Abstract
We investigated the effects of chronic compression (CCD) of the L3 and L4 dorsal root ganglion (DRG) on pain behavior in the mouse and on the electrophysiological properties of the small-diameter neuronal cell bodies in the intact ganglion. CCD is a model of human radicular pain produced by intraforaminal stenosis and other disorders affecting the DRG, spinal nerve, or root. On days 1, 3, 5, and 7 after the onset of compression, there was a significant decrease from preoperative values in the threshold mechanical force required to elicit a withdrawal of the foot ipsilateral to the CCD (tactile allodynia). Whole cell patch-clamp recordings were obtained, in vitro, from small-sized somata and, for the first time, in the intact DRG. Under current clamp, CCD neurons exhibited a significantly lower rheobase compared with controls. A few CCD but no control neurons exhibited spontaneous action potentials. CCD neurons showed an increase in the density of TTX-resistant and TTX-sensitive Na+ current. CCD neurons also exhibited an enhanced density of voltage-dependent K+ current, due to an increase in delayed rectifier K+ current, without a change in the transient or “A” current. We conclude that CCD in the mouse produces a model of radicular pain, as we have previously demonstrated in the rat. While the role of enhanced K+ current remains to be elucidated, we speculate that it represents a compensatory neuronal response to reduce ectopic or aberrant levels of neuronal activity produced by the injury.
Keywords: dorsal root ganglion compression, neuropathic pain, ion channels, whole cell recordings, mouse
a chronic compression (CCD) of the dorsal root ganglion (DRG) in the rat is a model of human radicular pain. Such pain may accompany an intraforaminal stenosis, a laterally herniated disk, and other disorders that affect the functional properties of the DRG, spinal nerve, or root. Previously, we have demonstrated that CCD in the rat produces ipsilateral pain behavior and tactile allodynia that is associated with an hyperexcitability at the cell bodies (somata) of DRG neurons, including those of small diameter, that are putatively nociceptive (Zhang et al. 1999). However, the biophysical basis for the hyperexcitability of small diameter neurons has not been identified.
Previous patch-clamp electrophysiological studies of hyperexcitability used acutely dissociated neurons in culture. The results obtained are difficult to interpret because dissociation, in itself, produces hyperexcitability in the absence of CCD (Ma and LaMotte 2005; Zheng et al. 2007). Thus, a major goal of the present study was to determine whether CCD alters voltage-gated Na+ and K+ currents in small-diameter neurons and to do so in the intact DRG. For this purpose, the mouse was used, and the CCD model was validated for the mouse, which then allows for future studies using transgenic animals.
METHODS
Behavioral testing.
Ten male C57BL6 mice (Charles River, Wilmington, MA) were used, each weighing between 21 and 30 g. For each mouse, the incidence of hindpaw withdrawal was measured in response to punctate mechanical stimuli before and after CCD surgery. Animals were placed in a transparent plastic chamber (7 × 7 × 4 cm) placed over a metal mesh wire mounted on a raised plastic platform. To habituate the animals to the experimental conditions, the mice were handled and placed in the experimental chambers, and their hindpaws were periodically stimulated by randomly selected Von Frey filaments. This was carried out once a day for 1 wk before the onset of behavioral data collection and subsequent CCD surgery.
Behavioral testing and data collection began 1 day before surgery. Before testing began each day, the animal was allowed to acclimatize to its surroundings for 30 min. Six modified Von Frey filaments were then used to deliver bending forces of 2, 4, 6, 8, 16, and 20 mN. Each filament was fitted with a metal cylinder with a flat tip and a diameter of 0.2 mm (Song et al. 1999; Zhang et al. 1999).Each filament was delivered from underneath the metal mesh to an area located on the plantar surface of the hindpaw between the heel and the walking pads. The weakest filament was delivered alternately to one paw and then to the other until each paw had received a total of five indentations. The procedure was repeated for the remaining four filaments tested in an ascending order. The duration of each indentation was 1 s, and the interval between stimulating one paw and then the other was 45–60 s. For each foot, the incidence of the foot withdrawal to each stimulus was expressed as a percentage of the five applications as a function of force. All 10 animals were tested on the day before CCD surgery (“pre-CCD”) and on postoperative days 1, 3, 5, and 7 after the surgery.
The percentage of foot withdrawal responses as a function of force was fitted by a Hill equation (Origin 8.1, Origin Lab). The withdrawal threshold was defined as the interpolated force eliciting a withdrawal incidence of 50%. The mechanical threshold values were statistically analyzed separately for each foot. For a given foot, thresholds obtained before and on different test days after CCD were analyzed with one-way ANOVA with repeated measures. Tukey's post hoc test determined the significance of the difference over the days of testing. Data are presented as means ± SE, and the criterion for significance was set at P < 0.05.
CCD surgery.
