Abstract
Production of reactive oxygen species (ROS) is linked to signalling in both developmental and stress responses. The level of ROS is controlled by both production and removal through various scavengers including ascorbic acid and glutathione. Here, the role of low ascorbic acid or glutathione concentrations was investigated on ozone-induced cell death, defence signalling, and developmental responses. Low ascorbic acid concentrations in vtc1 activated expression of salicylic acid (SA)-regulated genes, a response found to be dependent on the redox-regulated transcriptional co-regulator NPR1. In contrast, low glutathione concentrations in cad2 or pad2 reduced expression of SA-regulated genes. Testing different responses to jasmonic acid (JA) revealed the presence of at least two separate JA signalling pathways. Treatment of the vtc1 mutant with JA led to hyper-induction of MONODEHYDROASCORBATE REDUCTASE3, indicating that low ascorbic acid concentrations prime the response to JA. Furthermore, NPR1 was found to be a positive regulator of JA-induced expression of MDHAR3 and TAT3. The vtc1 and npr1 mutants were sensitive to glucose inhibition of seed germination; an opposite response was found in cad2 and pad2. Overall, low ascorbic acid concentrations mostly led to opposite phenotypes to low glutathione concentrations, and both antioxidants interacted with SA and JA signalling pathways.
Keywords: Ascorbic acid, defence signalling, glutathione, jasmonic acid, ozone, salicylic acid
Introduction
For successful survival, plants have to adapt and acclimatize to the surrounding environment. One common consequence of exposure to abiotic stress conditions, for example extreme temperatures or high light levels, is the production of reactive oxygen species (ROS). Far from being only damaging agents, ROS are also used by plants as signalling molecules in a variety of process ranging from defence against pathogens to root development (Apel and Hirt, 2004; Foyer and Noctor, 2005a; Jaspers and Kangasjärvi, 2010; Torres, 2010). It has become increasingly clear that signalling pathways in plants are not organized into linear pathways; instead, defence signalling is better described as a web of interactions. Not even individual ROS give uniform responses; instead, separate molecules (ozone, hydrogen peroxide, superoxide, and singlet oxygen), acting at different subcellular locations give rise to unique changes in gene expression (Gadjev et al., 2006; Wrzaczek et al., 2010). ROS production and scavenging are intimately linked, and the balance between them will determine defence signalling output as well as damage and cell-death responses. An extensive repertoire of enzymatic ROS scavengers (including catalase, superoxide dismutase, and ascorbate peroxidase) and low-molecular-weight scavengers (including ascorbic acid, glutathione, and α-tocopherol) protect plants from excessive ROS production. Ascorbic acid and glutathione are connected through the ascorbic acid-glutathione cycle (Noctor and Foyer, 1998) and are essential for plants; Arabidopsis thaliana mutants with very low concentrations of either compound have severe developmental defects (Vernoux et al., 2000; Dowdle et al., 2007).
In addition to ROS scavenging and redox chemistry, ascorbic acid also has a role in regulating the cell cycle (Potters et al., 2004) and is a substrate or cofactor of many enzymes (Arrigoni and De Tullio, 2002). Arabidopsis mutants with low ascorbic acid concentration were initially isolated based on increased sensitivity to the air pollutant ozone (Conklin et al., 2000). Further characterizations of these mutants have been instrumental in defining ascorbic acid biosynthesis in plants (Linster and Clarke, 2008) and have also shown a role for ascorbic acid in several conditions including pathogen defence and senescence (Veljovic-Jovanovic et al., 2001; Pastori et al., 2003; Barth et al., 2004).
