Abstract
Indirect evidence suggests that water supply to fleshy fruits during the final stages of development occurs through the phloem, with the xylem providing little water, or acting as a pathway for water loss back to the plant. This inference was tested by examining the water balance and vascular functioning of ripening kiwifruit berries (Actinidia chinensis var. chinensis ‘Hort16A’) exhibiting a pre-harvest ‘shrivel’ disorder in California, and normal development in New Zealand. Dye labelling and mass balance experiments indicated that the xylem and phloem were both functional and contributed approximately equally to the fruit water supply during this stage of development. The modelled fruit water balance was dominated by transpiration, with net water loss under high vapour pressure deficit (Da) conditions in California, but a net gain under cooler New Zealand conditions. Direct measurement of pedicel sap flow under controlled conditions confirmed inward flows in both the phloem and xylem under conditions of both low and high Da. Phloem flows were required for growth, with gradual recovery after a step increase in Da. Xylem flows alone were unable to support growth, but did supply transpiration and were responsive to Da-induced pressure fluctuations. The results suggest that the shrivel disorder was a consequence of a high fruit transpiration rate, and that the perception of complete loss or reversal of inward xylem flows in ripening fruits should be re-examined.
Keywords: Actinidia chinensis, berry shrivel, hydraulic conductance, kiwifruit, phloem, sap flow, transpiration, xylem
Introduction
The growth of fleshy fruits involves a balance between the supply or withdrawal of water via the vascular tissue, and losses to transpiration. In general, both the phloem and xylem contribute to this balance, but the current understanding for most fruits is that there is usually a transition from significant contributions by the xylem early in development to dominance by phloem flows during the later stages of development (Ho et al., 1987; Lang, 1990; Greenspan et al., 1994). The causes and functional significance of this change have been the subject of much recent interest (Matthews and Shackel, 2005; Choat et al., 2009; Greer and Rogiers, 2009; Tilbrook and Tyerman, 2009). The consequence for mature fruits at the end of their growth cycle is that xylem flows into the fruit are thought to be negligible, and may contribute little should the fruit water balance be altered; for example, if a fruiting plant is exposed to a hot, dry atmosphere, or heavy rainfall.
Unfortunately all the evidence for a minor contribution by the xylem to the maturing fruit water balance is indirect, obtained from mass balance approaches (Lang and Thorpe, 1989; Fishman and Genard, 2000), dye tracers (During et al., 1987), or nutrient budgets (Clark and Smith, 1988; Rogiers et al., 2006). Direct observation is rare because the flows and driving forces involved are small and difficult to measure (Windt et al., 2009). End of season ripening disorders connected to the fruit water balance, such as fruit shrivel and splitting, are relatively common (e.g. Milad and Shackel, 1992; Krasnow et al., 2010), but because of these methodological issues, their causes and the role of the vascular tissues in their development remain topics open to debate (Matthews and Shackel, 2005). Here a shrivel disorder of kiwifruit berries is examined, with particular emphasis on the contribution of the phloem and xylem to the water balance of ripening fruit.
The fruit of the kiwifruit (Actinidia sp.) vine is anatomically a berry, exhibiting a double sigmoid pattern of growth similar to fruits such as grape (Vitis sp.) and stone fruit (Prunus sp.), although the ‘lag’ (stage II) and second period of accelerated growth (stage III) in fresh weight are often quite indistinct in kiwifruit (Hopping, 1976; Richardson et al., 1997). Dry weight accumulates at a constant rate from soon after anthesis, with a shift from structural incorporation to rapid accumulation of starch as the main non-structural carbohydrate near the end of the first phase of growth (stage I; Richardson et al., 1997). Unlike in grapes and tomatoes, the two best known ‘model’ fruits, the concentration of soluble sugars does not rise until the end of stage III, when growth in both fresh and dry weight begins to decline, starch hydrolysis starts, and the fruit begins to ripen (or ‘mature’) (Boldingh et al., 2000; Nardozza et al., 2010). The familiar green kiwifruit of international trade [Actinidia deliciosa (A. Chev.) C.F. Liang et A.R. Ferguson var. deliciosa ‘Hayward’] is normally harvested at this point, ∼160 days after anthesis (DAA), while still relatively firm and growing slowly.
Fruit of a newer cultivar of yellow kiwifruit, from a closely related species (Actinidia chinensis Planch. var. chinensis ‘Hort16A’), are normally harvested after a longer period of development (180–190 DAA), but on a similar calendar date (the yellow cultivar flowers 1 month earlier than the green, but begins growth more slowly). At the end of the season, growth and firmness decline more rapidly in this cultivar, and the fruit are usually picked when growth is near zero (Minchin et al., 2003b). The primary reason for the later harvest is the need to wait until chlorophyll degradation has occurred and the yellow carotenoid flesh colour has been revealed (Mcghie and Ainge, 2002; Minchin et al., 2003a). This cultivar, selected and successfully commercialized under relatively mild, maritime New Zealand growing conditions, has since been widely introduced to other kiwifruit-growing regions around the world. Soon after its introduction to regions with warmer and drier summer climates, including Italy and California, a ‘shrivel’ disorder of the final stages of fruit growth was observed, with the cessation of normal growth and the onset of abnormal softening and shrinkage (a slowing or cessation of dry weight accumulation and a decline in fresh weight) occurring between 130 and 170 DAA, beginning first at the stylar end of the fruit (Thorp et al., 2007). Abnormal fruit growth occurred even though the cultivar was growing in the same orchards on apparently healthy irrigated vines, and progressed through the same stages of development with similar timing and climatic conditions, as the green cultivar.
Explanations for changes in vascular functioning during fruit development centre on the necessity for water potential gradients to remain favourable for growth and ripening (Matthews and Shackel, 2005). Earlier dye labelling and anatomical studies in grape led to the conclusion that the berry xylem was completely blocked or disrupted during the sudden transition from stage II to III (known as ‘véraison’ in grapes), hydraulically isolating the fruit from the vine, with further growth dependent on the phloem (During et al., 1987; Findlay et al., 1987). Hydraulic isolation was considered necessary to prevent the withdrawal to the vine (‘backflow’), via the xylem, of the solute-rich apoplasmic fluid which accumulates as the berry ripens (Lang and During, 1991). More recently it has been shown that the xylem remains functional (Bondada et al., 2005; Keller et al., 2006), although with a reduced hydraulic conductance (Tyerman et al., 2004; Choat et al., 2009). The decline in xylem flow into the fruit is instead attributed to a reduction in the forces driving flow, including a decline in berry transpiration (Bondada et al., 2005), hydraulic ‘buffering’ by phloem-derived water (Choat et al., 2009), and, in the later stages of ripening, the end of berry expansion. The situation appears to be similar in the kiwifruit berry. Nutrient budgets and mass balance studies suggest high xylem flows early in development, but declining or negligible xylem contributions during fruit maturation (Clark and Smith, 1988; Morandi et al., 2007). Initially, dye tracers were readily carried into excised yellow fruit, but complete xylem dysfunction was inferred by 120 DAA (Dichio et al., 2003). However, in contrast to dye tracer experiments, hydraulic measurements do show that appreciable xylem flows can still be induced into and out of excised fruit throughout the later stages of fruit development. Fruit hydraulic conductance reaches a maximum early in fruit development, but declines, particularly in the receptacle area, from 80 DAA (Mazzeo, 2008). Using a mass balance approach, Morandi et al. (2010) inferred xylem inflows during the later stages of kiwifruit berry growth, but found no relationship between apoplasmic pressure gradients and estimated flows, implying some form of hydraulic isolation. Kiwifruit berries do not exhibit the rapid softening and dramatic changes in solute accumulation that occur in grapes at véraison, but both symplasmic and apoplasmic solute potentials rise later during fruit maturation (Nardozza et al., 2010; N. Gould, unpublished data). Some degree of hydraulic isolation may therefore be necessary to reduce the potential for xylem backflow to the vine as apoplasmic solute potentials rise and turgor and transpiration decrease.