The mouse was anesthetized with isoflurane inhalation anesthesia. On the right side, the paraspinal muscles were separated from the mammillary process and the transverse process, and the intervertebral foramina of L4 and L3 were exposed. A fine, sharp, stainless steel needle, 0.3 mm in diameter with a right angle to limit penetration, was inserted ∼2 mm into the intervetebral foramen at L4 and again at L3 at a rostral direction at an angle of 30–40° with respect to the dorsal middle line and from −10 to −15° below the vertebral horizontal (Song et al. 1999; Hu and Xing 1998). Once the needle was withdrawn, a stainless steel rod, L-shaped, 2 mm in length and 0.3 mm in diameter, was implanted into each foramen, one at L4 and the other at the L3 ganglion. The incision was closed in layers. Another group of mice received no surgery and was used as the control group. All animals were in good health after the surgery and demonstrated no change in their posture or gait. Not withstanding conceivable interspecies differences, sham surgeries were not performed as these have previously been shown, in the rat, to have little or no effect on pain behavior or on the electrophysiological properties of cell bodies in the DRG (Song et al. 1999; Zhang et al. 1999).
All protocols were approved by the Institutional Animal Care and Use Committee of Yale University and were in accordance with guidelines provided by the National Institutes of Health and the International Association for the Study of Pain.
DRG preparation.
Control mice or CCD-treated mice on postoperative days 7–10 were anesthetized with pentobarbital sodium [Nembutal (50 mg/kg ip)], and the L4 and L3 lumbar DRGs were dissected free along with their corresponding dorsal roots, spinal nerves, and sciatic nerve. DRGs were placed in oxygenated artificial cerebrospinal fluid (ACSF), which consisted of (in mM) 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 10 dextrose, 1.2 MgCl2, and 1.2 CaCl2 (pH 7.3). Under a dissecting microscope, the epineurium and perineurium that form the capsule of each ganglion were removed with fine forceps and sharp scissors, and DRGs were placed for 30 min at 37°C in a 10-ml vial filled with ACSF with collagenase P (1 mg/ml, Roche, Mannheim, Germany) and protease XIV (0.2 mg/ml, Sigma Aldrich, St. Louis, MO) (Zhang et al. 1998) to loosen connective tissue and facilitate removal of the ganglionic sheath.
Electrophysiological recordings.
Fire-polished electrodes (1–2 MΩ) were fabricated from 1.2-mm borosilicate capillary glass (Sutter Instruments, Novato, CA) using a P-97 puller (Sutter Instruments). A single DRG was placed in a recording chamber that was mounted on the stage of an inverted microscope (Nikon Diaphot). The ganglion was fixed to the bottom of the chamber by gentle pressure applied by a pipette held by a manipulator (Hu and Xing 1998; Zhang et al. 1998). The DRG was continuously superfused at 2 ml/min with oxygenated ACSF. Although a suction electrode at the end of the nerve was originally intended to be used to electrically evoke action potentials (APs) for the purpose of obtaining conduction velocity, in most experiments the length of the nerve was too short (perhaps this problem could be remedied in the future by carrying out a more extensive dissection of the tissue in the animal surrounding the more distal portion of the nerve). Neurons were recorded at room temperature using a patch-clamp amplifier (HEKA EPC 10) under computer control (HEKA Patchmaster). An extacellular recording electrode (electrolyte-filled micropipette) was used to apply suction to a neuronal soma of interest. The suction was used either to pull off non-neuronal cells (e.g., satellite glia) to provide access of a patch pipette to the neuronal membrane or to remove a neuron on the surface to gain access to one located deeper within the ganglion.
For a given neuron, ∼20 s of extracellular recording was obtained, during which time the occurrence of any spontaneous AP discharges would define the neuron as “spontaneously active.” This electrode was then removed, a patch pipette was brought in place to obtain whole cell recordings, and the bath perfusion solution was switched accordingly for recording K+ or Na+ currents. Recordings were obtained from small-sized neurons with diameters of 18–25 μm. The capacitance artifact was canceled using computer-controlled circuitry of the patch-clamp amplifier. Linear leak subtraction was used for all voltage-clamp recordings. The liquid junction potential for recording Na+ current was 11.2 mV. Data were not corrected to account for liquid junction potential.
Excitability measurements were made under current-clamp conditions followed by voltage-clamp measurements of voltage-gated K+ currents using the same internal solution. Neurons that had a stable membrane potential that was more negative than −40 mV were accepted for further study. Resting membrane potential was measured 1 min after a stable recording was obtained. Current pulses from −0.4 to 0.35 nA (1,000-ms pulse duration) were delivered in increments of 0.05 nA until one or more APs were evoked. The rheobase was defined as the minimum current required to elicit an AP. AP voltage threshold was defined as the first point on the rising phase of an AP where the rising rate exceeded 50 mV/ms. The AP amplitude was measured between the peak and AP threshold level. The input resistance for each cell was obtained from the slope of a steady state current-voltage plot in response to a series of hyperpolarizing currents from −0.2 to −0.05 nA in increments of 0.05 nA. After measurement in the current-clamp configuration, the amplifier was switched to the whole cell, voltage-clamp mode to measure K+ currents. Patch-clamp recordings were filtered at 10 kHz and digitized at 50 kHz.