Similar to ascorbic acid, the tri-peptide glutathione has a role in redox chemistry and in addition has multiple roles in plant development, cell-cycle regulation, heavy-metal detoxification, and regulation of protein activity (Rouhier et al., 2008; Vivancos et al., 2010). The Arabidopsis cad2 (cadmium sensitive2) and pad2 (phytoalexin deficient 2) mutants were originally isolated based on sensitivity to cadmium and impaired biosynthesis of the secondary metabolite camalexin, respectively (Cobbett et al., 1998; Parisy et al., 2007). Both mutants have low glutathione concentrations and contain mutations in the same gene, γ-glutamylcysteine synthetase, the first step of glutathione biosynthesis. A third allelic mutant, rax1 (regulator of ASCORBATE PEROXIDASE2-1), was isolated based on constitutive expression of an antioxidant marker gene APX2 (Ball et al., 2004). These mutants contain contrasting amounts of glutathione (Parisy et al., 2007), which leads to somewhat different phenotypes; for example, rax1 and cad2 have different gene-expression patterns (Ball et al., 2004).
Ascorbic acid and glutathione are central components in regulating the redox balance of the plant cell. The stress hormone salicylic acid (SA) activates defence signalling pathway(s) through NON-EXPRESSOR OF PR-PROTEINS1 (NPR1), which is one of the few known redox-regulated signalling proteins in plants. Inactive NPR1 is present in a cytosolic oligomer complex through intermolecular disulfide bonds (Mou et al., 2003). SA signalling activates thioredoxin TRX-h5 leading to reduction in NPR1, thus converting it to active monomers that are translocated from the cytosol into the nucleus activating defence gene expression (Tada et al., 2008). In vtc1 grown under non-stressed control conditions (but not the wild type), a NPR1-GFP fusion can be detected in the nucleus, indicating that the altered redox status of vtc1 constitutively activates the NPR1 signalling pathway (Pavet et al., 2005). Consistent with this, vtc1 and vtc2 have a high expression of PATHOGENESIS RELATED1 (PR-1) (Colville and Smirnoff, 2008; Mukherjee et al., 2010). In contrast, PR-1 expression in cad2 is lower than the wild type; indicating that plants with low glutathione concentrations to some extent have opposite phenotypes to plants with low ascorbic acid concentrations (Ball et al., 2004). These contrasting phenotypes are also seen in response to infection with Pseudomonas syringae where vtc1 and vtc2 are more tolerant, while rax1, cad2, and pad2 are more sensitive (Ball et al., 2004; Pavet et al., 2005; Parisy et al., 2007). Defence-related phenotypes of mutants with low ascorbic acid and glutathione concentrations are summarized in Table 1. The linkage between ROS production and scavenging, and the role of ROS, ascorbic acid, and glutathione as signalling molecules themselves, makes it challenging (if even possible) to determine the exact role of individual molecules in plant defence responses. Hence, the terminology ‘oxidative signalling’ has been proposed to encompass the response to ROS, ascorbic acid, and glutathione (Foyer and Noctor, 2005a, b).
Table 1.
Stress-related phenotypes of Arabidopsis mutants with low ascorbic acid or glutathione concentrations
| vtc1 | vtc2-1 | vtc2 vtc5 | rax1 | cad2 | pad2 | rml1 | References | |
| Ascorbic acid | ≈30 | ≈20–30 | ≈0 | ND | WT | ND | ND | Conklin et al., 2000 |
| content compared | Veljovic-Jovanovic et al., 2001 | |||||||
| with WT (%) | Pavet et al., 2005 | |||||||
| Dowdle et al., 2007 | ||||||||
| Colville and Smirnoff, 2008 | ||||||||
| Glutathione | WT | WT | ND | ≈38 | ≈30 | ≈22 | ≈3 | Pavet et al., 2005 |
| content | Vernoux et al., 2000 | |||||||
| compared | Parisy et al., 2007 | |||||||
| with WT (%) | Colville and Smirnoff, 2008 | |||||||
| Ozone | Sensitive | Sensitive | ND | ND | WT | WT | ND | Conklin et al., 2000; this study |
| Virulent | Resistant | Resistant | ND | Sensitive | Sensitive | Sensitive | ND | Ball et al., 2004 |
| bacteria | Pavet et al., 2005 | |||||||
| Parisy et al., 2007 | ||||||||
| PR-1 gene | Increased | Increased | ND | Decreased | Decreased | ND | ND | Pastori et al., 2003 |
| expression | Ball et al., 2004 | |||||||
| Pavet et al., 2005 | ||||||||
| Colville and Smirnoff, 2008 | ||||||||
| Miscellaneous | Growth arrest | Defective root | Vernoux et al., 2000 | |||||
| after germination | growth | Dowdle et al., 2007 |
ND, not determined; WT, wild-type phenotype.
