Abstract
Background: Although serious health effects associated with particulate matter (PM) with aerodynamic diameter ≤ 10 μm (PM10) and ≤ 2.5 μm (PM2.5; fine fraction) are documented in many studies, the effects of coarse PM (PM2.5–10) are still under debate.
Objective: In this study, we estimated the effects of short-term exposure of PM2.5–10 on daily mortality in Stockholm, Sweden.
Method: We collected data on daily mortality for the years 2000 through 2008. Concentrations of PM10, PM2.5, ozone, and carbon monoxide were measured simultaneously in central Stockholm. We used additive Poisson regression models to examine the association between daily mortality and PM2.5–10 on the day of death and the day before. Effect estimates were adjusted for other pollutants (two-pollutant models) during different seasons.
Results: We estimated a 1.68% increase [95% confidence interval (CI): 0.20%, 3.15%] in daily mortality per 10-μg/m3 increase in PM2.5–10 (single-pollutant model). The association with PM2.5–10 was stronger for November through May, when road dust is most important (1.69% increase; 95% CI: 0.21%, 3.17%), compared with the rest of the year (1.31% increase; 95% CI: –2.08%, 4.70%), although the difference was not statistically significant. When adjusted for other pollutants, particularly PM2.5, the effect estimates per 10 μg/m3 for PM2.5–10 decreased slightly but were still higher than corresponding effect estimates for PM2.5.
Conclusions: Our analysis shows an increase in daily mortality associated with elevated urban background levels of PM2.5–10. Regulation of PM2.5–10 should be considered, along with actions to specifically reduce PM2.5–10 emissions, especially road dust suspension, in cities.
Keywords: coarse particles, health effects, mortality, PM2.5, PM10, road dust, short-term exposure
Particle effects on mortality. Hundreds of epidemiological studies have shown that the ambient particulate air pollution is associated with daily mortality, generally studied using the concentration of particulate matter (PM) with an aerodynamic diameter ≤ 10 μm (PM10) or fine PM with aerodynamic diameter of ≤ 2.5 μm (PM2.5) (Samoli et al. 2008). The effect of coarse PM (PM2.5–10) on mortality has been less studied. In their review article, Brunekreef and Forsberg (2005) concluded that most published mortality studies that applied two-pollutant models were unable to demonstrate independent PM2.5–10 effects on mortality after adjusting for PM2.5. However, PM2.5–10 levels are expected to be more spatially heterogeneous than are PM2.5 levels, which increase exposure misclassification when one or a few monitors provide exposure data (Monn 2001). Moreover, most time-series studies that have reported significant effects on mortality associated with PM2.5–10 were conducted in arid areas, including such places as Phoenix, Arizona (Mar et al. 2000), Coachella Valley, California (Ostro et al. 2000), and Mexico City (Castillejos et al. 2000). In arid areas, particle dust often originates from the surrounding land, not from local point sources, and particle levels are therefore expected to be more spatially homogeneous.
In a more recent study, Malig and Ostro (2009) used data from 15 counties in California and found an association between PM2.5–10 and daily mortality (both all-cause and cardiovascular mortality), particularly among demographic subgroups of lower socioeconomic status. In their study, only those participants who resided close to an air pollution monitor were included in the study in order to reduce exposure misclassification. Adjusting for PM2.5 had no effect on the effect estimates for PM2.5–10, likely due to its low correlation with PM2.5–10 in these California counties. An even larger study of U.S. cities found an association between PM2.5–10 and daily mortality that persisted after adjusting for PM2.5 (Zanobetti and Schwartz 2009). Recent studies from southern Europe have explored the effects of windblown Saharan dust, including a study conducted in Barcelona, Spain, that found evidence of an effect of PM2.5–10 on daily mortality during Saharan dust days, despite rather moderate particle concentrations (Perez et al. 2008).
European toxicological studies have indicated that PM2.5–10 has the same toxicological potential as PM2.5 on a mass basis (Gerlofs-Nijland et al. 2007; Sandström et al. 2005). It also has been suggested that particles of crustal origin are associated with markers of inflammation and acute toxicity in bioassays (Steerenberg et al. 2006). A cluster of European in vitro studies have shown that for mineral particles the composition and surface reactivity appeared to be most important for the proinflammatory potential of the particles (Schwarze et al. 2007).
PM2.5–10 sources and its importance for PM10. A directive from the European Union (EU; European Commission 2008) regulates the total mass of all PM10 irrespective of size, morphology, chemistry, and health effects. In the urban environment, different sources contribute differently to total PM10 because of variation in the size distribution of the emitted particles (Johansson et al. 2007). At roadside locations, most traffic exhaust particles are 10–30 nm in diameter, which is too small to result in a large aerosol mass, even when number concentrations are high (Gidhagen et al. 2004). Samples collected in Berlin showed that about 45% of local traffic contributions to roadside PM10 concentrations were due to suspended soil material, and the remaining traffic contribution was due to vehicle exhaust and tire abrasion (Lenschow et al. 2001). Likewise, about 50% of PM10 during summer months in Birmingham, United Kingdom, was due to PM2.5–10 (Harrison et al. 1997). In northern Europe, PM2.5–10 concentrations are generally elevated during winter and spring because of the use of studded tires, road salt, and traction sand. In Stockholm, road wear increases drastically because of the use of studded winter tires and traction sand on streets, such that up to 90% of the locally emitted PM10 during the winter may be due to road abrasion (Johansson et al. 2007). Suspension of road dust is a major contributor to PM2.5–10 and to the exceedances of the EU limit values for PM10 in Stockholm (Norman and Johansson 2006).