Solutions for electrophysiological recording.
For recordings under current clamp, the bath solution contained (in mM) 145 NaCl, 3 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.4 with NaOH, and the osmolarity was adjusted to 300–310 mOsm. The pipette solution contained (in mM) 100 K-aspartate, 40 KCl, 10 HEPES, 5 EGTA, 1 MgCl2, and 1 CaCl2.
The same pipette solution was used when recording K+ current, but the bath solution contained (in mM) 140 choline Cl, 3 KCl, 1 CaCl2, 1 MgCl2, 0.1 CdCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.4 with Tris base, and the osmolarity was adjusted to 300–310 mOsm.
Na+ currents were measured starting ∼3 min after a stable recording had been achieved. The bath solution contained (in mM) 35 NaCl, 10 HEPES, 105 choline-Cl, 10 glucose, 1 CaCl2, 1 MgCl2, 0.1 CdCl2, and 20 TEA-Cl. The pH was adjusted to 7.4 with NaOH. The pipette solution contained (in mM) 140 CsF, 10 NaCl, 2 MgCl2, 0.1 CaCl2, 1 EGTA, and 10 HEPES, and the pH was adjusted to 7.2 with CsOH.
Voltage protocols for measuring Na+ and K+ currents.
A voltage protocol was used to obtain separate current-voltage relationships for both TTX-sensitive (TTX-S) and TTX-resistant (TTX-R) Na+ currents in the absence of TTX (Cummins and Waxman 1997; Huang and Song 2008; Lopez-Santiago et al. 2006). The total Na+ current was obtained by using a series of 500-ms prepulses to −100 mV, each followed by a test pulse of 80-ms duration, incremented each time by 10 mV from −100 to +50 mV. The interpulse interval was 1 s. The TTX-R component was recorded using the same protocol except with a prepulse to −50 mV, which inactivated most of the TTX-S current. The TTX-S component was then obtained by digitally subtracting the TTX-R component from the total Na+ current. From the current traces, the current density was obtained by dividing the peak current by the cell capacitance.
K+ currents were evoked by a series of 500-ms test pulses ranging from −60 to +50 mV in 10 mV steps. The command potential protocol was initially undertaken from a holding potential of −100 mV and repeated from a holding potential of −40 mV.
Data analysis and statistics.
Electrophysiological data were analyzed using Fitmaster (HEKA) and Origin 8 (Origin Lab). Data are expressed as means ± SE. Statistical differences between CCD and control neurons were analyzed by Student's unpaired t-test. In the case of repeated measurements, the data were initially analyzed by ANOVA with repeated measures. Post hoc testing was by Tukey's multiple-comparison test or Student's t-test with a Holm-Bonferroni correction to the critical P value. Significance was set at P < 0.05.
For the analysis of steady-state activation, the Na+ conductance (gNa) at different potentials was calculated from the corresponding current (INa) as follows: gNa = INa/(V − VNa), where V is the voltage and VNa is the reversal potential for Na+ current. The conductance values at different potentials were normalized to the maximum conductance (gmax) and fitted to the following Boltzman function: g/gmax = 1/{1+ exp[(Vg − V)/Kg]}, where Vg is the voltage for half-maximum activation and Kg is the slope factor.
The steady-state inactivation of TTX-R and TTX-S Na+ currents was measured after delivering 100-ms prepulse potentials over the range of −130 to −10 mV with 5-mV increments followed by a 30-ms depolarization to a command potential of −10 mV. The inactivation of TTX-R current was assumed to occur positive to −50 mV. The steady-state inactivation parameter (h∞) was calculated at each prepulse potential by dividing the current evoked after a given prepulse potential by the current evoked after a −130-mV prepulse potential. The inactivation parameter was fitted to a Boltzman function for the determination of the half-inactivation potential (Vh) and the slope factor (kh), where h∞(V) = 1/{1+ exp[(V − Vh)/Kh]}.
RESULTS
Effects of CCD on behavioral withdrawal threshold.