Here, the properties of the oxidative signalling network are explored further using mutants with low ascorbic acid (vtc1) or glutathione (cad2, pad2) concentration. NPR1 is one of the few known redox-regulated defence signalling proteins, and the concentrations of SA, glutathione and H2O2 are proposed to be co-regulated (Mateo et al., 2006). Thus, double mutants npr1 vtc1, npr1 cad2, and npr1 pad2 were constructed to determine the contribution of NPR1 in oxidative signalling. Also, a second stress hormone, jasmonic acid (JA), is shown to interact with ascorbic acid and glutathione in the regulation of the defence-gene expression.
Materials and methods
Plant material
Wild type Arabidopsis accession Columbia-0 (Col-0) was used as an ozone-tolerant control plant. Mutant seeds were obtained from the Nottingham Arabidopsis Stock Centre (NASC; http://arabidopsis.info/) or were gifts from Dr Patricia Conklin (vtc1-1) and Dr Christopher S. Cobbett (cad2-1). Double mutants were generated using npr1-1 as the pollen acceptor. Double mutants were identified using PCR-based markers: npr1-1 (TGCTCTTCATTTCGCTGTTG and GAGTGCGGTTCTACCTTCCA, NlaIII cuts WT), pad2 (TCTATTTGCGAATTCCCCTTT and CAAATGGTAGCATTCCTGTGC, DdeI cuts WT), cad2-1 (mutant for primer CATTATGAGAAACTACATGCTTGG, WT for primer GAGAAACTACATGCCGAAAGTTGG, rev primer GGCAATGGTTAGTCAAAATCG), and vtc1-1 (TGCATTTTCAGGAAAAGGAGTT and TTAGCAAAATCAACAAGGGGCCTTG, StyI cuts WT). All genotypes were confirmed in the F3 generation.
Seeds were vernalized for 3 d and then grown on 1:1 v/v mixture of peat and vermiculite with subirrigation. Growth conditions were 23°C/19°C (day/night), 70%/90% relative humidity, under a 12 h photoperiod with 200 μmol m−2 s−1 irradiance in controlled growth chambers (Weiss Bio1300; Weiss Umweltstechnik, Reiskirchen-Lindenstruth, Germany). For fresh-weight and flower-time experiments, plants were grown in growth rooms under the same growth conditions.
Ozone and MeJA treatment
Three-week-old Arabidopsis plants were exposed to 375 nl l−1 ozone for 6 h. Samples were harvested at 8 h after the onset of the ozone treatment. Cell death was quantified by ion leakage as previously described (Overmyer et al., 2000). Samples for RNA isolation were isolated from 3-week-old plants sprayed with 0.5 mM methyl jasmonate (MeJA) in 0.01% Triton-X100, and controls were sprayed with 0.01% Triton-X100. After 8 h, leaves were harvested, frozen in liquid nitrogen, and stored at –80°C until RNA was isolated.
Total antioxidant assay
Plants were grown as above for 3 weeks, harvested into 2 ml screw-cap tubes containing glass beads and sand, and frozen in liquid nitrogen. Extracts were prepared by adding ice-cold 50% acetone and shaking at 6800 rpm for 2×20 s in a Precellys 24 (Bertin Technologies, Montigny le Bretonneux, France). The oxygen radical absorbance capacity (ORAC) assay was performed as described by Gillespie et al. (2007). A Victor 1420 Wallac plate reader (Perkin-Elmer, Waltham, MA, USA) was used to measure the fluorescence, and the data were analysed in WorkOut 2.5 (Dazdaq, Brighton, UK).