Grapes also provide a useful model for berry shrivel disorders, some of which have been linked to developmental changes in vascular functioning (McCarthy, 1999; Rogiers et al., 2004; Krasnow et al., 2010). The type of shrivel perhaps most analogous to weight loss of kiwifruit is late season berry dehydration, with grapes developing normally until late in ripening, followed by water loss and concentration of sugars (Krasnow et al., 2010). Particularly well known in the variety Shiraz, the phenomenon was originally attributed to the combined effects of a decline in phloem flows, in the absence of functional xylem (McCarthy and Coombe, 1999), and continued transpiration (Rogiers et al., 2006). However, Shiraz berries have also been shown to maintain a higher xylem hydraulic conductance during ripening than other varieties, leading to the suggestion that weight loss may also be attributed to the withdrawal of water back to the vine (Tyerman et al., 2004; Tilbrook and Tyerman, 2008). The current consensus for grapes appears to be that shrivel disorders, including late season dehydration, bunch stem necrosis, and berry shrivel, may often involve some degree of xylem backflow (Choat et al., 2009; Tilbrook and Tyerman, 2009; Hall et al., 2011). However, backflow from grapes has never been directly observed, and few studies have convincingly partitioned berry water loss between evaporation and xylem flows (Greer and Rogiers, 2009). Indeed, the prevalence of xylem backflow in any fruits, whether fruit growth is proceeding normally or not, has not been established (Matthews and Shackel, 2005).
This study examines the functioning of xylem and phloem during late season development of kiwifruit berries in California and New Zealand, with the goals of improving understanding of normal fruit development, and identifying the cause of the shrivel disorder in California. The hypothesis was that by the end of the season normal fruit growth was dependent primarily on the phloem, with xylem flows progressively reduced. The shrivel disorder was expected to be the result of a reduction or reversal in xylem fluxes contributing to a net loss of water. A dye was applied in situ to examine xylem functionality, and a mass balance approach was used to partition vascular flows. A water balance model, based on observations of fruit hydraulic conductance, apoplasmic pressure gradients, fruit respiration, fruit surface conductance, and meteorological conditions, was used to quantify the likely contribution of xylem and phloem flows to the fruit water balance. Finally, purpose-grown fruiting plants and a recently developed sap flow technique (Clearwater et al., 2009) were used to observe the dynamics of growth and pedicel vascular flows in response to controlled changes in fruit and plant water status.
Materials and methods
Plant material
All measurements were made on fruit and shoots of A. chinensis Planch. var. chinensis ‘Hort16A’, a commercial cultivar of yellow fleshed kiwifruit (Ferguson, 1999). Measurements in California were made on 10 mature plants growing on a T-bar trellis in two adjacent rows in a commercial kiwifruit orchard near Porterville (36°00′22′′N, 119°08′29′′W). Vine management was according to normal commercial practice, with daily microjet irrigation below the canopy and a standard fertilizer regime. The percentage of open flowers was recorded on 5 d over the flowering period, and the day of anthesis (50% open flowers) estimated by linear extrapolation through the midpoint of the flowering period. For comparison between growing seasons and locations, fruit developmental age was expressed as DAA. At the time of measurements in California (3–9 October 2004, 187–193 DAA), a small proportion of fruit were beginning to show signs of softening at the distal ends of the fruit (Thorp et al., 2007). Measurements on orchard-grown fruit in New Zealand were made on 10 mature plants growing on a pergola trellis in the Plant & Food Research Te Puke Research Orchard (37°49′15′′S, 176°19′08′′E). Vine management was also according to normal commercial practice. Vines were not irrigated during the measurement periods (May 2004 and 2005, 190–220 DAA) because evaporative demand was low and soil moisture deficits are rare at this time of year. Fruit were developing normally, with no abnormal softening observed.
Controlled environment experiments were conducted using potted plants with a single shoot and fruit, grown for the purpose. Industry-standard, bare-rooted, 2-year-old seedling rootstocks of A. deliciosa (A. Chev.) C.F. Liang et A.R. Ferguson var. deliciosa ‘Bruno’ were purchased from a commercial nursery with shoots removed in December 2007, potted in potting mix (a blend of bark fines, coco fibre, pumice, slow release fertilizer; Daltons, Matamata, New Zealand) in 5.0 l planter bags, kept moist, and allowed to recover in the shade before being grown on in a sheltered nursery in the full sun. On 27 February 2008, the rootstocks were re-cut 20 cm above the potting mix and whip grafted with a 15 cm ‘Hort16A’ scion with two buds. Scion wood was cut from dormant 1-year-old shoots collected in July 2007 and held in plastic bags at 1 °C until needed. Grafted plants were grown in a nursery under 30% shade, irrigated daily, and pruned to leave one emerging shoot with one flower. Anthesis occurred on 14 April, with open flowers hand pollinated using male A. chinensis pollen collected and frozen at –20 °C in October 2007. The plants were transferred to an unheated plastic house on 24 April, provided with supplemental lighting for 4 h d−1, and fertilized at 2-monthly intervals with 2.5 g per plant of slow release fertilizer (Triabon, Scotts, Nordhorn, Germany) until their use in experiments in October 2008, from 180 to 210 DAA.
Growth and vascular functioning in situ
The diameters of six fruit growing in California were recorded continuously using displacement transducers (DFg2.5, Solartron, Sussex, UK) mounted in custom-made callipers and connected to a datalogger (CR10X, Campbell Scientific, Utah, USA). After 3 d, three randomly selected fruit were partially enclosed in plastic bags to reduce transpiration (full enclosure was not possible because of the wires used to support the callipers). The pedicels of the other three were cut, after first bracing the pedicel so that the fruit remained in their original positions. The net change in diameter over 4 d of an additional 100 fruit, subjected to a range of treatments at the beginning of the measurement period, was recorded to the nearest micrometre, using a digital micrometer (model 293-331, Mitutoyo, Japan). Repeatability in diameter measurements was improved by marking the position of the measurement on each fruit, and by using the ratchet spindle of the micrometer to apply a repeatable measurement force. On each of four plants, five fruit were randomly selected and marked on each of five 1-year-old parent shoots (‘canes’). Within each plant, each cane was assigned to one of five treatments: control (untreated), cut shoot (parent cane cut at the proximal end and left to wilt in situ), non-transpiring (each fruit enclosed in a plastic bag, secured with a wire around the pedicel), girdled pedicel (2–3 mm of pedicel bark removed from each fruit using a razor blade), and detached (each fruit detached at the proximal end of the pedicel and re-suspended in the same position using wire). The same experiment was repeated in May 2005 on fruit growing in New Zealand. Fruit diameters were converted to estimated volumes based on the calculations of Minchin et al. (2003b), and treatment effects were examined by analysis of variance, based on a randomized complete block design.
To trace potential pathways for xylem flow, an apoplasmic dye (0.5% aqueous Safranin O) was applied to in situ fruit in California using cotton wicks dipped in water, pulled in a transverse direction through the point of interest using a sewing needle, with one end inserted into a microcentrifuge tube filled with 1 ml of dye solution and secured close to the fruit. Dye was applied at three positions: the midpoint of the pedicel (12 fruit), and the proximal (10 mm from the receptacle; nine fruit) and distal (20 mm from the stylar tip; six fruit) ends of the fruit. After 2–6 h, dye-loaded fruit were harvested with their shoot, sliced at 2 mm intervals, and the distance of dye travel in the xylem in both acropetal and basipetal directions recorded. Distance travelled was expressed relative to the length of the fruit or pedicel. The wick method labels only vascular bundles that are intercepted by the secant traversed by the wick. Dye movement is assumed to indicate the existence of functional xylem, but the procedure disrupts natural pressure gradients by supplying a solution of high water potential. Dye may therefore move in both directions away from the point of loading, but this movement is not interpreted as indicating the direction or magnitude of xylem flows in unlabelled fruit.
Estimates of the apoplasmic pressure gradient between the stem and fruit in California were obtained by measuring the pressure potentials of fruit and their subtending shoots over the course of a day (192 DAA, 06:00 h to 1830 h) using a pressure chamber (Water Status Console, Soil Moisture Equip. Corp., Santa Barbara, CA, USA). The subtending leaves of randomly selected fruit on three vines were enclosed in a plastic bag and covered in aluminium foil at dawn. At 3-hourly intervals, two fruit per vine plus their respective non-transpiring subtending leaf were harvested into a plastic bag and apoplasmic pressure measured immediately with the pressure chamber. For modelling of the fruit water balance, a sinusoidal curve describing daily variation in pressure potentials was fitted to the measured values, with the curve maximum, minimum, and period varied to minimize the sum of squares (Fishman and Genard, 1998).