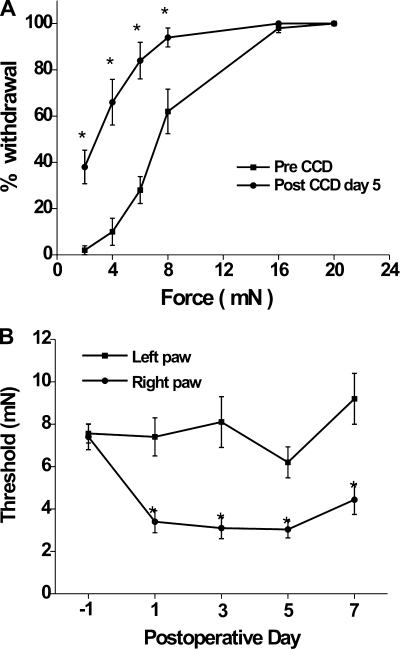
Before CCD, the mean incidence of ipsilateral foot (right foot) withdrawal increased monotonically with filament bending force (Fig. 1A). After CCD, there was a significant increase in the percentage of foot withdrawal to each bending force except in response to the two highest values, as shown in Fig. 1A for postoperative day 5.
Fig. 1.
Incidence of withdrawal and thresholds for withdrawal of the hindpaw to mechanical indentation of the plantar surface by Von Frey filaments before and after chronic compression (CCD). A: percent withdrawal of the right hindpaw as a function of the force of indentation 1 day before (pre-CCD) and 5 days after CCD (post-CCD; n = 10). Threshold was defined as the force eliciting 50% withdrawal. B: time course of changes in the mean mechanical threshold force on the hindpaw ipsilateral and contralateral to CCD.
The postoperative mechanical thresholds obtained for the foot contralateral to the CCD were not significantly changed over pre-CCD threshold values (P = 0.16 by repeated-measures ANOVA; Fig. 1B). For the ipsilateral foot, the thresholds decreased significantly below pre-CCD values on postoperative day 1 and remained decreased through day 7 (P < 0.05 by repeated-measures ANOVA followed by Tukey's multiple-comparison test; Fig. 1B).
The mice exhibited no obvious “spontaneous” behavior directed at the limb ipsilateral (or contralateral) to the CCD, in accordance with the lack of such behavior in certain other models of neuropathic pain in the mouse (Mogil et al. 2010) but contrary to results previously obtained in the rat after CCD (Song et al. 1999).
Effects of CCD on neuronal excitability.
Passive membrane properties and AP characteristics were recorded under current clamp (Table 1). Compared with control neurons, CCD neurons exhibited a significantly lower rheobase and a more negative voltage threshold (P < 0.01 and P < 0.05, respectively, by Student's t-tests). No significant differences between CCD and control neurons were found in cell capacitance, resting membrane potential, input resistance, AP amplitude, AP duration, and the number of APs evoked by current at 2× rheobase. In all of the cells we screened using extracellular recording, five CCD neurons but no control neurons exhibited spontaneous APs. All patch-clamp recordings, however, were obtained from silent neurons.
Table 1.
Membrane properties of control and CCD neurons measured in whole cell patch clamp experiments
| AP |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Cells | Diameter, μm | Cell Capacitance, pF | Membrane Potential, mV | Input Resistance, MΩ | Rheobase, pA | Voltage threshold, mV | Amplitude, mV | Duration, ms | MS/Non-MS | |
| Control | 9 | 24.4 ± 0.7 | 18.9 ± 1.1 | −54.1 ± 2.6 | 374.7 ± 70.4 | 101.7 ± 6.5 | −26.8 ± 1.4 | 105.6 ± 7.4 | 2.9 ± 0.4 | 2/7 |
| CCD | 8 | 23.3 ± 1.4 | 17.3 ± 2.1 | −57.0 ± 4.5 | 346.3 ± 38.4 | 62.5 ± 9.1† | −33.0 ± 1.5* | 103.8 ± 3.7 | 4.1 ± 0.6 | 3/5 |
Values are mean ± SE. CCD, chronic compression; AP, action potential; rheobase, current threshold; MS, number of cells firing multiple (≥2) spikes to current step at 2× rheobase; non-MS, number of cells discharging 1 spike.
P < 0.05 and
P < 0.01 by Student's t-tests (significant differences between control and CCD neurons).
Effects of CCD on voltage-gated Na+ currents.
Twenty-two small neurons, including 11 neurons from 5 control mice and 11 neurons from 6 CCD mice, were recorded and analyzed.
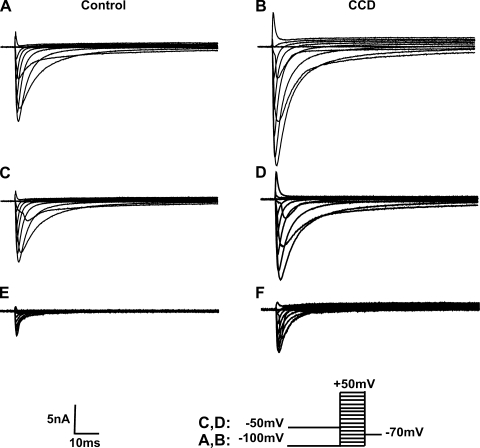
All of the neurons analyzed expressed both TTX-R and TTX-S type currents (Fig. 2). Two neurons from CCD mice were excluded where the ratio of peak TTX-R to TTX-S currents was <10% at −10 mV.