Real-time quantitative RT-PCR
RNA isolation and cDNA synthesis were performed as previously described (Wrzaczek et al., 2010). The reverse-transcription reaction was diluted to a final volume of 60 μl, and 1 μl was used as template for the PCR using LightCycler 480 SYBR Green I master mix (Roche Diagnostics, Basel, Switzerland). PCR was performed in triplicate on a LightCycler 480 (Roche Diagnostics) with the following cycling conditions: 1 cycle for 10 min at 95 °C, 55 cycles at 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s followed by a melting-curve analysis. The primers used for PCR are listed in Supplementary Table S1 at JXB online. The raw threshold cycle (Ct) values were normalized against a reference gene (At4g34270) selected from Czechowski et al. (2005) and used to compare the results from WT with mutant, or mock treated control samples with treated samples as previously described (Wrzaczek et al., 2010). Amplification efficiency was calculated for each primer pair from standard curves with serially diluted cDNA.
MeJA root growth
Age-matched seeds were sterilized and planted on ½ MS plates, vernalized for 2 d at +4 °C and grown for 3 d at 200 μmol m−2 s−1 for 16/8 h light/dark, at 23 °C. Subsequently, seedlings were transferred to half-strength MS plates supplemented with 10 μM or 50 μM MeJA (dissolved in ethanol), or the corresponding amount of ethanol for the control. The root length was measured after 5 d. The coi1-16 mutant (a gift from Dr John Turner; Ellis and Turner, 2002) was used as a JA-insensitive control.
Analysis of publicly available gene-expression data
A list of genes encoding ascorbic acid and glutathione biosynthesis genes and ascorbic acid–glutathione cycle genes was compiled from AraCyc pathways (ftp://ftp.arabidopsis.org/home/tair/Pathways/). In addition, genes used in the qPCR analysis were added to the list. Publicly available experiments using the Affymetrix ATH1-121501 platform were obtained from several data sources: NASC Arrays http://affymetrix.arabidopsis.info/narrays/experimentbrowse.pl (ABA—NASCARRAYS-176; SA—NASCARRAYS-192; BTH—NASCARRAYS-392; Senescence experiment 1 NASCARRAYS-52; Senescence experiment 2—NASCARRAYS-150). ArrayExpress http://www.ebi.ac.uk/microarrayas/ae/ (MeJA—EATMX-13; Paraquat—E-ATMX-28). Gene Expression Omnibus http://www.ncbi.nlm.nih.gov/geo/ (H2O2—GSE5530; Salt—GSE5623; Heat—GSE19603; High light—GSE7743; sid2—GSE9955; lht1—GSE19109; Pseudomonas syringae ES4326—GSE18978; sni1- GSE6827; csn3, csn4 and csn5—GSE9728; cs26 long day—GSE19241; SA 24 h—GSE14961; siz1—GSE6583; mkk1mkk2—GSE10646; Ethylene—GSE14247; npr1—GSE13833; Botrytis cinerea infection—GSE5684; Ozone—GSE5722; vtc1 and vtc2—GSE19257; oas-a1—GSE19245). The Integrated Microarray Database System http://ausubellab.mgh.harvard.edu/imds (experiment names: NPR1 direct targets full genome and local and systemic responses to Trichoplusia ni feeding). Raw data for acd11 (Palma et al., 2010) were obtained from John Mundy. The raw.cel files were robust mulitiarray average normalized, and for each experiment the log2-base fold changes of treatment versus control, or mutant versus wild type, were computed. The preprocessed data were clustered using Bayesian hierarchical clustering as described in Wrzaczek et al. (2010).
Flower time and fresh weight
Plants were germinated and grown as described above with 12 individuals of each genotype, one plant per pot (6×6cm pot), with the pots placed randomly on three different trays in the growth room. Plant positions were randomized every 3 days. Fresh weight was measured on 3-week-old plants. For flower time, the plants were monitored daily, and the day of bud emergence was recorded.
Germination on sugar plates
Age-matched seeds were sown on plates with half-strength MS-Agar and supplemented with 0 mM, 300 mM glucose or 300 mM mannitol and vernalized for 3 ds. Plates were transferred to growth room with 23/19 °C (day/night), 70/90% relative humidity, under a 12 h photoperiod with an irradiance of 200 μmol m−2 s−1. Germination was scored after 7 d.
