaii | 99 PD patients after 30 years of follow-up Median age at diagnosis, 73.7 years Range, 54–89 years | Baseline, 7,986 Japanese men in Hawaii | SR-R Occ/Non-Occ P | — | RR calculated from reported incidence numbers | |||||||
| Duzcan et al. 2003 | CCp | Turkey | 36 parkinsonism patients, > 50 years of age Age 50–59 years, 11.1% Age 60–69 years, 30.6% Age 70–79 years, 47.2% Age ≥ 80 years, 11.1% | 108 age/sex matched | SR-R Occ/Non-Occ P | — | — | |||||||
| continued next page | ||||||||||||||
| Table 1. continued | ||||||||||||||
| Study | Study design | Location | Cases | Controls | Exposure assessment | Adjustments | Remarks | |||||||
| Baldereschi et al. 2003 | CS | Italy | 113 PD patients Mean age , 78.1 years | Study among 4,496 randomly selected elderly | SR-E/N Occ only P | Age, sex, education, smoking | Having a pesticide-use license was used as a proxy for pesticide use | |||||||
| Baldi et al. 2003a | CCp | France | 84 PD patients, > 69 years of age Mean age, 75.6 years | 252 age/sex matched | JT Occ only P | Age, sex, smoking, education | — | |||||||
| Baldi et al. 2003b | Co | France | 24 PD patients after 5-year follow-up No age information | Baseline, 1,507 persons who were ≥ 65 years of age in specific area | JT Occ only P | Smoking, education | Reported results for men and women were pooled | |||||||
| Nuti et al. 2004 | CCp | Italy | 190 PD patients Mean age, 63.9 years | 190 age/sex/ sociocultural factors matched | SR-E/N Occ/Non-Occ P | — | OR calculated from reported numbers | |||||||
| Frigerio et al. 2006 | CCp | USA | 149 PD patients Age range, 41–97 years Mean age, 70.0 years Participation, 76% | 129 age/sex matched Participation, 66% | SR-E/N Occ/Non-Occ P, H, I | Age, sex | Also results occupational only (farming) | |||||||
| Ascherio et al. 2006 | Co | USA | 413 PD patients after 9-year follow-up Mean onset age, 70 years | Baseline: 184,190 persons | SR-R Occ/Non-Occ P | Age, sex, smoking, coffee, NSAIDs, education, physical activity | — | |||||||
| Kamel et al. 2007 | Co | USA | 78 PD patients after 5-year follow-up Age ≤ 50 years, 9% Age 51–60 years, 40% Age 61–70 years, 41% Age > 70 years, 10% | Baseline: 84,738 persons (applicants for pesticide use certification and their spouses) | SR-E/N Occ/Non-Occ P | Age, state, applicator or spouse | — | |||||||
| Dick et al. 2007 | CCo | Scotland, Sweden, Romania, Italy, Malta | 767 PD patients Mean age, 69.8 years Participation, 77% | 1,989 age/sex/country frequency matched Participation, 59% | SR-E/N (+ JT) Occ/Non-Occ P | Age, sex, country, smoking, family history, ever knocked unconscious | — | |||||||
| Fong et al. 2007 | CCh | Taiwan | 153 PD patients Mean age, 71.7 years | 155 age/sex/ birthplace matched | SR-R Occ only P | Age, sex, smoking | — | |||||||
| Brighina et al. 2008 | CCo | USA | 833 PD patients, Age range, 32–91 years Median age, 67.7 years | 361 age/sex/region matched and 472 siblings | SR-R Occ/Non-Occ P, H, I, F | Age, sex | — | |||||||
| Hancock et al. 2008 | CCo | USA | 319 PD patients Age range, 29–94 years Mean age, 65.6 years | 296 relatives and spouses | SR-E/N Occ/Non-Occ P, H, I | Age, sex, smoking, caffeine consumption | — | |||||||
| Petersen et al. 2008 | CCp | Faroe islands | 79 PD patients Mean age, 74.4 years | 154 age/sex matched | SR-E/N Occ only P | Smoking | Only OR in men is shown because no exposed women in study | |||||||