Fruit gas exchange
Fruit surface conductance to water vapour (gc) and respiration rate (Rf) under ambient conditions were measured using a portable photosynthesis system (LI6400, Licor, Lincoln, NE, USA) with a custom-made cylindrical chamber (inside diameter 80 mm, length 100 mm). Measurements were made between 11:00 h and 17:00 h on fruit still attached to the vine, or detached with pedicel intact and immediately installed in the chamber. Preliminary measurements found no detectable effect of detachment on gc or Rf for at least an hour after detachment (the fruit exocarp lacks functional stomata). Fruit surface temperature was measured with a thermocouple pushed against the fruit. The average fruit surface temperature during measurements was 28.4 °C, air temperature 28.5 °C, vapour pressure deficit (fruit to air) 2.0 kPa, and CO2 mol fraction 368 μmol mol−1. Gas exchange data were recorded 10 min after enclosing each fruit in the chamber, then the fruit was harvested for later measurement of fresh weight (wf, kg) and estimation of fruit surface area (Af=0.0752 wf+0.0040, m2, R2=99.1; D. Green, D. Tanner, and K. Maguire, unpublished data). Conductance estimates were corrected for fruit surface area, and respiration rates were expressed per unit of fruit fresh weight. The same measurements were made on fruit in New Zealand in 2004, with an average fruit surface temperature of 11.9 °C, vapour pressure deficit 0.6 kPa, and CO2 mol fraction 372 μmol mol−1.
A fruit respiration temperature response function was obtained in California by enclosing fruit harvested with pedicel intact in the gas exchange chamber overnight under laboratory conditions, while the chamber air temperature was varied over the maximum range achievable (17–35 °C) in ∼4 °C intervals using the auto program function of the gas exchange system. Rf was recorded after equilibration for an hour at each temperature step.
Fruit hydraulic conductance
Fruit xylem hydraulic conductance (Kf) was measured in New Zealand in 2005 using an evaporative flux method. Fruit were harvested with pedicel intact at dawn, the pedicel re-cut underwater, and bark stripped for 10 mm back from the cut surface and cleaned of mucilage (to prevent bark-exuded mucilage from blocking the xylem), and connected to the water supply (deionized water filtered to 0.2 μm) using an HPLC compression fitting and 1.15 mm inside diameter transparent PTFE tubing. The water supply was a reservoir of water on a balance (1 mg, PB303S; Mettler Toledo, Switzerland), 100 mm above the fruit, with weight recorded every 10 min by a datalogger (CR10X, Campbell Scientific) connected to the balance. The fruit were enclosed in a polystyrene-walled chamber ∼0.2 m3 in volume, and maintained at 25±0.5 °C by circulating water from a precision water bath (ZD, Grant, UK) through a copper heat exchanger also enclosed within the chamber, with air circulation provided by a 12 V fan. Laboratory temperature was controlled by an air conditioner, set to 20 °C. Temperature and humidity inside the chamber were monitored using a probe (HMP50Y, Vaisala, Finland) connected to the datalogger. Fruit water potential was recorded using stem psychrometers (Plant Water Status Instruments, Canada), connected to a water potential datalogger (Psypro, Wescor, Utah, USA), with the psychrometers sealed against the fruit exocarp with petroleum jelly, after first gently abrading the fruit exocarp with fine grit sandpaper and rinsing with distilled water. The purpose of the controlled temperature environment was to minimize temperature gradients between the fruit and psychrometer (the temperature compensation junction of the psychrometers cannot be read with the Psypro). Fruit water potential (Ψw) and flow rate from the balance were recorded when stable (at least 3 h). A second set of fruit was collected during the same period, sap expressed from the cut pedicel using the pressure chamber, and sap osmotic potential [Ψs(a)] measured using a vapour pressure osmometer (Vapro 5500, Wescor). Kf was calculated as the ratio of flow to fruit apoplasmic pressure potential [calculated as Ψw–Ψs(a) (Boyer, 1995)]. Measurements were made on 10 fruit, with one or two fruit collected fresh and measured each day.
Modelling of the fruit water balance
The fruit water balance was modelled as the sum of xylem (Ux), phloem (Up), and transpiration (Ef) flows for a 30 d period prior to typical harvest dates in California (14 October) and New Zealand (15 May). The aim was to quantify the likely relative magnitude of the three pathways for water uptake and loss, with growth estimated only as the net amount of water gained or lost during each time step. The model did not incorporate elastic and inelastic growth processes, or dynamic simulations of phloem transport and dry matter accumulation (Fishman and Genard, 1998). Xylem flows were approximated as the product of Kf and the difference in apoplasmic pressure between the stem [Ψp(a) stem] and fruit [Ψp(a) fruit], obtained from pressure chamber measurements:
The pressure difference in New Zealand was estimated to be 0.2 of the difference measured in California, in keeping with the difference between the two sites in atmospheric vapour pressure deficit. Minimum phloem flows were estimated as the flow required to support fruit respiration (Rf), assuming a phloem carbon concentration (cp) of 0.04 g g−1 H2O (Clark and Smith, 1988):
with respiration modelled as a linear function of ambient air temperature (see the Results). Assuming that the boundary layer conductance (gb), and therefore wind speed, have little effect on fruit transpiration (because gc <<gb) in mature fruit, transpiration was estimated as the product of gc and the vapour pressure deficit (Da):
Equations 1–3 were calculated and summed to give the fruit water balance on an hourly basis, with air temperature and Da for California and New Zealand obtained from automatic weather stations in Porterville (CIMIS Station 169) and Te Puke (Plant & Food Research), respectively. Sensitivity to hydraulic (Kf) and diffusive (gc) conductance was assessed by varying these parameters and observing their effect on the predicted water balance.
Growth and vascular flows under controlled conditions
The influence of transpiration on growth and vascular flows was assessed by observing fruit growth and pedicel sap flow while exposing fruiting plants to a constant temperature and controlled, step changes in Da. The constant temperature environment minimized the effects of fluctuating temperatures on fruit volume and sap flow measurements, facilitating measurement of the small fluxes involved. Individual potted plants were enclosed in a custom-made clear acrylic chamber (0.24 m3) heated to a constant 30±0.5 °C by fan circulation of air over a heating element controlled by a proportional temperature controller and illuminated from above by six 22 W compact fluorescent lamps [photosynthetic photon flux density (PPFD) ∼300 μmol m−2 s−1 at the shoot apex] for 12 h daily. Chamber temperature and humidity were monitored using a probe (HMP50Y, Vaisala, Finland). Humidity was lowered or raised by circulating air through a 0.012 m3 container filled with silica gel, or a second container housing a continuously moistened surface of polyester fabric. A displacement transducer was installed across the fruit equator, as described above, and an external heat pulse sap flow gauge was installed on the fruit pedicel, as described previously (Clearwater et al., 2009). In some experiments, a leaf psychrometer (L51, Wescor), connected to the water potential datalogger, was installed on the leaf subtending the same node as the fruit to give an estimate of stem water potential, and the leaf was covered in plastic and aluminium foil to prevent transpiration. Remote phloem girdling without opening the chamber and disturbing sap flow and growth measurements was achieved by applying 12 V for 1 min to two 47 W chip resistors (also used in the sap flow gauge), held with wires on opposite sides of the fruit pedicel, proximal to the sap flow gauge, thereby raising the pedicel temperature to ∼80 °C (monitored using a thermocouple). A datalogger and switch module (CR10X and SDMCD16AC, Campbell Scientific) were used to monitor temperature and humidity, fruit growth, and pedicel sap flow, and control chamber humidity by switching air flows through the humidifying or dehumidifying devices at 5 s intervals. For a typical experiment, a plant was installed in the chamber, held at a Da of 0.9 kPa while sap flow and fruit growth measurements were monitored for at least 24 h, then the Da was raised to 3.0 kPa for 10–12 h before returning to 0.9 kPa. The cycle was repeated before and after phloem girdling and pedicel severance treatments. During step changes, the targeted Da was normally achieved in <5 min, with little or no accompanying change in temperature, except when the chamber was opened to maintain equipment, to change the desiccant, or to cut the fruit pedicel. Five plants were examined over a 30 d period, from 178 DAA.