The aim of this study was to assess the effect of PM2.5–10 on daily mortality in Stockholm.
Material and Methods
Health data. This study of the greater Stockholm area (population ~ 1.3 million) was based on daily counts of deaths excluding external causes [International Classification of Diseases, 10th Revision (ICD-10) codes A00 through R99; World Health Organization 2007], for the years 2000 through 2008 from the Cause of Death Register at the Swedish National Board of Health and Welfare (Stockholm, Sweden).
Environmental data. Data on daily urban background concentrations of PM10, PM2.5, ozone (O3), and carbon monoxide (CO) were obtained from the Environment and Health Administration of Stockholm (2011). PM10, PM2.5, and O3 were measured in central Stockholm at Torkel Knutssonsgatan, a monitoring station located at rooftop level (at a height of 25 m) not directly affected by nearby emissions (Johansson et al. 2007). Measurements from the same monitoring station have been used to represent fluctuations in particle and O3 levels in Stockholm in previous studies, such as APHEA 2 (Air Pollution and Health: A European Approach; Gryparis et al. 2004; Le Tertre et al. 2002).
The mass concentrations of PM10 and PM2.5 were measured using tapered element oscillating microbalance (TEOM 14001; Thermo Fisher Scientific, East Greenbush, NY, USA). To account for losses of volatile material in the PM, all data were corrected following Areskoug (2007). Continuous measurement of O3 was based on its absorption of ultraviolet light (UV Absorption Ozone Analyzer, model 42M; Environnement S.A, Poissy, France). The urban background CO concentrations were based on continuous measurements of two rooftop stations (Hornsgatan and Sveavägen, both at a height of 25 m) in central Stockholm. The instruments were based on a nondispersive infrared technique (Carbon Monoxide Analyzer, model 48; Thermo Environmental Instrument Inc., Franklin, MA, USA). The coarse fraction of PM10 (PM2.5–10) is based on the difference between PM10 and PM2.5.
The contribution of road dust to the particle concentrations varies with the wetness of the road surfaces (Norman and Johansson 2006) and is not correlated with exhaust particles (Johansson et al. 2007). The number of days with high PM2.5–10 levels therefore depends on the meteorological conditions, especially during the late winter and spring. Therefore, we adjusted for meterological data that was collected from the Swedish Meterological and Hydrological Institute. Daily temperature and relative humidity were measured at Bromma Airport, a city airport, 9 km from Stockholm city center.
Statistical methods. We studied the association between daily mortality and PM2.5–10 concentrations averaged over the day of death and the day before death (lag01) with a time-series analysis. The exposure lag01 has been commonly used when effects of air pollution on mortality have been studied (Gryparis et al. 2004; Katsouyanni et al. 2001; Samoli et al. 2007). Time-series analysis allows estimation of relatively small acute effects in large study populations.
We applied additive Poisson regression models, controlling for long-term trend using a smooth function with eight degrees of freedom per year, and for day of the week and public holidays using indicator variables. We controlled for the effect of weather by adjusting for the current day’s temperature and relative humidity, together with smooth functions of mean temperature and relative humidity over the previous 2 days (each using six degrees of freedom). Influenza episodes were controlled by modeling the daily influenza hospital admissions as a smooth function. All influenza hospital admissions in Sweden were obtained from the Patient Register at the Swedish National Board of Health and Welfare (Stockholm, Sweden). Each of the smooth functions in the model was represented using penalized regression splines.
We modeled 24-hr average concentrations of PM2.5–10, PM2.5, and CO and the maximum of 8-hr moving-average (between 0600 hours and 2200 hours) concentrations of O3 on the same day and the previous day (lag01). Results are reported for single-pollutant models (adjusted for time trend, day of the week, public holidays, temperature, humidity, and influenza outbreaks) and for multipollutant models (including two pollutants in the same model, in addition to the covariates listed above). Results also are reported for a 10-μg/m3 increase as well as for an interquartile range (IQR) increase in each pollutant.
The analysis was stratified by period because the composition of particles varies seasonally. In Sweden, passenger cars, light-weight trucks, and light-weight buses are required to have winter tires from 1 December to 31 March. Heavy vehicles are not required to use winter tires. Winter tires can be studded or nonstudded, but studded winter tires were allowed from 1 October to 30 April during the study period 2000 through 2008 and were used by 70–75% of vehicles in Stockholm during those years. The share of studded winter tires usually increases from zero in September through October to its maximum in December through March and then falls back to zero in May (Norman and Johansson 2006), depending on weather and road conditions. Although studded tires are banned after 1 May, road dust remains elevated because of the accumulation of PM on the road surface during the winter (Ketzel et al. 2007; Norman and Johansson 2006). Therefore, we selected November through May as the period of interest for effects of studded tires (“road dust period,” which results in in high concentrations of PM2.5–10) and June through October as the reference season.