Statistics
Statistics were performed in GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). qPCR statistis was performed with Relative Expression Software Tool 2009 (Qiagen, Hilden, Germany). Ion-leakage data were analysed with a linear model and computing contrasts with Bonferroni corrections implemented in R (Bretz et al., 2010).
Results
Ozone-induced cell death
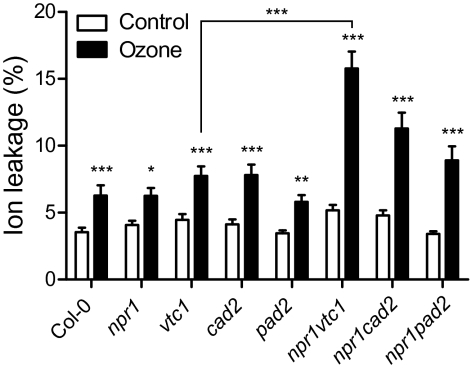
A short, high-concentration exposure to ozone causes visible lesions and cell death in sensitive plants (Wohlgemuth et al., 2002). Ascorbic acid is required for ozone defence (Conklin and Barth, 2004), but the role of glutathione is less clear. Wild-type Col-0 (WT) plants and mutants defective in ascorbic acid or glutathione biosynthesis and corresponding double mutants with npr1 were exposed to increasing amounts of ozone. Under our growth conditions, no visible damage was seen in any of the genotypes when exposed up to 300 nl l−1 ozone. After 375 nl l−1 ozone for 6 h, visible lesions were frequently observed only in npr1 vtc1. The magnitude of cell death was quantified with ion leakage (Fig. 1). Increased cell death was measured in all genotypes, which was further significantly increased in the double npr1 vtc1. The reduced concentration of glutathione in cad2 and pad2 did not seem to affect ozone sensitivity. The vtc1 mutant was originally isolated as an ozone-sensitive mutant. However, this ozone sensitivity was observed at higher doses and after a longer exposure (Conklin et al., 2000) than used in this study.
Fig. 1.
Ozone sensitivity of antioxidant mutants. Plants of the indicated genotypes were exposed to a single 6-h pulse of 375 nl l−1 of ozone and cell death was monitored as ion leakage at 8 h after the beginning of the exposure. The presented data are the average and ±SE of five biological repeats of this experiment, with each repeat consisting of five samples. Mean values of ozone treatment that differed significantly from controls were identified with a linear model and Bonferroni correction (*P <0.05, **P <0.01, ***P <0.001).
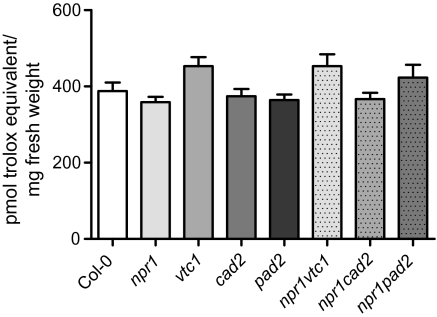
The ozone sensitivity of vtc mutants has been suggested to be a result of low ascorbic acid concentration leading to reduced scavenging of ozone derived ROS. The low ascorbic acid concentration in vtc1 and glutathione concentration in cad2 or pad2 could potentially be compensated by increases in other classes of antioxidants allowing the plant to maintain ROS-scavenging capacity. Measurements of glutathione in vtc mutants and ascorbic acid in cad2 have yielded conflicting results but often show the WT concentration of these compunds (Table 1; Veljovic-Jovanovic et al., 2001; Pavet et al., 2005; Colville and Smirnoff, 2008). Rather than measuring individual antioxidants, the total antioxidant capacity was measured based on ORAC (Fig. 2). This assay compares the capacity of a plant extract to prevent radical [2,2′-azobis-2-methyl-propanimidamide (AAPH)] induced destruction of a fluorescent reporter (fluorescein) with a reference, a water-soluble vitamin E analogue—Trolox (Gillespie et al., 2007). There was a trend towards a higher scavenging capacity of vtc1 and npr1 vtc1; however, no statistically significant differences were detected between any of the mutants and WT. Thus, the low ascorbic acid or glutathione concentrations in vtc1, cad2, pad2, and the double mutants might be compensated by an increase in other (unidentified) antioxidants.