Results
Growth and vascular functioning in situ
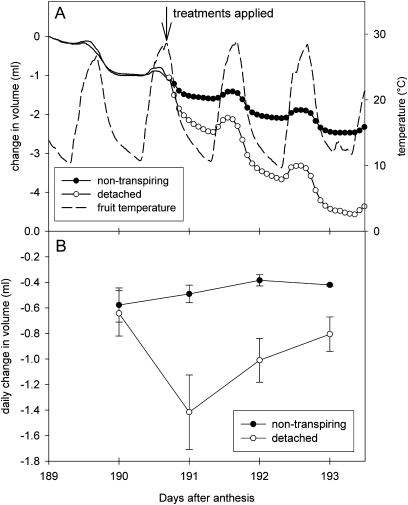
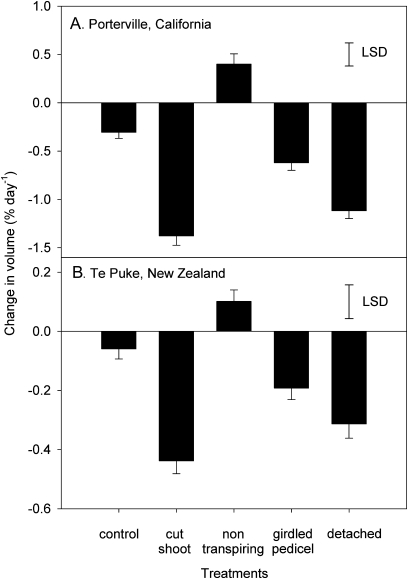
Berries of this A. chinensis cultivar were decreasing in volume 1 month before a normal harvest date in California (Fig. 1). Continuous measurement of fruit diameter from 189 DAA showed a daily net decrease in volume of fruit, with fruit volume increasing briefly with rising temperatures from dawn until early afternoon, before rapidly decreasing for the remainder of the day. Detaching fruit from the shoot approximately doubled the rate of decrease in volume (Fig. 1), with the most rapid decrease observed on the first day after detachment. Partially enclosing the fruit in a plastic bag caused a small reduction in the daily loss of volume. Similar results were obtained when the daily change in diameters of a larger number of fruit was monitored using a micrometer (Fig. 2). Detaching the parent shoot and allowing it to dehydrate in situ, with fruit still attached, caused the most rapid decrease in fruit volume. Preventing transpiration by completely enclosing individual fruit in plastic bags reversed the volume decline, leading to positive growth. Phloem girdling accelerated the decline in volume compared with that of control fruit, and severing both the phloem and xylem approximately doubled the rate of shrinkage compared with girdling alone. Compared with micrometer results, the displacement transducer experiment (Fig. 1) suggested higher rates of fruit shrinkage, possibly because fully enclosing the fruit in plastic was more difficult to achieve with the transducer in place, and because spring pressure from the core assembly resulted in some compression (visible indentations on the fruit suface by the end of the experiment). Using the micrometer approach, a near-identical response to the same treatments was observed in New Zealand, except that the growth of control fruit was closer to zero, and the responses to all treatments were 3–5 times smaller in magnitude than in California (Fig. 2).
Fig. 1.
Cumulative changes in volume (A) from the time of first measurement, and the daily (24 h) change in volume (B) of ripening Actinidia chinensis ‘Hort16A’ fruit growing in California, estimated from displacement transducer measurements of fruit diameter, before and after treatment by partial enclosure in a plastic bag (non-transpiring) or by severing the fruit pedicel (detached). Means ±SE, n=3 per treatment; average fruit weight after the experiment was 86 g.
Fig. 2.
Relative daily changes in volume of ripening Actinidia chinensis ‘Hort16A’ fruit growing in California (A) and New Zealand (B), subjected to a range of treatments that affect water flows to or from the fruit. Volumes were estimated from micrometer measurements of fruit diameter. Treatment effects were significant in both environments (P < 0.001), means ±SE, n=20 per treatment; LSD (Fisher's least significant difference), (α=0.05).
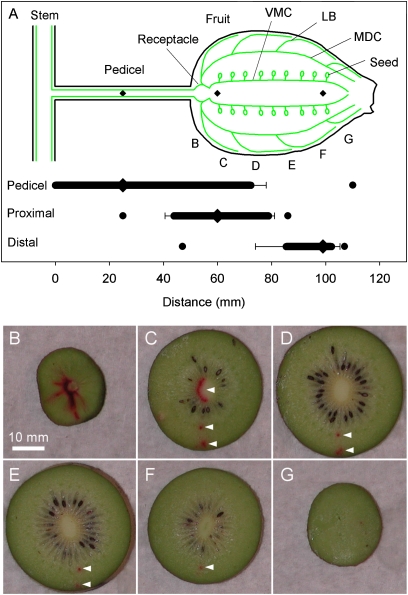
Apoplasmic dye applied to the fruit pedicel moved in the xylem both towards the shoot and into the fruit, readily crossing the receptacle zone (11 of 12 fruit), and in some fruit reaching the distal end of the fruit within 6 h of application (Fig. 3). Dye applied to the proximal and distal ends of the fruit moved within the fruit, in both directions, and in some fruit crossed the receptacle zone and entered the pedicel (three of nine for proximal, one of six for distal dye application; Fig. 3). Dye movement was most often observed in the ventromedian carpellary bundles, probably because these bundles are more likely to be labelled by a wick passing through the centre of the fruit, but was also observed in the median dorsal carpellary bundles and in lateral branch bundles directly beneath the exodermis.
Fig. 3.
(A) Diagram showing the median longitudinal arrangement of the major vascular bundles of an Actinidia berry (after Hopping, 1990), and the distances travelled in both directions by the apoplasmic dye Safranin O introduced into the xylem of Actinidia chinensis ‘Hort16A’ fruit using wicks (horizontal bars). (B–G) Slices of a fruit showing an example of dye transport from the pedicel into the fruit. The wick method labels bundles intercepted by the secant traversed by the wick (see the Materials and methods for an explanation). In this example, dye has passed through the receptacle area and entered ventromedian carpellary (VMC), median dorsal carpellary (MDC), and lateral branch bundles (LB) (arrowheads), and travelled to the distal end of the fruit in one MDC bundle. In A, letters indicate the approximate position of transverse slices shown in B–G, and the horizontal bars indicate the mean distance travelled (error bars=1 SE) in the basipetal and acropetal directions from three points of loading (pedicel, proximal, and distal, diamonds); circles represent the maximum distance travelled in both directions. Data from individual fruit were normalized to a fruit 70 mm long with a 50 mm pedicel. n=6–12 fruit for each loading position.
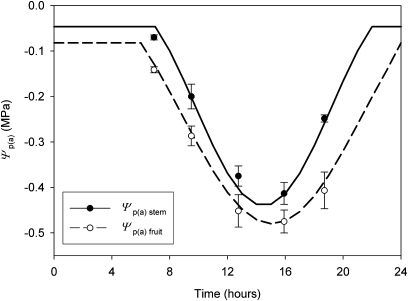
Stem and fruit xylem pressure potentials decreased from a maximum at dawn to a minimum during the mid-afternoon (Fig. 4). Fruit xylem pressure was always more negative than that of the stem to which it was attached, indicating a pressure gradient for xylem flow from stem to fruit, with the largest differential observed in the late afternoon/early evening (Fig. 4).
Fig. 4.
Apoplasmic pressure potentials of Actinidia chinensis ‘Hort16A’ fruit [Ψp(a) fruit] and the stem at the point of attachment of the fruit [Ψp(a) stem] over the course of a day in California, 192 DAA. Lines represent sinusoidal curves fitted to the data for the purpose of modelling the fruit water balance (see the Materials and methods). n=6 for each point, ±1 SE.
Measurement and modelling of the fruit water balance
Fruit surface conductance to water vapour was similar in California and Te Puke, with slightly higher values measured in the more humid New Zealand environment (Table 1). Fruit respiration rates were approximately three times higher in California than in New Zealand (Table 1). The response of fruit respiration to temperature in California was best approximated as linear over the range of temperatures used (Rf= –0.0105Tfruit+0.0736, R2=0.93), with the Te Puke measurements falling on the same line as the California response function. The difference between the two environments in respiration rate measured under ambient conditions was therefore entirely accounted for by the difference in fruit temperature (in California, mean Tfruit=28.4 °C; in Te Puke Tfruit=11.9 °C). Mean fruit hydraulic conductance (Kf), measured in New Zealand fruit, was 0.4±0.1×10−4 g MPa−1 s−1.
Table 1.