| Elbaz et al. 2009 | CCp | France | 224 PD patients < 76 years of age Median age, 69.0 years Participation, 83% | 557 age/sex/region matched Participation, 75% | SR-E/N Occ only, and Non-Occ only P, H, I, F | Smoking, Mini Mental State Examination score | Reported I-OR, H-OR, and F-OR for men and women were pooled The OR for Non-Occ only is unadjusted | |||||||
| Tanner et al. 2009 | CCo | USA | 519 parkinsonism patients Age range, 30–88 years Median age, 65 years | 511 age/sex/ location frequency matched | SR-E/N Occ only P | Age, sex, ethnicity, smoking, alcohol, caffeine, head injury | — | |||||||
| Vlajinac et al. 2010 | CCh | Serbia | 110 PD patients Mean age, 60.8 years Participation, 100% | 220 age/sex/urban or rural living matched Participation, 100% | SR-E/N Occ/Non-Occ P, H, I, F | I-OR is adjusted for gardening, rural living, well and spring water drinking, dyes or naphtha exposure, service-sector work | OR, H-OR, and F-OR calculated from reported numbers | |||||||
| Firestone et al. 2005, 2010 | CCh | USA | 404 PD patients Age range, 29–88 years Median age, 69 years Participation, 70% | 526 age/sex frequency matched Participation, 60% | SR-E/N Occ only, and Non-Occ only P, H, I, F | Age, ethnicity, smoking | Reported results for all pesticides were pooled for men and women Only results for men are shown for the subgroups for Occ only | |||||||
| Manthripragada et al. 2010 | CCp | USA | 351 PD patients Age ≤ 60 years, 22% Age > 60 years, 78% | 363 controls from same region | SR-E/N (+ JT) Occ only P | Age, sex, ethnicity, smoking, education, county | — | |||||||
| Abbreviations: CCh, case–control study with hospital controls; CCo, case–control study with controls from other sources or a combination of sources; CCp, case–control study with population controls; CNS, central nervous system; Co, cohort study; CS, cross-sectional study; F, fungicides; H, herbicides; I, insecticides; JT, job titles; Non-Occ only, only nonoccupational exposure included in the exposed group; NSAIDs, nonsteroidal anti-inflammatory drugs; Occ only, only occupational exposure included in the exposed group; Occ/Non-Occ, nonoccupational exposure included in the exposed group; P, pesticides; SR-E/N, self-report ever/never; SR-R, self-report regular. | ||||||||||||||
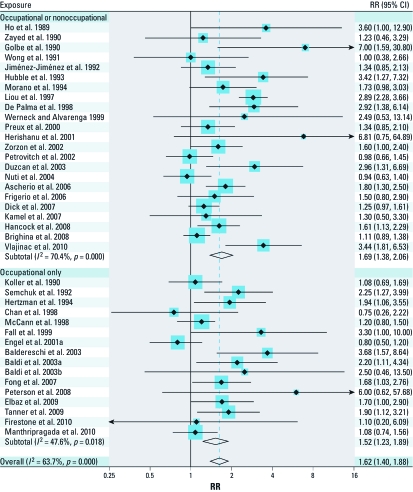
Figure 1 shows PD relative risk estimates for any pesticide exposure based on studies of occupational and/or nonoccupational exposures, and studies of occupational exposures only. The summary risk ratios (sRRs) between these two groups were very similar, with sRRs of 1.69 (95% CI: 1.38, 2.06) and 1.52 (95% CI: 1.23, 1.89), respectively, and an overall sRR for all studies combined of 1.62 (95% CI: 1.40, 1.88). The I2 for all studies combined was 63.7%. Only three studies estimated effects of nonoccupational exposure only (Chaturvedi et al. 1995; Elbaz et al. 2009; Firestone et al. 2005), with an sRR of 1.18 (95% CI: 0.86, 1.63).
Figure 1.
Forest plot for study-specific RRs and sRRs (95% CIs) of PD associated with the use of pesticides. The studies are ordered by publication year and stratified by studies that did or did not include nonoccupational exposure in the exposed group. Studies were pooled with the random effects method. The size of the squares reflects the statistical weight of the study in the meta-analyses.