Fig. 2.
Antioxidant status of antioxidant mutants. The total antioxidant capacity of 22-d-old plants was determined by the ORAC assay. Values are the mean ±SE of seven biological repeats (n=35). No statistical significances were found in the one-way ANOVA and Tukey post-hoc test.
Therefore, the massive ozone-induced cell death observed in npr1 vtc1 (Fig. 1) and of vtc mutants (Conklin et al., 2000) may not be a result of deficient ROS scavenging per se but rather a consequence of an altered balance of defence and cell-death signalling pathways brought about by low ascorbic acid concentrations. Alternatively, the specific localization of the antioxidant may be important. The ORAC measurements were performed on whole leaf extracts, and previously the ascorbic acid concentration of the apoplast has been suggested to be a determinant of ozone sensitivity (Yoshida et al., 2006; Foyer and Noctor, 2009).
Gene expression under control conditions in antioxidant mutants
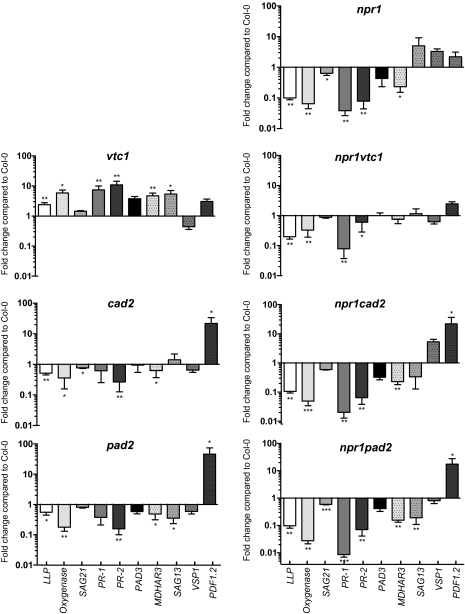
If a low ascorbic acid or glutathione concentration alter defence-signalling pathways, this should be visible as changes in the expression of defence-related genes. Indeed, previous results from gene-expression analysis using microarrays in vtc1, rax1, and cad2 indicated that SA-responsive genes had a higher expression in vtc1 and lower expression in rax1 and cad2 (Pastori et al., 2003; Ball et al., 2004). Marker genes were selected based on previous array analysis (Pastori et al., 2003; Ball et al., 2004; Ahlfors et al., 2009) and from public microarray data from experiments using MeJA or the SA analogue benzothiadiazole (https://www.genevestigator.com/). Plants were grown for 22 d and gene expression analysed with real-time quantitative reverse transcription-PCR (qPCR). Several SA-regulated genes, LECTIN-LIKE PROTEIN (LLP), Oxygenase, SENESCENCE ASSOCIATED 21 (SAG21), PR-1, and PR-2, showed a higher expression in vtc1 (Fig. 3). Furthermore, the low expression of these genes in npr1 indicated that they were regulated through the NPR1 signalling pathway. The elevated expression of these genes was missing in the double mutant npr1 vtc1, suggesting that signals resulting in altered gene expression in vtc1 are mediated through SA and NPR1. The cad2 and pad2 mutants had a lower expression of the SA marker genes and thus resembled npr1, suggesting that the low glutathione concentration of cad2 and pad2 could lead to a more inactive oligomeric state of NPR1. MONODEHYDROASCORBATE REDUCTASE3—MDHAR3 represents a marker gene regulated by SA or JA as well as multiple other stress treatments (Wrzaczek et al., 2010). This gene had a higher expression in vtc1 and lower in npr1, thus resembling the SA marker genes. However, in the npr1 vtc1 double mutant, this gene showed intermediate expression, suggesting that other signalling pathways in addition to SA signalling were activated in vtc1.
Fig. 3.
Gene expression in antioxidant mutants. Expression of SA and JA marker genes was analysed by qPCR in 3-week-old antioxidant mutants. Transcript levels were normalized to the reference gene and expressed relative to Col-0 grown under control conditions. Values are the mean ±SE of five biological repeats. Statistical significance was calculated using Relative Expression Software Tool 2009 (*P <0.05; **P <0.01; ***P <0.001).