Average fruit surface conductance (gc), respiration rate (Rf), air temperature, humidity, vapour pressure deficit (Da), and modelled daily fruit transpiration (Ef), for Porterville, CA, USA and Te Puke, New Zealand, for the month before harvest in 2004
| Variable | Porterville, CA | Te Puke, NZ |
| gc (mmol H2O m−2 s−1) | 4.1±0.3 | 4.6±0.3 |
| Rf (nmol CO2 g FW−1 s−1) | –0.167±0.008 | –0.061±0.006 |
| Air temperature (°C) | 19.0 (28.8) | 13.9 (18.6) |
| Humidity (%) | 58.5 (26.8) | 90.7 (70.4) |
| Da (kPa) | 1.21 (3.00) | 0.19 (0.67) |
| Ef (g d−1) | 0.89 (0.51–1.40) | 0.15 (0.00–0.36) |
Values are given with ±SE for gc and Rf, n=20 and 8, respectively; average daily maxima for temperature and Da, average daily minima for humidity, and range for Ef, in parentheses.
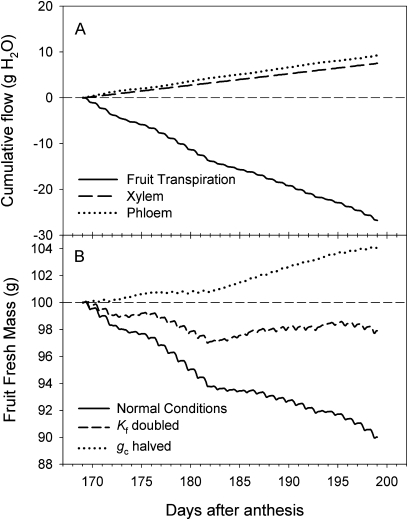
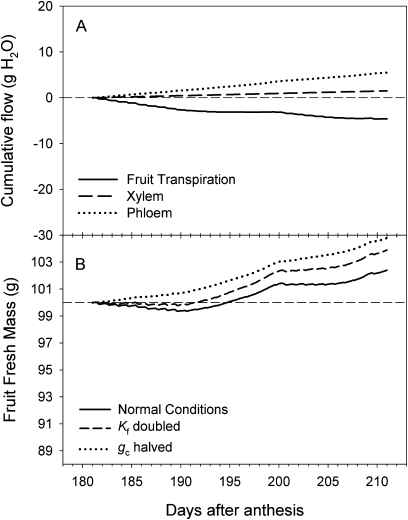
During the final month of the fruit growth cycle California was a much warmer, lower humidity environment than New Zealand (Table 1). Daily average and maximum vapour pressure deficits were between four and six times higher in California than in New Zealand, resulting in a 6-fold higher average estimated daily fruit transpiration in California (Table 1). The fruit water balance model predicted approximately equal fluxes of water into the fruit via the xylem and phloem (Fig. 5A), but a higher rate of loss of water via transpiration, resulting in a net decrease in fruit fresh mass of ∼10 g over the 1 month period prior to harvest (Fig. 5B). The predicted water balance was very sensitive to estimates of both the fruit surface conductance and the hydraulic conductance—halving of fruit surface conductance decreased transpiration and shifted the water balance to a net gain, and doubling of hydraulic conductance increased xylem fluxes and reduced the net loss of water to 2 g (Fig. 5B). When the same water balance model was run using New Zealand meteorological conditions, all predicted fluxes were reduced and the net water balance was positive for most of the period prior to harvest (Fig. 6). In particular, transpirational water losses were a less dominant feature of the water balance, and xylem fluxes were reduced compared with phloem fluxes because of the (estimated) reduction in the xylem pressure gradient.
Fig. 5.
Model predictions of the water balance of a 100 g Actinidia chinensis ‘Hort16A’ fruit for the final 30 d of fruit development under Californian meteorological conditions, incorporating the effect of changes in parameter values for fruit hydraulic conductivity (Kf) and surface conductance to water vapour (gc). (A) Fruit transpiration, and xylem and phloem flows under normal conditions of measured Kf and gc. (B) Change in fruit fresh mass calculated as the starting fresh mass plus the cumulative sum of transpiration+xylem flow+phloem flow, using measured Kf and gc, with Kf doubled, or gc halved.
Fig. 6.
Model predictions of the water balance of a 100 g Actinidia chinensis ‘Hort16A’ fruit for the final 30 d of fruit development under New Zealand meteorological conditions, incorporating the effect of changes in parameter values for fruit hydraulic conductivity (Kf) and surface conductance to water vapour (gc). (A) Fruit transpiration, and xylem and phloem flows under normal conditions of measured Kf and gc. (B) Change in fruit fresh mass calculated as the starting fresh mass plus the cumulative sum of transpiration+xylem flow+phloem flow, using measured Kf and gc, with Kf doubled, or gc halved.
Growth and vascular flows under controlled conditions
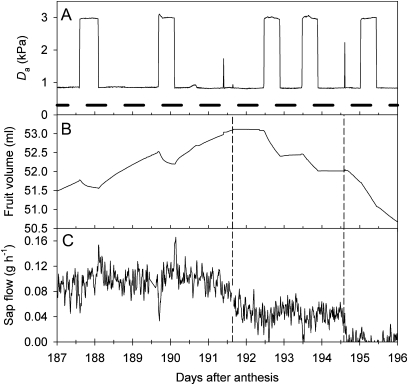
Consistent fruit growth and pedicel sap flow responses were observed when fruiting plants were exposed to controlled changes in Da (Figs 7, 8). Fruit volume immediately began to decrease when growing fruit were exposed to a step increase in Da. With the pedicel intact, the growth response to increased Da was curvilinear, with an initial rapid transition to negative growth, followed by a gradual decrease in the rate of volume loss. A return to the lower Da caused volume growth to recover, initially at higher rates than observed before the step increase (Fig. 7). Phloem girdling caused an immediate cessation in growth, and a more linear decrease in volume with step increases in Da. After severing the xylem, volume declined linearly at a rate proportional to the driving force for evaporation (Fig. 7).
Fig. 7.
Changes in (A) vapour pressure deficit (Da), (B) fruit volume, and (C) pedicel sap flow, for a fruiting Actinidia chinensis ‘Hort16A’ plant temporarily exposed to controlled conditions. Da was varied abruptly between 0.9 kPa and 3.0 kPa, and the resulting changes in fruit growth and sap flow observed. The pedicel phloem was heat girdled on day 191 (left-hand vertical dashed line; sap flow measurements were briefly interrupted by the resulting temperature gradients) and the pedicel was cut, severing the xylem, on day 194 (right-hand dashed line). Horizontal bars in A indicate dark periods.
Fig. 8.
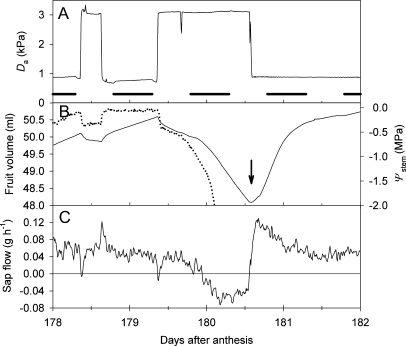
Changes in (A) vapour pressure deficit (Da), (B) fruit volume (solid line) and stem water potential (Ψstem, dotted line), and (C) pedicel sap flow, for a fruiting Actinidia chinensis ‘Hort16A’ plant temporarily exposed to controlled conditions. Experimental conditions were similar to those described for Fig. 7, except that watering was withheld, resulting in wilting on days 179 and 180, followed by recovery after re-watering (downward-facing arrow). Ψstem is the output from a leaf psychrometer installed on a non-transpiring leaf. The psychrometer failed on day 180 as the leaf desiccated, returning a value of 0 MPa after the vapour seal to the leaf was lost.
The pedicel sap flow response to step changes in Da was usually biphasic, with a transient decrease in flow towards the fruit when Da was raised, followed by a more gradual return to pre-existing or higher levels while the Da remained high (Figs 7, 8). The opposite response, a transient increase in flow towards the fruit, was observed when the Da was lowered. These transient changes in sap flow were observed with and without intact pedicel phloem, indicating that they were occurring in the xylem (Fig. 7). However, girdling caused a reduction in sap flow under steady-state conditions, suggesting that at least half or more of the flow in an intact pedicel was occurring in the phloem. Sap flow reversal (flow from the fruit to the plant) and a rapid decline in fruit volume were observed when a plant was allowed to dehydrate during a prolonged period of elevated Da. Upon re-watering, a surge in sap flow towards the fruit and a rapid recovery in fruit volume were observed. Psychrometer measurements confirmed that the step changes in Da were causing step changes in stem water potential, although leaf wound responses usually caused the leaf psychrometer to fail within a day or two of installation on a kiwifruit leaf (Fig. 8).