Fig. 2.
Representative waveforms of voltage-gated Na+ currents for a control neuron and a CCD neuron. A and B: total Na+ current. Traces were recorded in response to a series of 80-ms test pulses in 10-mV increments from −100 to 50 mV preceded by a 500-ms prepulse of −100 mV. C and D: TTX-resistant (TTX-R) currents were recorded in response to the same series of command potentials pulses but preceded by a 500-ms prepulse of −50 mV. E and F: TTX-sensitive (TTX-S) currents obtained by subtraction of TTX-R currents from the total Na+ current.
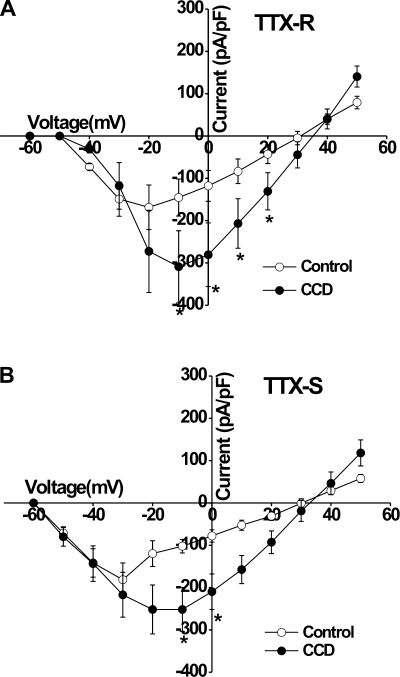
TTX-R currents were activated at approximately −40 mV for CCD and control and reached maximal levels at −10 and −20 mV, respectively (Fig. 3A). The mean current density of the TTX-R Na+ current at −10, 0, +10, and +20 mV was significantly greater for CCD neurons than for control neurons (P < 0.05 by Student's t-tests; Fig. 3A).
Fig. 3.
Mean voltage-current relationships for voltage-gated Na+ currents in control and CCD neurons. The current density of both TTX-R (A) and TTX-S (B) currents evoked by each voltage was obtained from control and CCD neurons (11 cells from 5 control mice and10 cells from 6 CCD mice).
TTX-S currents were activated at approximately −50 mV for CCD and control neurons and reached peak current values at about −20 and −30 mV, respectively (Fig. 3B). The mean current density of TTX-S Na+ currents was significantly greater for CCD neurons than for control neurons at −10 and 0 mV (P < 0.05 by Student's t-tests; Fig. 3B).
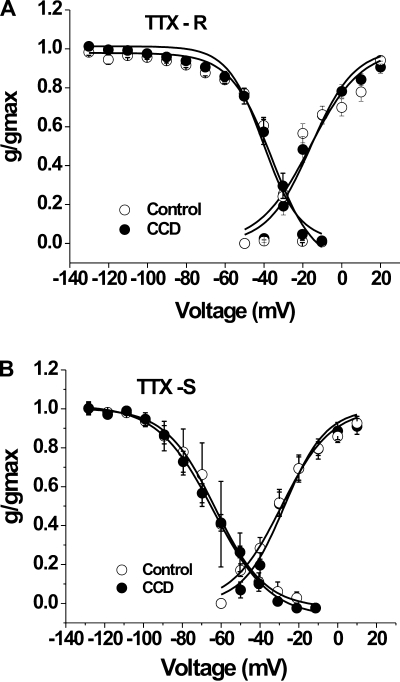
There were no significant differences in the levels of activation of TTX-S or TTX-R currents between CCD and control neurons. For TTX-R current, the mean midpoint of activation (V1/2) and the mean slope for activation (k) for control neurons was −16.2 ± 2.7 and 13.2 ± 2.6 mV (n = 11), respectively, and for CCD neurons was −15.9 ± 1.6 and 11.2 ± 1.5 mV (n = 10, P > 0.05 by Student's t-tests; Fig. 4A). For TTX-S currents, V1/2 and k for control neurons was −28.9 ± 1.3 and 12.7 ± 1.2 mV (n = 11), respectively, and for CCD neurons was −27.4 ± 1.4 and 10.7 ± 1.3 mV (n = 10, P > 0.05 by Student's t-tests; Fig. 4B).
Fig. 4.
Activation and steady-state inactivation characteristics of Na+ currents in control and CCD neurons. The mean relative peak conductance of TTX-R and TTX-S Na+ currents was normalized to the maximal conductance (g/gmax) and plotted against voltage. A: steady-state activation and inactivation characteristics of TTX-R currents. B: steady-state activation and inactivation characteristics of TTX-S currents. There were no significant differences in the levels of activation or inactivation for either type of current between CCD and control neurons (see main text for further details).