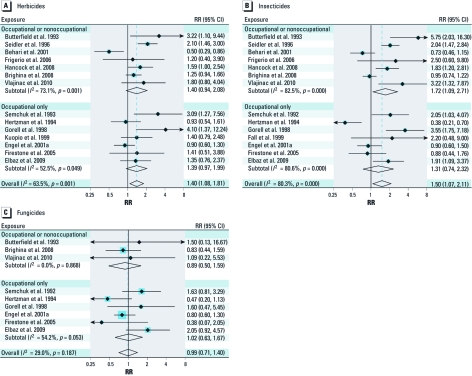
Meta-analyses by herbicide, insecticide, and fungicide exposure are shown in Figure 2. In line with the results for any pesticide exposure, we did not observe noticeable differences between studies of occupational exposures only and studies of nonocccupational and occupational exposures combined. The sRR for exposure to fungicides did not indicate an association with PD (overall sRR = 0.99; 95% CI: 0.71, 1.40; Figure 2C), in contrast with positive sRRs for exposure to herbicides (overall sRR = 1.40; 95% CI: 1.08, 1.81; Figure 2A) and insecticides (overall sRR = 1.50; 95% CI: 1.07, 2.11; Figure 2B).
Figure 2.
Forest plots for study-specific RRs and sRRs (95% CIs) of PD associated with the use of herbicides (A), insecticides (B), and fungicides (C). The studies are ordered by publication year and stratified by studies that did or did not include nonoccupational exposure in the exposed group. Studies were pooled with the random effects method. The size of the squares reflects the statistical weight of the study in the meta-analyses.
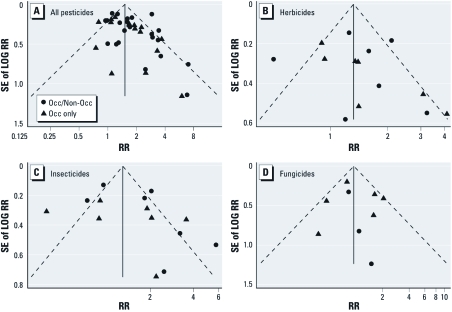
Funnel plots of effect estimates for exposure to pesticides and pesticide subcategories were suggestive of small study effects, with a tendency for smaller studies to report higher relative risks compared with larger studies (Figure 3), with Egger’s test p-values of 0.057, 0.338, 0.208, and 0.680 for pesticide, herbicide, insecticide, and fungicide effect estimates, respectively.
Figure 3.
Funnel plots of studies included in the meta-analysis for the risk of PD associated with the use of pesticides (A), herbicides (B), insecticides (C), and fungicides (D). Circles represent studies that included nonoccupational exposure in the exposed group, and triangles represent studies that were based on occupational exposure only. Egger’s test p-values were 0.057, 0.338, 0.208, and 0.680 for pesticide, herbicide, insecticide, and fungicide effect estimates, respectively.
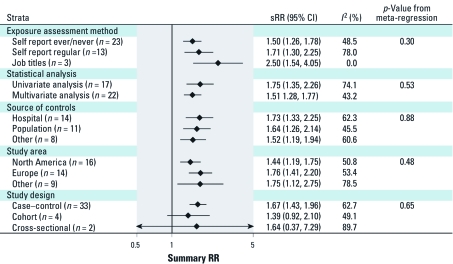
Figure 4 presents subgroup sRR estimates for those factors a priori hypothesized to be related to the observed heterogeneity in study results. The only study characteristic that was suggestive of contributing to heterogeneity was the exposure assessment method, with the lowest summary estimates observed for self-reported exposures (n = 36) and highest sRR for studies with exposures estimated based on reported job titles (n = 3). However, these differences were not statistically significant (p = 0.30). There was no evidence for a difference in summary estimates by adjustment of results for potential confounders, type of control population source, geographical area, or by study design. We also investigated whether adjustment for smoking had an effect on the summary risk estimate. Almost identical results were found for studies that did or did not correct for smoking (data not shown). Similar analyses for the subcategories herbicides and insecticides rendered similar results as for all pesticides (data not shown).
Figure 4.
sRRs (95% CIs) for strata of exposure assessment method, statistical analysis, source of controls, study area, and study design. The p-value from meta-regression represents the p-value of the F-test in case of more than two categories, whereas it represents the p-value for the t-test in the case of the two statistical analysis strata.