To summarize observations across all five plants examined, intact phloem was required for fruit growth—in two plants, positive fruit growth ceased soon after the experiment began, and no fruit growth was observed in the other plants after phloem girdling. Intact xylem was required for maintenance of fruit volume in the absence of positive growth (before or after phloem girdling). Transient fluctuations in sap flow in response to step changes in Da, before and after phloem girdling, and with or without positive growth occurring, indicated that the xylem was still functional and that flows were responsive to changes in pressure induced by changes in evaporation from the leaves and fruit.
Discussion
The initial hypothesis was that late season fruit growth in kiwifruit was primarily dependent on phloem flows, and that the shrivel disorder was caused by the cessation or reversal of xylem flows. Instead, the results demonstrated that both the phloem and xylem remained functional and contributed positively to the water balance of the fruit. Fruit growth in the orchard was negatively affected by disruption of both the phloem and the xylem. Shoot dehydration and dye infiltration treatments confirmed that the xylem was functional and able to conduct water to or from the fruit, and pressure potential measurements showed that the pressure gradients driving apoplastic flows remained in favour of fluxes from the vine to the fruit. The model of the fruit water balance suggested that fluxes of water in the phloem are usually equal to or higher than xylem flows at this stage of development, but that both tissues contribute significantly to hydration and growth of the fruit. This situation contrasts with current understanding of vascular flows to the post-véraison grape berry, which are thought to be dominated by phloem flows, even though the xylem remains functional (Bondada et al., 2005; Chatelet et al., 2008; Choat et al., 2009). What then was the cause of abnormal fruit development in California, and what are the usual contributions of the phloem and xylem to kiwifruit berry growth late in the season?
Measurement and modelling of fruit transpiration indicate that the late season water balance of fruit in California was dominated by transpiration. An immediate transition from negative to positive growth when transpiration was restricted by bagging supports this conclusion. Predicted daily transpiration exceeded estimates of phloem and xylem flows, based on measured rates of respiration and apoplasmic pressure gradients, respectively. In the cooler, higher humidity orchard environment in New Zealand, the model predicted lower vascular fluxes, but also lower rates of transpiration, and a slightly positive water balance over the same period. Phloem fluxes may have been underestimated in both environments, because they did not include allowance for growth in dry weight in the fruit. However, growth in fresh and dry weight of this cultivar typically slows as it begins maturing on the vine, and it is not unusual to observe slight decreases in fruit volume at this time of the season in New Zealand (Minchin et al., 2003b). Addition of moderate phloem fluxes to the model to account for possible accumulation in dry weight, or variation from the assumed phloem sap concentration (Clark and Smith, 1988; Morandi et al., 2010), is therefore unlikely to compensate for the high rates of transpiration observed in California.
Three lines of evidence indicate that phloem and xylem water contributed approximately equally to the water balance of the maturing fruit. Detachment resulted in approximately double the decrement in fruit volume per day compared with phloem girdling (Fig. 2); the water balance model suggested that the phloem flux required to sustain respiration equalled or exceeded xylem flows (Fig. 5); and in the controlled environment experiments, pedicel sap flow declined by approximately half when the phloem was girdled (Fig. 7). If girdling caused damage and reduced the hydraulic conductance of the xylem, the contribution by xylem water may have been underestimated. Subtractive girdling and severance methods for estimating the relative contributions of phloem and xylem flows to the water balance of growing fruit (Lang and Thorpe, 1989) rely on the tenuous assumption that the two vascular tissues are independent, with disruption of one not affecting flows in the other (Fishman and Genard, 2000). However, for reasons discussed below, it did appear that at this stage of development the behaviour of vascular flows in response to phloem girdling was additive. A recent study of berry growth in a different kiwifruit cultivar using a subtraction technique described a gradual increase in the contribution of phloem to the fruit water balance, from ∼10% early in development to 50% during fruit maturation (Morandi et al., 2010), a finding in agreement with the present study. In comparison, estimated phloem contributions in tomato increased from 90% to 98% during tomato fruit development (Ho et al., 1987; but see also Windt et al., 2009), and from 10% to 85% in grapes, with an abrupt shift occurring at veraison (Greenspan et al., 1994). Xylem contributions to total water flux in kiwifruit are therefore relatively high compared with those in these two well studied fruits, an observation which may be linked to the accumulation of starch, rather than high amounts of soluble sugars throughout most of development, and associated potential differences in phloem unloading mechanisms (Patrick, 1997; Nardozza et al., 2010).
The most likely explanation for the abnormal late season fruit development in California is that this cultivar has a high surface conductance and transpiration rate compared with the standard green kiwifruit (A. deliciosa ‘Hayward’), which can be grown successfully in the same environment (Walton and DeJong, 1990). The present study was not a comparison of the two species, but in New Zealand the yellow cultivar consistently has a higher surface conductance than the green cultivar when the two are grown in the same environment (25–40% higher; MJC, unpublished data). The surface conductance of the kiwifruit berry declines exponentially during fruit development as the surface hairs die and the exocarp thickens and suberizes (Hallett and Sutherland, 2005). Conductance would normally have reached low and relatively stable values by the time of season examined in this study (Smith et al., 1995; Montanaro et al., 2006). Xylem functionality (inferred from dye uptake by excised fruit) and hydraulic conductance also decline, for unknown reasons (Dichio et al., 2003; Mazzeo, 2008). The onset of negative growth may therefore be the consequence of a gradual decline in xylem hydraulic conductance, resulting in reduced xylem flows that eventually reach a threshold where they no longer balance the rate of loss of water through continued transpiration. Whether a decline in phloem flows also contributes is unknown, but the orchard-based and controlled environment experiments indicate that both tissues do still contribute positively to the water balance of the fruit, and that xylem backflow to the vine does not contribute significantly to negative growth. This pattern of continuing, but inadequate, vascular fluxes would explain the longitudinal pattern of shrivel within the fruit, which is observed earlier and more severely at the stylar end of the fruit (Thorp et al., 2007).
Role of the xylem in late season fruit development
Inward xylem flows are necessary to maintain a positive fruit water balance during normal late season fruit development. The controlled environment experiment confirmed that inward xylem flows were still occurring in vivo, and in the absence of phloem flows were usually adequate to prevent a decrease in fruit volume, provided Da was low. In many fruits, xylem flows are correlated with fruit transpiration rates (Morandi et al., 2010). While fruit surface conductance generally declines in most fruits during development, transpiration remains a significant component of the water balance in mature fruits (Greenspan et al., 1994). Late season kiwifruit berry surface conductance to water vapour (4 mmol m−2 s−1; this study) is comparable with that in tomato (0.4–6 mmol m−2 s−1; Leonardi et al., 1999; Leide et al., 2007) and grapes (1 mmol m−2 s−1; Rogiers et al., 2004). It is likely that xylem flows in response to transpiration will also continue in these fruits, particularly during the later stages of ripening when dry matter accumulation, and by inference phloem flows, decrease. However, recent literature for tomato and the post-véraison grape emphasizes the role of the phloem in late season fruit development, with the xylem instead viewed as a potential pathway for loss of phloem water by backflow to the plant (Guichard et al., 2005; Choat et al., 2009; Tilbrook and Tyerman, 2009). Dye infiltration and hydraulic measurements have confirmed that the xylem remains functional in grapes as a potential pathway for backflow to occur (Tilbrook and Tyerman, 2009), but it is important to note that there have been no direct observations of sustained backflow in grapes during normal development. The only direct observations of xylem flow from fruit to plant, under non-stressed conditions, of which the present authors are aware, are transient morning reversals in sap flow observed in mangoes (Higuchi and Sakuratani, 2006) and kiwifruit (Clearwater et al., 2009) in response to diurnal stem xylem pressure fluctuations. Sustained reversal of xylem flows from fruit probably only occurs when plants undergo severe water stress (Fig. 8; Greenspan et al., 1996). A recent magnetic resonance imaging (MRI) study also concluded that the role of the xylem in supplying truss development in tomato has been underestimated (Windt et al., 2009). It is concluded that an imbalance of inward flows relative to transpirational loss is a better explanation for most instances of negative growth in kiwifruit, grapes (Greer and Rogiers, 2009), and other fruits. There is also a clear need for additional studies that better quantify the water balance of developing fruit and directly measure flows in both vascular tissues.