To test the hypothesis that PM2.5–10 may affect mortality with a longer lag than lag01, we also fitted a distributed lag model for up to 7 days (same day and up to 6 days earlier) for PM2.5–10.
We used p-values < 0.05 to define statistically significant effect estimates and p-values between 0.05 and 0.10 to define borderline significant effect estimates. Data were analyzed using the mgcv package in R (version 2.11.1; R Core Development Team 2010).
Results
There were 93,398 deaths (excluding deaths due to external causes) during the 2000 through 2008 study period (3,285 days). On average, there were 28.4 deaths per day (range, 12–52). The average number of deaths per day for the road dust period (November through May) was 29.6 (range, 13–52), and for the reference season (June through October) was 26.8 (range, 12–50). Days with missing values for environmental variables during the study period were not included, most often because of missing PM2.5 (260 days) and PM10 data (106 days) that did not allow calculation of PM2.5–10. In total, 2,789 days were included in the analysis. A statistical summary of the air pollution measurements for the whole period (2000 through 2008) is presented in Table 1.
Table 1.
Summary of daily air pollution and meteorological data.
| Variable | Season | Mean ± SD | IQR | Maximum | ||||
|---|---|---|---|---|---|---|---|---|
| PM10 (μg/m3) | Overall | 15.5 ± 9.6 | 9.4 | 95.2 | ||||
| Nov–May | 17.0 ± 10.8 | 11.9 | 95.2 | |||||
| Jun–Oct | 13.5 ± 7.0 | 6.4 | 67.0 | |||||
| PM2.5 (μg/m3) | Overall | 8.6 ± 5.7 | 4.9 | 46.2 | ||||
| Nov–May | 8.9 ± 6.1 | 5.3 | 46.2 | |||||
| Jun–Oct | 8.2 ± 5.0 | 4.5 | 43.0 | |||||
| PM2.5–10 (μg/m3) | Overall | 7.1 ± 6.4 | 5.4 | 61.9 | ||||
| Nov–May | 8.3 ± 7.8 | 8.0 | 61.9 | |||||
| Jun–Oct | 5.5 ± 3.2 | 3.5 | 36.7 | |||||
| PM2.5–10/PM10a | Overall | 0.42 ± 0.18 | 0.25 | 0.93 | ||||
| Nov–May | 0.44 ± 0.20 | 0.31 | 0.93 | |||||
| Jun–Oct | 0.40 ± 0.13 | 0.18 | 0.81 | |||||
| CO (μg/m3) | Overall | 281 ± 85 | 108 | 812 | ||||
| Nov–May | 300 ± 85 | 109 | 812 | |||||
| Jun–Oct | 254 ± 78 | 95 | 612 | |||||
| O3 (μg/m3) | Overall | 60.0 ± 22.4 | 31.7 | 142.0 | ||||
| Nov–May | 57.8 ± 24.0 | 35.9 | 142.0 | |||||
| Jun–Oct | 63.1 ± 19.5 | 26.0 | 126.6 | |||||
| Temperature (°C) | Overall | 7.7 ± 8.0 | 12.7 | 26.2 | ||||
| Nov–May | 2.8 ± 5.9 | 7.6 | 19.0 | |||||
| Jun–Oct | 14.4 ± 5.0 | 6.6 | 26.2 | |||||
| Relative humidity (%) | Overall | 0.75 ± 0.13 | 0.20 | 0.99 | ||||
| Nov–May | 0.77 ± 0.14 | 0.20 | 0.99 | |||||
| Jun–Oct | 0.73 ± 0.12 | 0.17 | 0.97 | |||||
| aFraction of PM10 that is PM2.5–10. | ||||||||
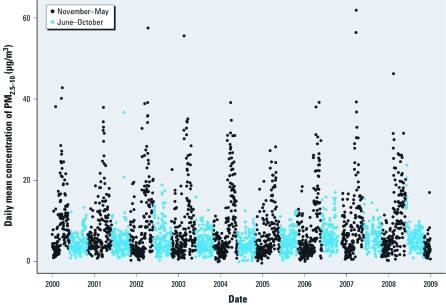
Figure 1 shows that the daily mean concentrations of PM2.5–10 are highest during late winter and spring. For PM2.5–10, 148 days with a daily mean concentration of 20 μg/m3 (95th percentile) or higher were observed within the road dust period, but only 4 days outside that season (Figure 1). On average, PM2.5–10 is somewhat less than half (42%) of the total PM10 concentration. PM2.5–10 contributes 44% on average in November through May and 40% in June through October (Table 1).
Figure 1.
Seasonal variation in PM2.5–10 concentrations in Stockholm, Sweden, over the study period, 2000 through 2008.