CCD did not change V1/2 of inactivation of TTX-R or TTX-S currents. For TTX-R current, the mean V1/2 was −35.6 ± 3.3 mV for CCD neurons (n = 10) and −40.1 ± 1.4 mV for control neurons (n = 11; Fig. 4A). The slope factor for TTX-R inactivation was not significantly different between CCD and control neurons (10.8 ± 2.4 vs. 9.2 ± 1.2 mV, respectively). For TTX-S current, the mean V1/2 of inactivation was −63.0 ± 0.5 mV for control neurons (n = 4) and −64.2 ± 0.8 mV for CCD neurons (n = 7; Fig. 4B). There were no significant differences in the slope factor for TTX-S inactivation after CCD (14.4 ± 0.8 vs. 12.4 ± 0.4 mV, P > 0.05 by Student's t-test).
Effects of CCD on voltage-gated K+ currents.
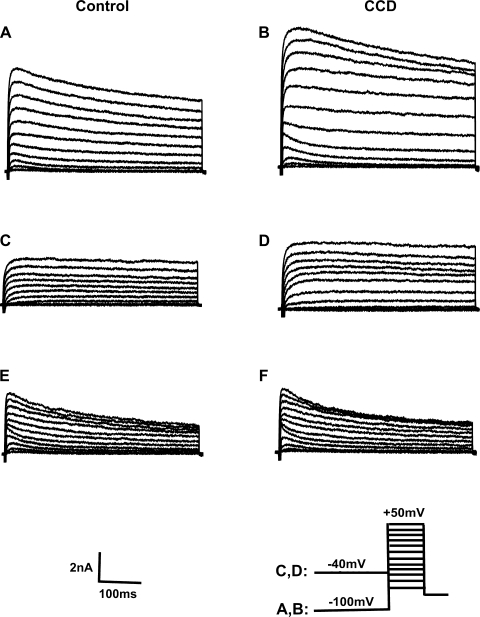
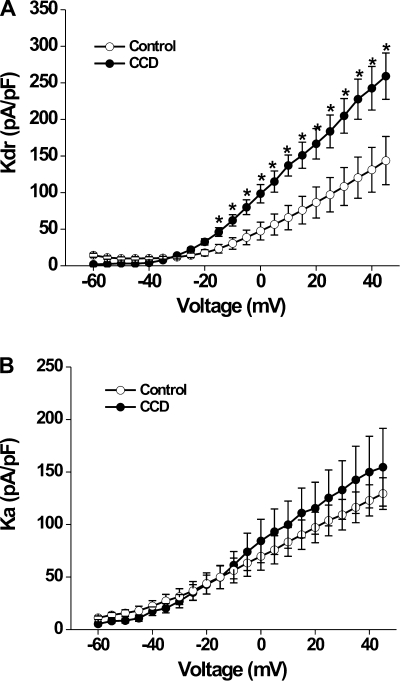
The command potential after −100-mV holding potential evoked both a transient current and a sustained outward current (Fig. 5, A and B). The −40-mV holding potential was used to inactivate the transient current (Fig. 5, C and D). A subtraction of the current traces evoked at the two holding potentials yielded the transient current (Fig. 5, E and F). The density of sustained K+ current for CCD neurons (n = 13, 7 mice) was nearly double that for control neurons (n = 19, 8 mice, P = 0.014 by repeated-measures ANOVA; Fig. 6A). Post hoc comparisons at each voltage revealed that the magnitude of sustained outward current was larger in CCD neurons at each potential above −20 mV (Fig. 6A). The sustained K+ current density also increased in spontaneously firing neurons (n = 3; data not shown). In contrast, there were no significant differences between CCD and control neurons in the density of the transient K+ current (P = 0.66 by repeated-measures ANOVA; Fig. 6B).
Fig. 5.
Representative waveforms of voltage-gated K+ currents for a control neuron and a CCD neuron. Whole cell K+ currents were elicited by a series of 500-ms test pulses ranging from −60 to + 50 mV in 10-mV steps. A and B: neurons were held at −100 mV before each pulse. C and D: neurons were held at −40 mV before each pulse. E and F: transient K+ currents were obtained by subtraction currents in C and D from those in A and B, respectively.
Fig. 6.
Mean current-voltage relationships for voltage-gated K+ currents in control and CCD neurons. A: the mean density of sustained K+ current (Kdr) was significantly greater for CCD neurons than for control neurons. B: the mean density of fast-inactivating K+ current (Ka) was not significantly changed after CCD.
The fast and slow inactivating components of the transient currents were compared after fitting the inactivating phase to a two-term exponential function. The mean values of the slow and fast time constants were unchanged by CCD (609.3 ± 155.1 and 37.2 ± 6.8 ms before and 549.9 ± 74.2 and 24.8 ± 4.2 ms after CCD, respectively).
DISCUSSION
The principal finding of the present study is that CCD in a mouse model resulted in tactile allodynia and an increase in the excitability of small-diameter DRG neurons that is accompanied by, and perhaps caused by, enhanced expression of Na+ currents.