Dynamic vascular flows in ripening fruit
The combination of a controlled environment and continuous measurements of both fruit diameter and pedicel sap flow provided a unique insight into the role of vascular flows in fruit development. Apart from the recent MRI study of a single tomato truss (Windt et al., 2009), it is believed these are the first direct observations of fruit pedicel vascular flows in response to manipulative treatments. The combination of relatively high phloem compared with xylem fluxes in a stable environment also allows the reporting of the first verifiable observations of phloem flow using a heat-based sap flow technique (Clearwater et al., 2009). The results show that intact pedicel phloem was required for positive fruit growth, and phloem girdling caused a pronounced change in the dynamics of the growth response to a step change in evaporative demand. Prior to girdling, curvilinear changes in fruit volume following a change in evaporative demand suggest active adjustment of phloem flows in response to fruit water status. Whether phloem unloading in kiwifruit is entirely symplasmic, or includes an apoplasmic step, is unknown, but it can be hypothesized that a decrease in water potential caused an up-regulation of the unloading process, resulting in a gradual recovery of growth in the low humidity environment. Maintaining the low humidity environment for an extended period was cumbersome with the controlled environment chamber used in this study, but, on at least one occasion, a recovery from negative to positive fruit growth was observed after 12 h when the Da was held low for >30 h (not shown). The time constant for the growth response to low Da appeared to be much longer than the more passive pressure-driven responses observed after phloem girdling. This observation suggests that increased fruit transpiration stimulates phloem unloading, as observed in grapes (Rebucci et al., 1997), and that the response may be more active than an increase in flow resulting from a decrease in sieve element or sink cell turgor.
Growth responses to evaporation after girdling suggest that the xylem provided water for evaporation but could not support growth. After phloem girdling, the growth response to Da could be described as passive, with steady-state xylem flows equalling (no net growth at low Da) or less than (negative growth at high Da) losses to transpiration. Transient fluctuations in xylem sap flow in response to changes in Da resemble the response of a capacitor (the fruit) discharging or recharging across a resistor (the fruit to shoot hydraulic resistance). When Da is raised, increasing transpiration from both the shoot and fruit, shoot xylem pressure falls faster than pressure at the distal end of the pedicel, because of the large and relatively elastic volume of the fruit. The pressure gradient across the pedicel is reduced and sap flow to the fruit is transiently reduced until the capacitor has discharged and the pressure gradient is restored. The opposite process of capacitor recharge occurs when Da is lowered (Fig. 8). These transient responses demonstrate that xylem flow is responsive to pressure gradients between the fruit and shoot, even at this late stage of development (cf. Morandi and Grappadelli, 2009). If growth occurs when water moves towards expanding cells down a water potential gradient (Boyer and Silk, 2004), why do xylem fluxes not substitute for phloem water after girdling and allow growth to continue, at least temporarily? A potential explanation is that turgor in the maturing fruit is already close to zero (Thomas et al., 2008); following girdling, a decrease in turgor and growth-sustaining water potential large enough to compete for already limited xylem water may not be possible (Boyer and Silk, 2004). Girdling may also prevent continued solute accumulation by expanding cells. In contrast, earlier in berry development (60 DAA), both turgor and xylem hydraulic conductance are higher and kiwifruit berry growth can continue after phloem girdling, at similar rates to pre-girdling, for at least 3 d (MJC, unpublished data).
Overall these results suggest that the normal development of ripening kiwifruit berries requires the continued functioning of both phloem and xylem tissues, with inward flows predominating in both tissues. Ripening fruit may be partially hydraulically buffered by phloem-derived water (sensu Choat et al., 2009), in that xylem flows alone are insufficient to support transpiration, but recycling of water to the plant via the xylem does not appear to be a significant component of the fruit water balance in this system. Further understanding of the role of the phloem and xylem in fruit ripening and softening will require better quantification and more sophisticated modelling of fruit growth and the fluxes in both tissues.
Acknowledgments
We thank Mike Currie, Kevin Patterson, Bill Snelgar, Murray Judd, Phil Martin, and Peter Minchin for assistance with various aspects of this work. Research was funded by ZESPRI Group Ltd and the New Zealand Foundation for Research Science and Technology (Contract C06X0706).
References
- Boldingh H, Smith GS, Klages K. Seasonal concentrations of non-structural carbohydrates of five Actinidia species in fruit, leaf and fine root tissue. Annals of Botany. 2000;85:469–476. [Google Scholar]
- Bondada BR, Matthews MA, Shackel KA. Functional xylem in the post-veraison grape berry. Journal of Experimental Botany. 2005;56:2949–2957. doi: 10.1093/jxb/eri291. [DOI] [PubMed] [Google Scholar]
- Boyer JS. Measuring the water status of plants and soils. San Diego: Academic Press; 1995. [Google Scholar]
- Boyer JS, Silk WK. Hydraulics of plant growth. Functional Plant Biology. 2004;31:761–773. doi: 10.1071/FP04062. [DOI] [PubMed] [Google Scholar]
- Chatelet DS, Rost TL, Matthews MA, Shackel KA. The peripheral xylem of grapevine (Vitis vinifera) berries. 2. Anatomy and development. Journal of Experimental Botany. 2008;59:1997–2007. doi: 10.1093/jxb/ern061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choat B, Gambetta GA, Shackel KA, Matthews MA. Vascular function in grape berries across development and its relevance to apparent hydraulic isolation. Plant Physiology. 2009;151:1677–1687. doi: 10.1104/pp.109.143172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CJ, Smith GS. Seasonal accumulation of mineral nutrients by kiwifruit. 2. Fruit. New Phytologist. 1988;108:399–409. [Google Scholar]
- Clearwater MJ, Luo ZW, Mazzeo M, Dichio B. An external heat pulse method for measurement of sap flow through fruit pedicels, leaf petioles and other small-diameter stems. Plant, Cell and Environment. 2009;32:1652–1663. doi: 10.1111/j.1365-3040.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- Dichio B, Remorini D, Lang S. Developmental changes in xylem functionality in kiwifruit fruit: implications for fruit calcium accumulation. Acta Horticulturae. 2003;610:191–195. [Google Scholar]
- During H, Lang A, Oggionni F. Patterns of water flow in Riesling berries in relation to developmental changes in their xylem morphology. Vitis. 1987;26:123–131. [Google Scholar]
- Ferguson AR. Kiwifruit cultivars: breeding and selection. Acta Horticulturae. 1999;498:43–51. [Google Scholar]
- Findlay N, Oliver KJ, Nii N, Coombe BG. Solute accumulation by grape pericarp cells. Journal of Experimental Botany. 1987;38:668–679. [Google Scholar]
- Fishman S, Genard M. A biophysical model of fruit growth: simulation of seasonal and diurnal dynamics of mass. Plant, Cell and Environment. 1998;21:739–752. [Google Scholar]
- Fishman S, Genard M. Simulation of fruit growth as a tool for estimation of systematic errors in experiments separating the xylem and phloem flows. Proceedings of the 14th European Simulation Multiconference on Simulation and Modelling: Enablers for A Better Quality of Life. 2000:702–705. [Google Scholar]
- Greenspan MD, Schultz HR, Matthews MA. Field evaluation of water transport in grape berries during water deficits. Physiologia Plantarum. 1996;97:55–62. [Google Scholar]
- Greenspan MD, Shackel KA, Matthews MA. Developmental-changes in the diurnal water-budget of the grape berry exposed to water deficits. Plant, Cell and Environment. 1994;17:811–820. [Google Scholar]
- Greer DH, Rogiers SY. Water flux of Vitis vinifera L. cv. Shiraz bunches throughout development and in relation to late-season weight loss. American Journal of Enology and Viticulture. 2009;60:155–163. [Google Scholar]