The correlations between PM2.5–10 and the other pollutants are given in Table 2. We found a 1.68% increase [95% confidence interval (CI): 0.20%, 3.15%; p = 0.026] in daily mortality per 10-μg/m3 increase in PM2.5–10 (lag01) based on the single-pollutant model (Table 3). Adjusting for O3, PM2.5, or CO resulted in only minor decreases in effect estimates for PM2.5–10, with borderline significance (corresponding p-values = 0.06, 0.10, and 0.05, respectively).
Table 2.
Correlation coefficients between variables in the study.
| Pollutant | Season | PM2.5 | PM2.5–10 | CO | O3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PM2.5 | Overall | |||||||||
| Nov–May | ||||||||||
| Jun–Oct | ||||||||||
| PM2.5–10 | Overall | 0.273 | ||||||||
| Nov–May | 0.229 | |||||||||
| Jun–Oct | 0.475 | |||||||||
| CO | Overall | 0.522 | 0.126 | |||||||
| Nov–May | 0.515 | 0.031 | ||||||||
| Jun–Oct | 0.546 | 0.239 | ||||||||
| O3 | Overall | 0.209 | 0.387 | –0.192 | ||||||
| Nov–May | 0.187 | 0.478 | –0.259 | |||||||
| Jun–Oct | 0.287 | 0.293 | –0.003 | |||||||
| Temperature | Overall | 0.050 | –0.003 | –0.257 | 0.435 | |||||
| Nov–May | 0.079 | 0.244 | –0.172 | 0.441 | ||||||
| Jun–Oct | 0.260 | 0.177 | 0.036 | 0.663 | ||||||
| Relative humidity | Overall | –0.006 | –0.418 | 0.278 | –0.729 | |||||
| Nov–May | –0.055 | –0.569 | 0.248 | –0.777 | ||||||
| Jun–Oct | 0.054 | –0.212 | 0.251 | –0.630 | ||||||
Table 3.
Mortality and PM2.5–10 association for lag01: overall estimates.
| Model type | Pollutant | Percent increase per 10 μg/m3 (95% CI) | Percent increase per IQR (95% CI)a | |||
| Single pollutant | ||||||
| PM2.5–10 | PM2.5–10 | 1.68 (0.20, 3.15) | 0.88 (0.11, 1.64) | |||
| PM2.5 | PM2.5 | 1.46 (0.07, 2.84) | 0.68 (0.03, 1.33) | |||
| Two pollutant | ||||||
| PM2.5–10 + PM2.5 | PM2.5–10 | 1.33 (–0.26, 2.92) | 0.69 (–0.13, 1.52) | |||
| PM2.5 | 0.90 (–0.62, 2.41) | 0.42 (–0.29, 1.13) | ||||
| PM2.5–10 + O3 | PM2.5–10 | 1.47 (–0.07, 3.00) | 0.77 (–0.04, 1.57) | |||
| O3 | 0.31 (–0.32, 0.93) | 0.94 (–0.98, 2.85) | ||||
| PM2.5–10 + CO | PM2.5–10 | 1.53 (–0.02, 3.09) | 0.80 (–0.01, 1.61) | |||
| CO | 0.04 (–0.09, 0.16) | 0.37 (–0.87, 1.60) | ||||
| All models adjusted for time trend, day of the week, public holidays, temperature, humidity, and influenza outbreaks. aIQR values: PM2.5–10, 5.2 μg/m3; PM2.5, 4.7 μg/m3; O3, 30.5 μg/m3; CO, 100 μg/m3. | ||||||
The effect estimate for a 10-μg/m3 increase in PM2.5–10 (1.33%; 95% CI: –0.26%, 2.92%; p = 0.10) was higher than the effect estimate for a 10-μg/m3 increase in PM2.5 (0.90%; 95% CI: –0.62%, 2.41%; p = 0.25) when both pollutants were included in the same model. In addition, the estimated percent change in daily mortality for an IQR increase was larger for PM2.5–10 (5.2 μg/m3) than for PM2.5 (4.7 μg/m3; Table 3).
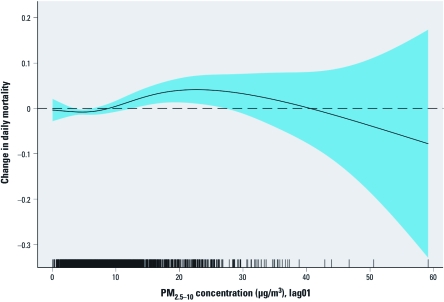
The smooth function of PM2.5–10 (lag01)from the single-pollutant model, adjusted for the covariates listed above (Figure 2), suggests that the more precisely estimated part of the curve does not deviate from linearity.
Figure 2.
The smooth function of the relationship between PM2.5–10 (lag01) and daily mortality from the single-pollutant model, adjusted for time trend, day of the week, public holidays, temperature, humidity, and influenza outbreaks. The shaded area represents 95% CI.
We estimated a 1.69% increase (95% CI: 0.21%, 3.17%; p = 0.025) in daily mortality per 10-μg/m3 increase in PM2.5–10 for the period November through May (Table 4). The effect estimate for the reference time period was lower (1.31%; 95% CI: –2.08%, 4.70%), but the difference between estimates was not statistically significant (p = 0.81).
Table 4.