Discussion
Our systematic review indicated that PD is related to pesticide exposure with an sRR of 1.62 (95% CI: 1.40, 1.88). However, there was substantial heterogeneity among individual study estimates (I2 = 63.7%). Summary estimates also indicated positive associations of PD with herbicides and insecticides, but not with fungicides. We systematically investigated several factors that could explain heterogeneity in study results, but none appeared to be related to the observed heterogeneity, with the possible exception of the method of exposure assessment. Studies that based their exposure assessment on job titles reported somewhat higher risk estimates than studies that used self-reported exposures, but the difference did not reach statistical significance, in part because of low numbers of studies relying on job title and expert judgment.
Including persons who were nonoccupationally exposed to pesticides together with those occupationally exposed resulted in a very similar sRR. Given that occupational pesticide applications are in general more frequent and on larger areas than are nonoccupational exposures, one would have anticipated higher RRs for studies focusing only on occupational exposures. On the other hand, use of protective equipment during nonoccupational applications may be less. The fact that summary results were similar for both types of studies could indicate that nonoccupational and occupational pesticide exposures carry similar risks, or that most of the exposures in the combined studies were occupational. In the three studies that exclusively reported on nonoccupational pesticide exposures, only a small increase in relative risk was observed (sRR = 1.18; 95% CI: 0.86, 1.63), suggesting that risks associated with nonoccupational pesticide exposures are lower than from occupational exposures. Nevertheless, nonoccupational pesticide exposure cannot be ruled out as a risk factor for PD based on these analyses.
Studies used different methods for exposure assessment and assignment. Most studies (36 of 39) were based on self-reported exposure to pesticides, defined as ever versus never use or as regular versus nonregular use. No difference in sRR was seen between these two definitions of self-reported exposure, although it could have been expected that using a more stringent definition of exposure would have resulted in stronger associations. Studies that used reported job titles and expert judgment, and/or that used a job-exposure matrix to estimate exposures, resulted in a higher sRR compared with studies using self-reported pesticide exposures. This difference cannot be explained by recall bias, because in that case, higher risk ratios would have been expected for studies relying on self-reported exposures. A more likely explanation is that subjects are not able to reliably report exposures to pesticides, resulting in nondifferential exposure misclassification and bias toward the null (Daniels et al. 2001; Engel et al. 2001b). The fact that some heterogeneity is observed in study results by exposure assessment method indicates that this may be an important factor that should be taken into consideration when designing or interpreting studies.
A broad range of different pesticides exist with different chemical compositions and working mechanisms. In line with the conclusions of Brown et al. (2006), we found that both herbicides and insecticides, but not fungicides, were associated with PD. However, it is difficult to disentangle the effect of herbicides and insecticides given that the use of these two pesticide groups is often highly correlated. This is illustrated by the fact that we observed a correlation coefficient of 0.79 between the study-specific RRs of herbicides and insecticides. Few studies have focused on specific pesticides precluding any meaningful meta-analyses (Brighina et al. 2008; Elbaz et al. 2009; Engel et al. 2001a; Firestone et al. 2010; Hancock et al. 2008; Hertzman et al. 1994; Kamel et al. 2007; Liou et al. 1997; Seidler et al. 1996; Semchuk et al. 1992; Tanner et al. 2009; Vlajinac et al. 2010). However, it is interesting to note that the subgroup of organochlorines was significantly associated with PD in three studies (Elbaz et al. 2009; Hancock et al. 2008; Seidler et al. 1996). This is also in line with studies on biomarkers in serum (Richardson et al. 2009; Weisskopf et al. 2010) and in the brains of deceased patients (Corrigan et al. 2000; Fleming et al. 1994). Organochlorines are mainly insecticides, including DDT (dichlorodiphenyltrichloroethane), dieldrin, and heptachlor.