- Guichard S, Gary C, Leonardi C, Bertin N. Analysis of growth and water relations of tomato fruits in relation to air vapor pressure deficit and plant fruit load. Journal of Plant Growth Regulation. 2005;24:201–213. [Google Scholar]
- Hall GE, Bondada BR, Keller M. Loss of rachis cell viability is associated with ripening disorders in grapes. Journal of Experimental Botany. 2011;62:1145–1153. doi: 10.1093/jxb/erq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett IC, Sutherland PW. Structure and development of kiwifruit skins. International Journal of Plant Sciences. 2005;166:693–704. [Google Scholar]
- Higuchi H, Sakuratani T. Water dynamics in mango (Mangifera indica L.) fruit during the young and mature fruit seasons as measured by the stem heat balance method. Journal of the Japanese Society for Horticultural Science. 2006;75:11–19. [Google Scholar]
- Ho LC, Grange RI, Picken AJ. An analysis of the accumulation of water and dry matter in tomato fruit. Plant, Cell and Environment. 1987;10:157–162. [Google Scholar]
- Hopping ME. Structure and development of fruit and seeds in Chinese gooseberry (Actinidia chinensis Planch.) New Zealand Journal of Botany. 1976;14:63–68. [Google Scholar]
- Hopping ME. Floral biology, pollination, and fruit set. In: Warrington IJ, Weston GC, editors. Kiwifruit: science and management. Auckland: NZ Society for Horticultural Science and Ray Richards Publisher; 1990. pp. 71–96. [Google Scholar]
- Keller M, Smith JP, Bondada BR. Ripening grape berries remain hydraulically connected to the shoot. Journal of Experimental Botany. 2006;57:2577–2587. doi: 10.1093/jxb/erl020. [DOI] [PubMed] [Google Scholar]
- Krasnow MN, Matthews MA, Smith RJ, Benz J, Weber E, Shackel KA. Distinctive symptoms differentiate four common types of berry shrivel disorder in grape. California Agriculture. 2010;64:155–159. [Google Scholar]
- Lang A. Xylem, phloem and transpiration flows in developing apple fruits. Journal of Experimental Botany. 1990;41:645–651. [Google Scholar]
- Lang A, During H. Partitioning control by water potential gradient: evidence for compartmentation breakdown in grape berries. Journal of Experimental Botany. 1991;42:1117–1122. [Google Scholar]
- Lang A, Thorpe MR. Xylem, phloem and transpiration flows in a grape: application of a technique for measuring the volume of attached fruits to high resolution using Archimedes’ principle. Journal of Experimental Botany. 1989;40:1069–1078. [Google Scholar]
- Leide J, Hildebrandt U, Reussing K, Riederer M, Vogg G. The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: effects of a deficiency in a beta-ketoacyl-coenzyme A synthase (LeCER6) Plant Physiology. 2007;144:1667–1679. doi: 10.1104/pp.107.099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi C, Baille A, Guichard S. Effects of fruit characteristics and climatic conditions on tomato transpiration in a greenhouse. Journal of Horticultural Science and Biotechnology. 1999;74:748–756. [Google Scholar]
- Matthews MA, Shackel KA. Growth and water transport in fleshy fruit. In: Holbrook NM, Zwieniecki MA, editors. Vascular transport in plants. San Diego: Elsevier Academic Press; 2005. pp. 181–197. [Google Scholar]
- Mazzeo M. 2008. Xylem transport efficiency and calcium accumulation in fruit of Actinidia deliciosa: implications for fruit quality. PhD Thesis, University of Basilicata. [Google Scholar]
- McCarthy MG. Weight loss from ripening berries of Shiraz grapevines (Vitis vinifera L. cv. Shiraz) Australian Journal of Grape and Wine Research. 1999;5:10–16. [Google Scholar]
- McCarthy MG, Coombe BG. Is weight loss in ripening grape berries cv. Shiraz caused by impeded phloem transport? Australian Journal of Grape and Wine Research. 1999;5:17–21. [Google Scholar]
- Mcghie TK, Ainge GD. Color in fruit of the genus Actinidia: carotenoid and chlorophyll compositions. Journal of Agricultural and Food Chemistry. 2002;50:117–121. doi: 10.1021/jf010677l. [DOI] [PubMed] [Google Scholar]
- Milad RE, Shackel KA. Water relations of fruit end cracking in French prune (Prunus-Domestica l Cv French) Journal of the American Society for Horticultural Science. 1992;117:824–828. [Google Scholar]
- Minchin PEH, De Silva N, Snelgar WP, Richardson AC, Thorp TG. Modelling of colour development in the fruit of Actinidia chinensis ‘Hort16A’. New Zealand Journal of Crop and Horticultural Science. 2003a;31:41–53. [Google Scholar]
- Minchin PEH, Richardson AC, Patterson KJ, Martin PJ. Prediction of final weight for Actinidia chinensis ‘Hort16A’ fruit. New Zealand Journal of Crop and Horticultural Science. 2003b;31:147–157. [Google Scholar]
- Montanaro G, Dichio B, Xiloyannis C, Celano G. Light influences transpiration and calcium accumulation in fruit of kiwifruit plants (Actinidia deliciosa var. deliciosa) Plant Science. 2006;170:520–527. [Google Scholar]
- Morandi B, Grappadelli LC. Source and sink limitations in vascular flows in peach fruit. Journal of Horticultural Science and Biotechnology. 2009 ISAFRUIT Special Issue, 150–156. [Google Scholar]
- Morandi B, LoSciale P, Manfrini L, Zibordi M, Studhalter M, Grappadelli LC. The growth of the kiwifruit in its final stages. Acta Horticulturae. 2007;753:369–374. [Google Scholar]
- Morandi B, Manfrini L, LoSciale P, Zibordi M, Grappadelli L. Changes in vascular and transpiration flows affect the seasonal and daily growth of kiwifruit (Actinidia deliciosa) berry. Annals of Botany. 2010;105:913–923. doi: 10.1093/aob/mcq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardozza S, Boldingh HL, Richardson AC, Costa G, Marsh H, MacRae EA, Clearwater MJ. Variation in carbon content and size in developing fruit of Actinidia deliciosa genotypes. Functional Plant Biology. 2010;37:545–554. doi: 10.1071/FP10158. [DOI] [PubMed] [Google Scholar]
- Patrick JW. Phloem unloading: sieve element unloading and post-sieve element transport. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:191–222. doi: 10.1146/annurev.arplant.48.1.191. [DOI] [PubMed] [Google Scholar]
- Rebucci B, Poni S, Intrieri C, Magnanini E, Lakso AN. Effects of manipulated grape berry transpiration on post-veraison sugar accumulation. Australian Journal of Grape and Wine Research. 1997;3:57–65. [Google Scholar]
- Richardson AC, McAneney KJ, Dawson TE. Carbohydrate dynamics in kiwifruit. Journal of Horticultural Science. 1997;72:907–917. [Google Scholar]
- Rogiers SY, Greer DH, Hatfield JM, Orchard BA, Keller M. Solute transport into Shiraz berries during development and late-ripening shrinkage. American Journal of Enology and Viticulture. 2006;57:73–80. [Google Scholar]
- Rogiers SY, Hatfield JM, Jaudzems VG, White RG, Keller M. Grape berry cv. shiraz epicuticular wax and transpiration during ripening and preharvest weight loss. American Journal of Enology and Viticulture. 2004;55:121–127. [Google Scholar]
- Smith GS, Klages KU, Green TGA, Walton EF. Changes in abscisic acid concentration, surface conductance, and water content of developing kiwifruit. Scientia Horticulturae. 1995;61:13–27. [Google Scholar]
- Thomas TR, Shackel KA, Matthews MA. Mesocarp cell turgor in Vitis vinifera L. berries throughout development and its relation to firmness, growth, and the onset of ripening. Planta. 2008;228:1067–1076. doi: 10.1007/s00425-008-0808-z. [DOI] [PubMed] [Google Scholar]
- Thorp TG, Clearwater MJ, Barnett AM, Martin PJ, Blattmann P, Currie MB. ‘Hort16A’ fruit beak end softening and shrivel in California. Acta Horticulturae. 2007;753:389–396. [Google Scholar]
- Tilbrook J, Tyerman SD. Cell death in grape berries: varietal differences linked to xylem pressure and berry weight loss. Functional Plant Biology. 2008;35:173–184. doi: 10.1071/FP07278. [DOI] [PubMed] [Google Scholar]
- Tilbrook J, Tyerman SD. Hydraulic connection of grape berries to the vine: varietal differences in water conductance into and out of berries, and potential for backflow. Functional Plant Biology. 2009;36:541–550. doi: 10.1071/FP09019. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Tilbrook J, Pardo P, Kotula L, Sullivan W, Steudle E. Direct measurement of hydraulic properties in developing berries of Vitis vinifera L. cv Shiraz and Chardonnay. Australian Journal of Grape and Wine Research. 2004;10:170–181. [Google Scholar]
- Walton EF, DeJong TM. Growth and compositional changes in kiwifruit berries from three Californian locations. Annals of Botany. 1990;66:285–298. [Google Scholar]
- Windt CW, Gerkema E, Van As H. Most water in the tomato truss is imported through the xylem, not the phloem: a nuclear magnetic resonance flow imaging study. Plant Physiology. 2009;151:830–842. doi: 10.1104/pp.109.141044. [DOI] [PMC free article] [PubMed] [Google Scholar]