Mortality and PM2.5–10 association for lag01: by season.
| Model type | Pollutant (season) | Percent increase per 10 μg/m3 (95% CI) | Percent increase per IQR (95% CI)a | |||
|---|---|---|---|---|---|---|
| Single pollutant | ||||||
| PM2.5–10 | PM2.5–10 (Nov–May) | 1.69 (0.21, 3.17) | 1.37 (0.17, 2.57) | |||
| PM2.5–10 (Jun–Oct) | 1.31 (–2.08, 4.70) | 0.41 (–0.65, 1.46) | ||||
| Two pollutant | ||||||
| PM2.5–10 + PM2.5 | PM2.5–10 (Nov–May) | 1.38 (–0.21, 2.97) | 1.12 (–0.17, 2.41) | |||
| PM2.5–10 (Jun–Oct) | –0.28 (–5.06, 4.50) | –0.09 (–1.57, 1.40) | ||||
| PM2.5 (Nov–May) | 0.72 (–0.92, 2.37) | 0.37 (–0.48, 1.22) | ||||
| PM2.5 (Jun–Oct) | 1.79 (–1.30, 4.88) | 0.75 (–0.55, 2.05) | ||||
| PM2.5–10 + O3 | PM2.5–10 (Nov–May) | 1.48 (–0.07, 3.02) | 1.20 (–0.05, 2.45) | |||
| PM2.5–10 (Jun–Oct) | 1.26 (–2.13, 4.65) | 0.39 (–0.66, 1.45) | ||||
| O3 | 0.30 (–0.33, 0.93) | 0.92 (–1.01, 2.85) | ||||
| PM2.5–10 + CO | PM2.5–10 (Nov–May) | 1.55 (–0.01, 3.11) | 1.26 (–0.01, 2.52) | |||
| PM2.5–10 (Jun–Oct) | 1.17 (–2.25, 4.59) | 0.37 (–0.70, 1.43) | ||||
| CO | 0.04 (–0.09, 0.16) | 0.36 (–0.87, 1.59) | ||||
| All models adjusted for time trend, day of the week, public holidays, temperature, humidity, and influenza outbreaks. aIQR values: PM2.5–10, 8.1 μg/m3 in November through May, 3.1 μg/m3 in June through October; PM2.5, 5.2 μg/m3 in November through May, 4.2 μg/m3 in June through October; O3, 30.5 μg/m3; CO, 100 μg/m3. | ||||||
After adjusting for other pollutants, there were only minor changes in the effect estimates for PM2.5–10 for the period November through May, and the magnitudes of the estimates were similar to the corresponding estimates for the whole year (Table 4).
Also, for the reference period June through October, changes in effect estimates were small after adjustments for CO and O3. However, no indication of an effect of PM2.5–10 was found when PM2.5 was adjusted for. The larger change in the estimated effect of PM2.5–10 may be explained by the larger effect estimate for PM2.5 and the higher correlation between PM2.5 and PM2.5–10 during the reference period (correlation coefficient r = 0.47) compared with the road dust period (r = 0.23; Table 2).
When we examined the distributed lag model with 6 lag days for PM2.5–10, we found the largest coefficient for a 1-day lag and little evidence of mortality effects at longer lags (Figure 3). The association between mortality and the sum of the distributed lag (1.12%; 95% CI: –0.83%, 3.11%) was somewhat lower than the results for lag01 (1.68%; 95% CI: 0.20%, 3.15%).
Figure 3.
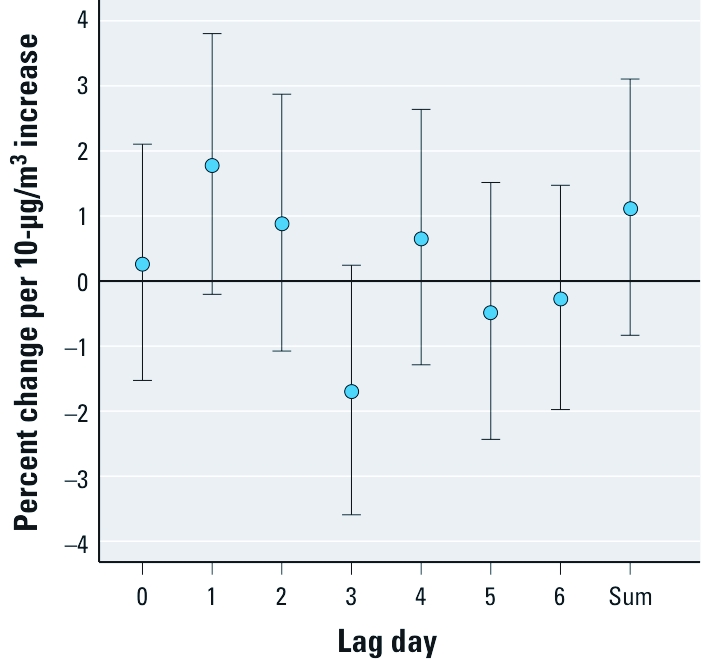
Percent change (95% CI) in daily mortality per 10-μg/m3 increase in PM2.5–10 for the 7-day distributed lag model.