This is the first study to apply the CCD model to the mouse. Although we cannot entirely rule out some contribution to the findings of the surgery (per se) required to implant the compressing rods, such effects (in the absence of compression) were shown at most to be transient in the rat (Song et al. 1999). In confirmation of results obtained in rats using a similar behavioral testing protocol (Song et al. 1999), a significant decrease in the threshold to punctate mechanical indentation was observed in the foot ipsilateral to the chronically compressed ganglion as early as 1 day after surgery and lasting for the entire period of testing. Thus, the CCD model produced in the mouse neuropathic pain behavior that was characterized by tactile allodynia. Extension of the CCD model to the mouse opens the possibility of exploring the mechanisms of CCD-type injuries using genetically engineered animals as in other models of pathological changes in neuronal excitability (Raouf et al. 2010).
In this study, we used a relatively new procedure that allowed for whole cell recording from intact DRG neurons. Technically the recordings were more challenging than those for dissociated neurons since they required the removal of one or a few non-neuronal cells before achieving a gigaseal. Such a constraint limits the number of cells that can be recorded in a given experiment. Previous studies of Na+ and K+ current in injured or inflamed peripheral neurons were conducted primarily after enzymatic and mechanical dissociation of DRG neuronal somata from their axons and from satellite glia and other cells in the ganglion. Although dissociation does not appear to alter the excitability properties of medium- and large-sized neurons, it does increase the excitability of small-diameter neurons to the same extent as does the CCD injury model of neuropathic pain (Ma and LaMotte 2005; Song et al. 2003; Zheng et al. 2007). The recording configuration used in the present study minimized this complication and is similar to the configuration we recently described for the in vivo recording of visualized DRG cell bodies with intact nerve fibers and peripheral innervation (Ma et al. 2010).
CCD also increased the excitability of small DRG neurons in the mouse as it does in the rat (Zhang et al. 1999), as characterized by a reduction in rheobase, a negative shift in the voltage threshold, and a probable increase in the incidence of ectopic spontaneous discharges. However, in contrast to the rat, there is no difference in firing multiple APs between CCD and control neurons in response to a suprathreshold current stimulus of 2× rheobase. Whether this discrepancy was due to the use of patch-clamp recording in the mouse versus the sharp-electrode intracellular recording in the rat or due to other factors, such as an insufficient sample size, requires further study.
Na+ currents.
In the present study, CCD produced a significant increase in the magnitude of TTX-S and TTX-R current. This is consistent with the demonstration that TTX-R current was increased in medium-sized cutaneous neurons after CCD (Tan et al. 2006). However, another study observed a decrease in TTX-R current 10–14 days after CCD surgery in small-sized neurons (Huang and Song 2008). The reason for the discrepancy is not immediately apparent. Perhaps the longer time period between the onset of CCD and electrophysiological recording or the use of acutely dissociated neurons in the earlier study contributed to the differences in results.
Although axonal Na+ current may contribute to the observed response, its role is likely to be minor. The surface area is much less for the axon than for the soma, and prolonged inward currents due to cable properties of the axon were not observed, as might be expected if currents were evoked and generated significantly away from the soma.
The increase in TTX-S and TTX-R Na+ currents observed in the present study is consistent with results obtained in models of peripheral inflammatory pain. Peripheral inflammation of the skin by complete Freund's adjuvant or carrageenan (Black et al. 2004; Gould et al. 1998; Tanaka et al. 1998) or a local inflammation of the DRG by the application of zymosan (Wang et al., 2007) was accompanied by an increase in TTX-S and TTX-R Na+ currents without shifting the voltage dependence of activation or inactivation. It seems likely that the local inflammation caused by CCD may be an important component of this type of injury (Song et al. 1999; Song et al. 2003; Zhang et al. 1999).
The CCD model differs in some respects from other models of neuropathic pain. For instance, sciatic nerve axotomy resulted in a downregulation in the mRNA for TTX-R isoforms and a downregulation of TTX-R current measured in dissociated DRG neurons (Cummins and Waxman 1997; Dib-Hajj et al. 1996; Rizzo et al. 1995). In addition, the TTX-R isoform Nav1.8 redistributes away from the soma to the axon of nerve fibers, which are in proximity to injured nerve fibers but not directly injured (Gold et al. 2003).
K+ currents.
Previous studies have demonstrated that voltage-gated K+ currents are dynamically regulated after peripheral inflammation or nerve injury. After transection of the sciatic nerve, voltage-gated K+ current were downregulated (Abdulla and Smith 2001; Dang et al. 2004; Everill and Kocsis 1999; Takeda et al. 2006). In contrast, exposure of the DRG to an inflammatory agent (zymosan, applied in vivo) resulted in an increase in sustained and transient K+ currents in dissociated small-sized neurons (Wang et al. 2007). In present study, we also observed an increase of sustained K+ current after CCD in small-sized DRG neurons. As with the changes in Na+ current, our results are most consistent with those observed after peripheral inflammation compared with axonal transection, suggesting that a major component of the CCD model is due to local inflammation of the DRG.