Funnel plots gave some indication for a small-study effect, such that larger effect estimates appeared to be associated with smaller studies, which suggests that the sRR might be slightly overestimated. In addition, the studies included were generally small, resulting in imprecise effect estimates that could have contributed to the substantial heterogeneity in study results. Meta-regression analyses provided no evidence for a difference in sRRs based on study design, geographical area, adjustment for potential confounders, or type of control population. As such, factors explaining the heterogeneity observed remain largely elusive. We were not able to investigate the effect of differences in criteria used for the diagnosis of PD because there was substantial variation in the exact inclusion criteria among the studies that reported on the criteria used. However, in most of the studies, the diagnosis was made by a physician and included the presence of two or three of the cardinal symptoms of PD, often with some additional inclusion and exclusion criteria. Variations in participation rates could also contribute to study heterogeneity. The ability to investigate this factor was limited because only 13 of the case–control studies reported participation rates. The same is true for differences across sex. Only 8 studies showed separate results for men and women, but the results were not conclusive: RRs were higher for men than for women in 3 studies (Baldi et al. 2003b; Frigerio et al. 2006; Hertzman et al. 1994), higher for women than men in 3 other studies (Chan et al. 1998; Elbaz et al. 2009; Firestone et al. 2010), and comparable between men and women in the remaining 2 studies (Ascherio et al. 2006; Brighina et al. 2008). Heterogeneity in the results could also arise from both quantitative and qualitative differences in types of agriculture in the study areas. Although we compared large regions (i.e., North America, Europe, and other), this analysis would not have captured regional differences in the types of agriculture and pesticides used. Analyses by time periods might provide some clues because pesticide use has changed over the decades, but data were insufficient to perform a meaningful analysis of changes over time.
Conclusion
Our overall summary risk estimates strongly suggest that exposure to pesticides, and to herbicides and/or insecticides in particular, increases the risk of developing PD. Heterogeneity among study-specific RRs could not easily be explained by methodological differences, except for a suggestive effect of exposure assessment characteristics. Future studies should therefore focus on using more objective semiquantitative methods for exposure assessment such as job- or crop-exposure matrices, rather than relying solely on self-report. Although classes of pesticides have been linked to PD, it remains important to identify the specific chemicals responsible for this association. Therefore, in new, preferably prospective studies, attention should be given to collecting detailed information on specific pesticide use.
Footnotes
This work was supported by the Internationaal Parkinson Fonds (the Netherlands).
The authors declare they have no actual or potential competing financial interests.
References
- Ascherio A, Chen H, Weisskopf MG, O’Reilly E, McCullough ML, Calle EE, et al. Pesticide exposure and risk for Parkinson’s disease. Ann Neurol. 2006;60(2):197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Baldereschi M, Di Carlo A, Vanni P, Ghetti A, Carbonin P, Amaducci L, et al. Lifestyle-related risk factors for Parkinson’s disease: a population-based study. Acta Neurol Scand. 2003;108(4):239–244. doi: 10.1034/j.1600-0404.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Baldi I, Cantagrel A, Lebailly P, Tison F, Dubroca B, Chrysostome V, et al. Association between Parkinson’s disease and exposure to pesticides in southwestern France. Neuroepidemiology. 2003a;22(5):305–310. doi: 10.1159/000071194. [DOI] [PubMed] [Google Scholar]
- Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003b;157(5):409–414. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- Behari M, Srivastava AK, Das RR, Pandey RM. Risk factors of Parkinson’s disease in Indian patients. J Neurol Sci. 2001;190(1–2):49–55. doi: 10.1016/s0022-510x(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Bekris LM, Mata IF, Zabetian CP.2010The genetics of Parkinson disease. J Geriatr Psychiatry Neurol 234228–242.; doi: 10.1177/0891988710383572[Online 11 October 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighina L, Frigerio R, Schneider NK, Lesnick TG, de Andrade M, Cunningham JM, et al. α-synuclein, pesticides, and Parkinson disease: a case-control study. Neurology. 2008;70(16 pt 2):1461–1469. doi: 10.1212/01.wnl.0000304049.31377.f2. [DOI] [PubMed] [Google Scholar]
- Brown TP, Rumsby PC, Capleton AC, Rushton L, Levy LS. Pesticides and Parkinson’s disease—is there a link? Environ Health Perspect. 2006;114:156–164. doi: 10.1289/ehp.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield PG, Valanis BG, Spencer PS, Lindeman CA, Nutt JG. Environmental antecedents of young-onset Parkinson’s disease. Neurology. 1993;43(6):1150–1158. doi: 10.1212/wnl.43.6.1150. [DOI] [PubMed] [Google Scholar]
- Chan DK, Woo J, Ho SC, Pang CP, Law LK, Ng PW, et al. Genetic and environmental risk factors for Parkinson’s disease in a Chinese population. J Neurol Neurosurg Psychiatry. 1998;65(5):781–784. doi: 10.1136/jnnp.65.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi S, Ostbye T, Stoessl AJ, Merskey H, Hachinski V. Environmental exposures in elderly Canadians with Parkinson’s disease. Can J Neurol Sci. 1995;22(3):232–234. doi: 10.1017/s0317167100039901. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson’s disease. J Toxicol Environ Health A. 2000;59(4):229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Daniels JL, Olshan AF, Teschke K, Hertz-Picciotto I, Savitz DA, Blatt J. Comparison of assessment methods for pesticide exposure in a case-control interview study. Am J Epidemiol. 2001;153(12):1227–1232. doi: 10.1093/aje/153.12.1227. [DOI] [PubMed] [Google Scholar]
- De Palma G, Mozzoni P, Mutti A, Calzetti S, Negrotti A. Case-control study of interactions between genetic and environmental factors in Parkinson’s disease. Lancet. 1998;352(9145):1986–1987. doi: 10.1016/s0140-6736(05)61332-3. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64(10):666–672. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzcan F, Zencir M, Ozdemir F, Cetin GO, Bagci H, Heutink P, et al. Familial influence on parkinsonism in a rural area of Turkey (kizilcaboluk-denizli): a community-based case-control study. Mov Disord. 2003;18(7):799–804. doi: 10.1002/mds.10440. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66(4):494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- Engel LS, Checkoway H, Keifer MC, Seixas NS, Longstreth WT, Jr, Scott KC, et al. Parkinsonism and occupational exposure to pesticides. Occup Environ Med. 2001a;58(9):582–589. doi: 10.1136/oem.58.9.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LS, Seixas NS, Keifer MC, Longstreth WT, Jr, Checkoway H.2001bValidity study of self-reported pesticide exposure among orchardists. J Expo Anal Environ Epidemiol 115359–368.; doi:. 10.1038/sj.jea.7500176 [DOI] [PubMed] [Google Scholar]
- Fall PA, Fredrikson M, Axelson O, Granerus AK. Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Mov Disord. 1999;14(1):28–37. doi: 10.1002/1531-8257(199901)14:1<28::aid-mds1007>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Firestone JA, Lundin JI, Powers KM, Smith-Weller T, Franklin GM, Swanson PD, et al. 2010Occupational factors and risk of Parkinson’s disease: a population-based case-control study. Am J Ind Med 533217–223.; doi: 10.1002/ajim.20788[Online 18 December 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone JA, Smith-Weller T, Franklin G, Swanson P, Longstreth WT, Jr, Checkoway H. Pesticides and risk of Parkinson disease: a population-based case-control study. Arch Neurol. 2005;62(1):91–95. doi: 10.1001/archneur.62.1.91. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR.1994Parkinson’s disease and brain levels of organochlorine pesticides. Ann Neurol 361100–103.; doi: 10.1002/ana.410360119[Online 8 October 2004] [DOI] [PubMed] [Google Scholar]
- Fong CS, Cheng CW, Wu RM. Pesticides exposure and genetic polymorphism of paraoxonase in the susceptibility of Parkinson’s disease. Acta Neurol Taiwan. 2005;14(2):55–60. [PubMed] [Google Scholar]
- Fong CS, Wu RM, Shieh JC, Chao YT, Fu YP, Kuao CL, et al. 2007Pesticide exposure on southwestern Taiwanese with MnSOD and NQO1 polymorphisms is associated with increased risk of Parkinson’s disease. Clin Chim Acta 3781–2136–141.; doi: 10.1016/j.cca.2006.11.006[Online 17 November 2006] [DOI] [PubMed] [Google Scholar]