Discussion
The daily mean concentrations of PM2.5–10 are highest during late winter and spring, presumably due to increased suspension of road dust particles during dry road conditions (Ketzel et al. 2007; Omstedt et al. 2005). This is mainly because of the wear of stone materials in the asphalt by studded winter tires (Hussein et al. 2008; Omstedt et al. 2005).
We estimated a 1.7% increase in daily mortality per 10-μg/m3 increase in lag01 PM2.5–10, both for the whole year and during November through May, the high road dust period. This is a larger estimated effect than typically reported for PM10, for example, 0.6% (95% CI: 0.4%, 0.8%) per 10-μg/m3 increase in PM10 in the European APHEA 2 study (Katsouyanni et al. 2001). The effect estimates for O3 and CO were similar to those reported for APHEA 2 (Gryparis et al. 2004; Samoli et al. 2007).
When data were split into two different time periods, the estimated effect associated with PM2.5–10 was higher during November through May, the road dust period, consistent with recent findings reported for PM2.5–10 and PM10 that indicated stronger associations during springtime (Zanobetti and Schwartz 2009; Zeka et al. 2006). Seasonal variation in associations could reflect greater indoor penetration during months when windows are open (Zeka et al. 2006) or seasonal variation in the composition of PM from different sources. In our case, the seasonal difference is not likely explained by indoor penetration, because associations were stronger during the winter, when windows are likely to be closed. However, the chemical composition is likely important, because PM produced during the winter period is dominated by stone minerals from road dust.
Detailed chemical analyses of sampled PM2.5–10 during that period have shown that the PM is dominated by quartzite, which was the most common stone mineral in the pavements in Stockholm (Furusjö et al. 2007; Sjödin et al. 2010).
We did not directly monitor PM2.5–10 but estimated its concentration as the difference between PM10 and PM2.5. This means that part of the variability in the concentration of PM2.5–10 is due to the measurement errors in both PM10 and PM2.5. Comparison with a gravimetric method has shown that the relative uncertainty of the determination of PM10 [according to the EU guidance for demonstrating equivalence of ambient air monitoring methods (European Commission Working Group on Particulate Matter 2002)] in Stockholm using the TEOM instrument is between 11% and 27% at the daily limit value (50 μg/m3). The uncertainty of PM2.5–10 has not been determined, but most of the uncertainty in PM10 is likely due to the correction for volatile PM, which is mainly present in PM2.5 fraction and not in the PM2.5–10 fraction.
A strength of the present study is that PM2.5–10 during the road dust period originates from the road network covering the whole city, not from a few point sources. Thus, in Stockholm, where the main contributor to PM2.5–10 is road traffic, the urban background daily mean concentrations in different parts of the city should fluctuate in a similar way.
The present study and other recent studies for which exposure can be expected to be spatially homogeneous support the hypothesis that there is a short-term effect on mortality, and it is at least as strong as typically found for PM10. Other recent studies also have estimated large effects on daily mortality of crustal PM2.5–10 (Malig and Ostro 2009; Perez et al. 2008; Zanobetti and Schwartz 2009).
There is support for cardiopulmonary effects of PM2.5–10 from recent human experimental studies. When 14 healthy young volunteers were exposed to concentrated ambient PM2.5–10 (2 hr; mean, 89 μg/m3) and filtered air in a single-blind, crossover study, exposure produced a mild pulmonary inflammation (Graff et al. 2009) 20 hr after exposure; a decrease in blood tissue plasminogen activator, which is involved in fibrinolysis; and a decrease in heart rate variability, which indicates an effect on the autonomic nervous system. Reduced heart rate variability is a prognostic marker for the development of cardiac arrhythmias (van Boven et al. 1998).
In a Swedish experimental study, Gustafsson et al. (2008) generated particles from the wear of studded tires on two pavements and traction sanding using a road simulator. A chemical analysis showed that the generated wear particles consisted almost entirely of minerals from the pavement stone material. It is well known that silica, which is part of this stone material, is capable of producing reactive oxygen species, either directly on the particle surface or by cells in response to exposure (Hamilton et al. 2008). In a recent study, Karlsson et al. (2011) examined the toxicoproteomic effects on human monocyte-derived macrophages after exposure to wear particles generated from the interface of studded tires and a granite-containing pavement. They showed that overall, proteins associated with inflammatory response were increased and proteins involved in cellular functions such as redox balance, antiinflammatory response, and glycolysis were decreased. Activation of the inflammatory pathway is one potential explanation for cardiopulmonary effects associated with exposure to mineral particles.
Road dust is an important traffic-related pollutant, because road wear particles contributes substantially to local particle emissions in cities. In cities where studded tires are used, road dust may cause violations of limit values for PM10. The effect of PM2.5–10 on mortality has been questioned because of many inconsistent findings when controlling for PM2.5 (Brunekreef and Forsberg 2005). This has influenced discussions on limit values and abatement strategies. Several recent studies (Malig and Ostro 2009; Perez et al. 2008; Samoli et al. 2011; Zanobetti and Schwartz 2009; Zauli Sajani et al. 2010) have, like the present one, produced evidence of a short-term effect of PM2.5–10 and crustal PM10 (not originating from combustion processes) on mortality. Results regarding the effect modification of Saharan dust days on a PM10–mortality relationship are inconsistent, despite positive associations, with negative effects and no interaction effects also reported. These inconsistent findings could reflect differences in the composition of other PM10 fractions, but also differences in correlations with other pollutants.