In accordance with other studies of neuropathic pain, CCD produced an increase in excitability, as demonstrated by a lowered rheobase. Although an increase in Na+ current likely contributed to the lowered rheobase, one cannot rule out the possible participation of other channels. However, because no change was observed in the transient or sustained K+ currents below −20 mV, it is unlikely that changes in voltage-gated K+ current affected neuronal excitability. On the other hand, the increase in sustained current may represent a compensatory mechanism for the enhanced neuronal activity after CCD injury. Such a mechanism might act to prevent the occurrence of ectopic discharges originating within the DRG.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- Abdulla FA, Smith PA. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J Neurophysiol 85: 644–658, 2001 [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Tanaka M, Cummins TR, Waxman SG. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain 108: 237–247, 2004 [DOI] [PubMed] [Google Scholar]
- Cummins TR, Waxman SG. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J Neurosci 17: 3503–3514, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol 286: G573–G579, 2004 [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S, Black JA, Felts P, Waxman SG. Down-regulation of transcripts for Na channel α-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci USA 93: 14950–14954, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol 82: 700–708, 1999 [DOI] [PubMed] [Google Scholar]
- Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, Lai J. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J Neurosci 23: 158–166, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, 3rd, England JD, Liu ZP, Levinson SR. Rapid sodium channel augmentation in response to inflammation induced by complete Freund's adjuvant. Brain Res 802: 69–74, 1998 [DOI] [PubMed] [Google Scholar]
- Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain 77: 15–23, 1998 [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Song XJ. Differing alterations of sodium currents in small dorsal root ganglion neurons after ganglion compression and peripheral nerve injury. Mol Pain 4: 20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, Wiley J, Decosterd I, Isom LL. Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci 26: 7984–7994, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Donnelly DF, LaMotte RH. In vivo visualization and functional characterization of primary somatic neurons. J Neurosci Methods 191: 60–65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, LaMotte RH. Enhanced excitability of dissociated primary sensory neurons after chronic compression of the dorsal root ganglion in the rat. Pain 113: 106–112, 2005 [DOI] [PubMed] [Google Scholar]
- Mogil JS, Graham AC, Ritchie J, Hughes SF, Austin JS, Schorscher-Petcu A, Langford DJ, Bennett GJ. Hypolocomotion, asymmetrically directed behaviors (licking, lifting, flinching, and shaking) and dynamic weight bearing (gait) changes are not measures of neuropathic pain in mice. Mol Pain 6: 34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf R, Quick K, Wood JN. Pain as a channelopathy. J Clin Invest 120: 3745–3752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Selective loss of slow and enhancement of fast Na+ currents in cutaneous afferent dorsal root ganglion neurones following axotomy. Neurobiol Dis 2: 87–96, 1995 [DOI] [PubMed] [Google Scholar]
- Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol 82: 3347–3358, 1999 [DOI] [PubMed] [Google Scholar]
- Song XJ, Vizcarra C, Xu DS, Rupert RL, Wong ZN. Hyperalgesia and neural excitability following injuries to central and peripheral branches of axons and somata of dorsal root ganglion neurons. J Neurophysiol 89: 2185–2193, 2003 [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M, Nasu M, Kadoi J, Yoshida S, Matsumoto S. Enhanced excitability of rat trigeminal root ganglion neurons via decrease in A-type potassium currents following temporomandibular joint inflammation. Neuroscience 138: 621–630, 2006 [DOI] [PubMed] [Google Scholar]
- Tan ZY, Donnelly DF, LaMotte RH. Effects of a chronic compression of the dorsal root ganglion on voltage-gated Na+ and K+ currents in cutaneous afferent neurons. J Neurophysiol 95: 1115–1123, 2006 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cummins TR, Ishikawa K, Dib-Hajj SD, Black JA, Waxman SG. SNS Na+ channel expression increases in dorsal root ganglion neurons in the carrageenan inflammatory pain model. Neuroreport 9: 967–972, 1998 [DOI] [PubMed] [Google Scholar]
- Wang JG, Strong JA, Xie W, Zhang JM. Local inflammation in rat dorsal root ganglion alters excitability and ion currents in small-diameter sensory neurons. Anesthesiology 107: 322–332, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Donnelly DF, LaMotte RH. Patch clamp recording from the intact dorsal root ganglion. J Neurosci Methods 79: 97–103, 1998 [DOI] [PubMed] [Google Scholar]
- Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 82: 3359–3366, 1999 [DOI] [PubMed] [Google Scholar]
- Zheng JH, Walters ET, Song XJ. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J Neurophysiol 97: 15–25, 2007 [DOI] [PubMed] [Google Scholar]