- Frigerio R, Sanft KR, Grossardt BR, Peterson BJ, Elbaz A, Bower JH, et al. Chemical exposures and Parkinson’s disease: a population-based case-control study. Mov Disord. 2006;21(10):1688–1692. doi: 10.1002/mds.21009. [DOI] [PubMed] [Google Scholar]
- Golbe LI, Farrell TM, Davis PH. Follow-up study of early-life protective and risk factors in Parkinson’s disease. Mov Disord. 1990;5(1):66–70. doi: 10.1002/mds.870050116. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50(5):1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, et al. 2008Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol 86; doi: 10.1186/1471-2377-8-6[Online 28 March 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herishanu YO, Medvedovski M, Goldsmith JR, Kordysh E. A case-control study of Parkinson’s disease in urban population of southern Israel. Can J Neurol Sci. 2001;28(2):144–147. doi: 10.1017/s0317167100052835. [DOI] [PubMed] [Google Scholar]
- Hertzman C, Wiens M, Snow B, Kelly S, Calne D. A case-control study of Parkinson’s disease in a horticultural region of British Columbia. Mov Disord. 1994;9(1):69–75. doi: 10.1002/mds.870090111. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.2003Measuring inconsistency in meta-analyses. BMJ 3277414557–560.; doi: 10.1136/bmj.327.7414.557[Online 4 September 2003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SC, Woo J, Lee CM. Epidemiologic study of Parkinson’s disease in Hong Kong. Neurology. 1989;39(10):1314–1318. doi: 10.1212/wnl.39.10.1314. [DOI] [PubMed] [Google Scholar]
- Hubble JP, Cao T, Hassanein RE, Neuberger JS, Koller WC. Risk factors for Parkinson’s disease. Neurology. 1993;43(9):1693–1697. doi: 10.1212/wnl.43.9.1693. [DOI] [PubMed] [Google Scholar]
- Jiménez-Jiménez FJ, Mateo D, Gimenez-Roldan S. Exposure to well water and pesticides in Parkinson’s disease: a case-control study in the Madrid area. Mov Disord. 1992;7(2):149–152. doi: 10.1002/mds.870070209. [DOI] [PubMed] [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165(4):364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Koller W, Vetere-Overfield B, Gray C, Alexander C, Chin T, Dolezal J, et al. Environmental risk factors in Parkinson’s disease. Neurology. 1990;40(8):1218–1221. doi: 10.1212/wnl.40.8.1218. [DOI] [PubMed] [Google Scholar]
- Kuopio AM, Marttila RJ, Helenius H, Rinne UK. Environmental risk factors in Parkinson’s disease. Mov Disord. 1999;14(6):928–939. doi: 10.1002/1531-8257(199911)14:6<928::aid-mds1004>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Li AA, Mink PJ, McIntosh LJ, Teta MJ, Finley B. Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. J Occup Environ Med. 2005;47(10):1059–1087. doi: 10.1097/01.jom.0000174294.58575.3e. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, et al. Environmental risk factors and Parkinson’s disease: a case-control study in Taiwan. Neurology. 1997;48(6):1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Manthripragada AD, Costello S, Cockburn MG, Bronstein JM, Ritz B.2010Paraoxonase 1, agricultural organophosphate exposure, and Parkinson disease. Epidemiology 21187–94.; doi: 10.1097/EDE.0b013e3181c15ec6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SJ, LeCouteur DG, Green AC, Brayne C, Johnson AG, Chan D, et al. The epidemiology of Parkinson’s disease in an Australian population. Neuroepidemiology. 1998;17(6):310–317. doi: 10.1159/000026185. [DOI] [PubMed] [Google Scholar]
- Menegon A, Board PG, Blackburn AC, Mellick GD, Le Couteur DG. Parkinson’s disease, pesticides, and glutathione transferase polymorphisms. Lancet. 1998;352(9137):1344–1346. doi: 10.1016/s0140-6736(98)03453-9. [DOI] [PubMed] [Google Scholar]
- Morano A, Jiménez-Jiménez FJ, Molina JA, Antolin MA. Risk-factors for Parkinson’s disease: case-control study in the province of Caceres, Spain. Acta Neurol Scand. 1994;89(3):164–170. doi: 10.1111/j.1600-0404.1994.tb01655.x. [DOI] [PubMed] [Google Scholar]