Conclusions
Given our results on road dust and other recent findings showing an impact of PM2.5–10 on daily mortality in studies of U.S. cities (Malig and Ostro 2009; Zanobetti and Schwartz 2009) and desert dust on daily mortality in Barcelona (Perez et al. 2008), it seems appropriate to separately regulate and control PM2.5–10. One must keep in mind that a large proportion of PM2.5 in many cities is transported over long distances and is difficult to avoid, whereas PM2.5–10, as in Stockholm, may be largely of local origin. Therefore, it also may be easier to improve health by reducing exposures to PM2.5–10.
Footnotes
This work was partly funded by the Stockholm County Council and the City of Stockholm.
The authors declare they have no actual or potential competing financial interest.
References
- Areskoug H. Stockholm: Stockholm University, Department of Applied Environmental Science; 2007. Bestämming av PM10—En jämförelse av de vanligaste mätmetoderna använda i Sverige och den europeiska referensmetoden. ITM-rapport 168 [in Swedish] [Google Scholar]
- Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. 2005;26:309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- Castillejos M, Borja-Aburto VH, Dockery DW, Gold DR, Loomis D. Airborne coarse particles and mortality. Inhal Toxicol. 2000;12:61–72. [Google Scholar]
- European Commission. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. 2008. Available: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:152:0001:01:EN:HTML [accessed 5 February 2012]
- European Commission Working Group on Particulate Matter. A Report on Guidance to Member States on PM10 Monitoring and Intercomparisons with the Reference Method. 2002. Available: http://www.ec.europa.eu/environment/air/pdf/finalwgreporten.pdf [accessed 16 August 2011]
- Environment and Health Administration, Stockholm, Sweden. Stockholm—Uppsala County Air Quality Management Association monitors regions air quality. 2011. Available: http://www.slb.nu/elvf [accessed 5 February 2012]
- Furusjö E, Sternbeck J, Cousins AP. PM10 source characterization at urban and highway roadside locations. Sci Total Environ. 2007;387:206–219. doi: 10.1016/j.scitotenv.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, Dormans JA, Bloemen HJ, Leseman DLAC, John A, Boere F, et al. Toxicity of coarse and fine particulate matter from sites with contrasting traffic profiles. Inhal Toxicol. 2007;19:1055–1069. doi: 10.1080/08958370701626261. [DOI] [PubMed] [Google Scholar]
- Gidhagen L, Johansson C, Langner J, Olivares G. Simulation of NOx and ultrafine particles in a street canyon in Stockholm, Sweden. Atmos Environ. 2004;38:2029–2044. doi: 10.1021/es0498134. [DOI] [PubMed] [Google Scholar]
- Graff DW, Cascio WE, Rappold A, Zhou H, Huang YC, Devlin RB. Exposure to concentrated coarse air pollution particles causes mild cardiopulmonary effects in healthy young adults. Environ Health Perspect. 2009;117:1089–1094. doi: 10.1289/ehp0900558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, et al. Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am J Respir Crit Care Med. 2004;170:1080–1087. doi: 10.1164/rccm.200403-333OC. [DOI] [PubMed] [Google Scholar]
- Gustafsson M, Blomqvist G, Gudmundsson A, Dahl A, Swietlicki E, Bohgard M, et al. Properties and toxicological effects of particles from the interaction between tyres, road pavement, and winter traction material. Sci Total Environ. 2008;393:226–240. doi: 10.1016/j.scitotenv.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44:1246–1258. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RM, Deacon AR, Jones MR. Sources and processes affecting concentrations of PM10 and PM2.5 particulate matter in Birmingham (U.K.). Atmos Environ. 1997;31:4103–4117. [Google Scholar]
- Hussein T, Johansson C, Karlsson H, Hansson H-C. Factors affecting nontailpipe aerosol particle emissions from paved roads: on-road measurements in Stockholm, Sweden. Atmos Environ. 2008;42:688–702. [Google Scholar]
- Johansson C, Norman M, Gidhagen L. Spatial and temporal variations of PM10 and particle number concentrations in urban air. Environ Monit Assess. 2007;127:477–487. doi: 10.1007/s10661-006-9296-4. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Lindbom J, Ghafouri B, Lindahl M, Tagesson C, Gustafsson M, et al. Wear particles from studded tires and granite pavement induce proinflammatory alterations in human monocyte-derived macrophages: a proteomic study. Chem Res Toxicol. 2011;24:45–53. doi: 10.1021/tx100281f. [DOI] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Le Tertre A, Monopolis Y, et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12:521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Ketzel M, Omstedt G, Johansson C, Düring I, Pohjola M, Oettl D, et al. Estimation and validation of PM2.5/PM10 exhaust and nonexhaust emission factors for practical street pollution modelling. Atmos Environ. 2007;41:9370–9385. [Google Scholar]
- Lenschow P, Abraham H-J, Kutzner K, Lutz M, Preuß J-D, Reichenbächer W. Some ideas about the sources of PM10. Atmos Environ. 2001;35(suppl 1):S23–S33. [Google Scholar]
- Le Tertre A, Medina S, Samoli E, Forsberg B, Michelozzi P, Boumghar A, et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Commun Health. 2002;56:773–779. doi: 10.1136/jech.56.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malig BJ, Ostro B. Coarse particles and mortality: evidence from a multicity study in California. Occup Environ Med. 2009;66:832–839. doi: 10.1136/oem.2008.045393. [DOI] [PubMed] [Google Scholar]
- Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn C. Exposure assessment of air pollutants: a review on spatial heterogeneity and indoor/outdoor/personal exposure to suspended particulate matter, nitrogen dioxide and ozone. Atmos Environ. 2001;35:1–32. [Google Scholar]
- Norman M, Johansson C. Studies of some measures to reduce road dust emissions from paved roads in Scandinavia. Atmos Environ. 2006;40:6154–6164. [Google Scholar]
- Omstedt G, Bringfelt B, Johansson C. A model for vehicle- induced nontailpipe emissions of particles along Swedish roads. Atmos Environ. 2005;39:6088–6097. [Google Scholar]
- Ostro BD, Broadwin R, Lipsett MJ. Coarse and fine particles and daily mortality in the Coachella Valley, California: a follow-up study. J Expo Anal Environ Epidemiol. 2000;10:412–419. doi: 10.1038/sj.jea.7500094. [DOI] [PubMed] [Google Scholar]
- Perez L, Tobias A, Querol X, Künzli N, Pey J, Alastuey A, et al. Coarse particles from Saharan dust and daily mortality. Epidemiology. 2008;19:800–807. doi: 10.1097/ede.0b013e31818131cf. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. The R Project for Statistical Computing. Vienna, Austria:R Foundation for Statistical Computing. 2010. Available: http://www.r-project.org/ [accessed 14 October 2010]
- Samoli E, Kougea E, Kassomenos P, Analitis A, Katsouyanni K. Does the presence of desert dust modify the effect of PM10 on mortality in Athens, Greece? Sci Total Environ. 2011;409:2049–2054. doi: 10.1016/j.scitotenv.2011.02.031. [DOI] [PubMed] [Google Scholar]
- Samoli E, Peng R, Ramsay T, Pipikou M, Touloumi G, Dominici F, et al. Acute effects of ambient particulate matter on mortality in Europe and North America: results from the APHENA study. Environ Health Perspect. 2008;116:1480–1486. doi: 10.1289/ehp.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoli E, Touloumi G, Schwartz J, Anderson HR, Schindler C, Forsberg B, et al. Short-term effects of carbon monoxide on mortality: an analysis within the APHEA project. Environ Health Perspect. 2007;115:1578–1583. doi: 10.1289/ehp.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström T, Cassee FR, Salonen R, Dybing E. Recent outcomes in European multicentre projects on ambient particulate air pollution. Toxicol Appl Pharmacol. 2005;207(2) suppl:261–268. doi: 10.1016/j.taap.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Schwarze PE, Øvrevik J, Hetland RB, Becher R, Cassee FR, Låg M, et al. Importance of size and composition of particles for effects on cells in vitro. Inhal Toxicol. 2007;19(suppl 1):17–22. doi: 10.1080/08958370701490445. [DOI] [PubMed] [Google Scholar]
- Sjödin Å, Ferm M, Björk A, Rahmberg M, Gudmundsson A, Swietlicki E, et al. Gothenburg, Sweden: Swedish Environmental Research Institute; 2010. WEAREM. Wear Particles from Road Traffic—a Field, Laboratory and Modeling Study. IVL Report B1830. [Google Scholar]
- Steerenberg PA, van Amelsvoort L, Lovik M, Hetland RB, Alberg T, Halatek T, et al. Relation between sources of particulate air pollution and biological effect parameters in samples from four European cities: an exploratory study. Inhal Toxicol. 2006;18:333–346. doi: 10.1080/08958370500515913. [DOI] [PubMed] [Google Scholar]
- van Boven AJ, Jukema JW, Haaksma J, Zwinderman AH, Crijns HJ, Lie KI. Depressed heart rate variability is associated with events in patients with stable coronary artery disease and preserved left ventricular function. Am Heart J. 1998;135:571–576. doi: 10.1016/s0002-8703(98)70269-8. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2007. International Statistical Classification of Diseases and Health Related Problems. 10th Revision. Available: http://www.who.int/classifications/icd/en/ [accessed 5 February 2012]
- Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environ Health Perspect. 2009;117:898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauli Sajani S, Miglio R, Bonasoni P, Cristofanelli P, Marinoni A, Sartini C, et al. Saharan dust and daily mortality in Emilia-Romagna (Italy). Occup Environ Med. 2010;68:446–451. doi: 10.1136/oem.2010.058156. [DOI] [PubMed] [Google Scholar]
- Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163:849–859. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]