- Nuti A, Ceravolo R, Dell’Agnello G, Gambaccini G, Bellini G, Kiferle L, et al. Environmental factors and Parkinson’s disease: a case-control study in the Tuscany region of Italy. Parkinsonism Relat Disord. 2004;10(8):481–485. doi: 10.1016/j.parkreldis.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, et al. 2008Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. Neurotoxicology 294584–590.; doi:. 10.1016/j.neuro.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Petrovitch H, Ross GW, Abbott RD, Sanderson WT, Sharp DS, Tanner CM, et al. Plantation work and risk of Parkinson disease in a population-based longitudinal study. Arch Neurol. 2002;59(11):1787–1792. doi: 10.1001/archneur.59.11.1787. [DOI] [PubMed] [Google Scholar]
- Preux PM, Condet A, Anglade C, Druet-Cabanac M, Debrock C, Macharia W, et al. Parkinson’s disease and environmental factors. Matched case-control study in the Limousin region, France. Neuroepidemiology. 2000;19(6):333–337. doi: 10.1159/000026273. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson’s disease and exposure to pesticides. Neurotoxicology. 2000;21(4):435–440. [PubMed] [Google Scholar]
- Richardson JR, Shalat SL, Buckley B, Winnik B, O’Suilleabhain P, Diaz-Arrastia R, et al. 2009Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol 667870–875.; doi:. 10.1001/archneurol.2009.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J, et al. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: a case-control study in Germany. Neurology. 1996;46(5):1275–1284. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42(7):1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease: a test of the multifactorial etiologic hypothesis. Neurology. 1993;43(6):1173–1180. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB.2011Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6193–222.; doi: 10.1146/annurev-pathol-011110-130242 [DOI] [PubMed] [Google Scholar]
- Smargiassi A, Mutti A, De Rosa A, De Palma G, Negrotti A, Calzetti S. A case-control study of occupational and environmental risk factors for Parkinson’s disease in the Emilia-Romagna region of Italy. Neurotoxicology. 1998;19(4–5):709–712. [PubMed] [Google Scholar]
- Stern M, Dulaney E, Gruber SB, Golbe L, Bergen M, Hurtig H, et al. The epidemiology of Parkinson’s disease. A case-control study of young-onset and old-onset patients. Arch Neurol. 1991;48(9):903–907. doi: 10.1001/archneur.1991.00530210029018. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Ross GW, Jewell SA, Hauser RA, Jankovic J, Factor SA, et al. Occupation and risk of parkinsonism: a multicenter case-control study. Arch Neurol. 2009;66(9):1106–1113. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Saint-Hilaire MH, Cupples LA, Thomas CA, Burchard AE, Feldman RG, et al. Environmental, medical, and family history risk factors for Parkinson’s disease: a New England-based case control study. Am J Med Genet. 1999;88(6):742–749. [PubMed] [Google Scholar]
- Vlaanderen J, Lan Q, Kromhout H, Rothman N, Vermeulen R. Occupational benzene exposure and the risk of lymphoma subtypes: a meta-analysis of cohort studies incorporating three study quality dimensions. Environ Health Perspect. 2011;119:159–167. doi: 10.1289/ehp.1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlajinac HD, Sipetic SB, Maksimovic JM, Marinkovic JM, Dzoljic ED, Ratkov IS, et al. 2010Environmental factors and Parkinson’s disease: a case-control study in Belgrade, Serbia. Int J Neurosci 1205361–367.; doi: 10.3109/00207451003668374 [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Knekt P, O’Reilly EJ, Lyytinen J, Reunanen A, Laden F, et al. 2010Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology 74131055–1061.; doi: 10.1212/WNL.0b013e3181d76a93[Online 30 March 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneck AL, Alvarenga H. Genetics, drugs and environmental factors in Parkinson’s disease. A case-control study. Arq Neuropsiquiatr. 1999;57(2B):347–355. doi: 10.1590/s0004-282x1999000300001. [DOI] [PubMed] [Google Scholar]
- Wong GF, Gray CS, Hassanein RS, Koller WC. Environmental risk factors in siblings with Parkinson’s disease. Arch Neurol. 1991;48(3):287–289. doi: 10.1001/archneur.1991.00530150055018. [DOI] [PubMed] [Google Scholar]
- Zayed J, Ducic S, Campanella G, Panisset JC, Andre P, Masson H, et al. Environmental factors in the etiology of Parkinson’s disease. Can J Neurol Sci. 1990;17(3):286–291. [in French] [PubMed] [Google Scholar]
- Zorzon M, Capus L, Pellegrino A, Cazzato G, Zivadinov R. Familial and environmental risk factors in Parkinson’s disease: a case-control study in north-east Italy. Acta Neurol Scand. 2002;105(2):77–82. doi: 10.1034/j.1600-0404.2002.1o040.x. [DOI] [PubMed] [Google Scholar]