JA responses of antioxidant mutants
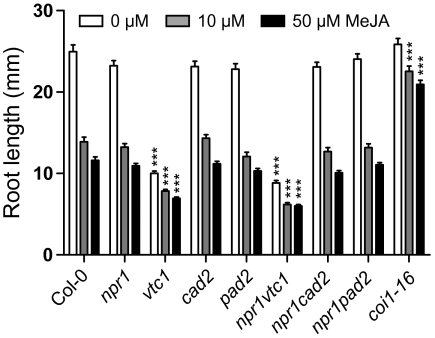
The JA-responsive marker gene PLANT DEFENSIN 1.2 (PDF1.2) exhibited a high expression in cad2 and pad2 (Fig. 3), suggesting that the response to JA might be altered in the antioxidant mutants. Furthermore, JA is a proposed regulator of ascorbic acid and glutathione biosynthesis (Xiang and Oliver, 1998; Sasaki-Sekimoto et al., 2005; Suza et al., 2010). MeJA inhibition of root growth, a classical JA assay used in several JA mutant screens, was used to test a general response of the mutants to JA. The roots of vtc1 and npr1 vtc1 were significantly smaller than WT under control conditions (Fig. 4), a growth response that is not dependent on ascorbic acid (Barth et al., 2010). Root growth of cad2, pad2, and npr1 single and corresponding double mutants was inhibited by MeJA to the same extent as WT.
Fig. 4.
MeJA inhibition of root growth: root growth of antioxidant mutants on control media, 10 μM MeJA and 50 μM MeJA. The coi1-16 mutant was included as a JA-insensitive mutant. Values are the mean ± SE of four biological repeats; n=50–70. Mean values that differed significantly from the WT were identified by the one-way ANOVA and Tukey’s post-hoc test (***P <0.001).
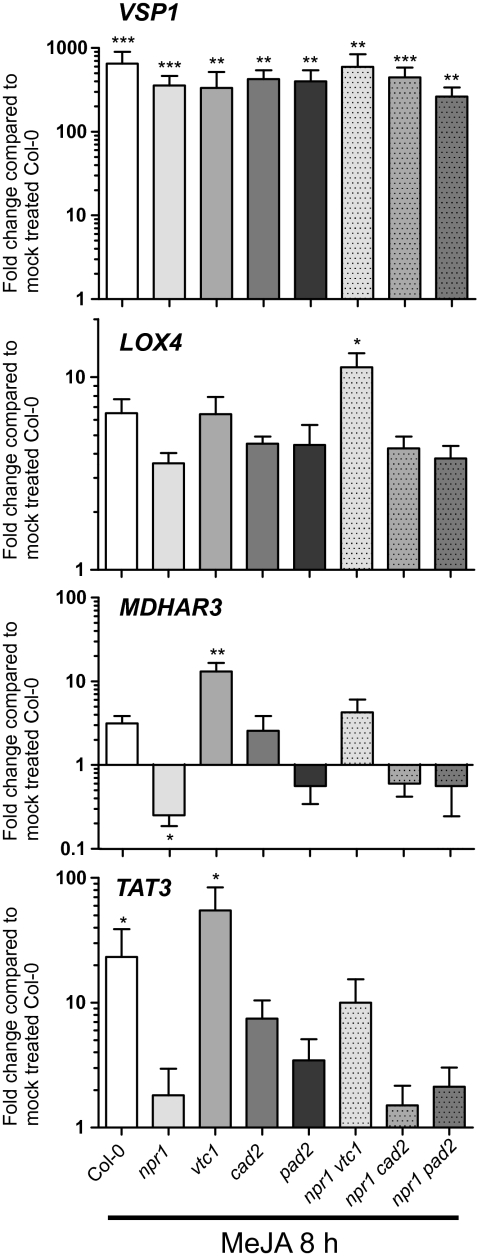
Although one general response to JA (root growth) appeared to be intact in the antioxidant mutants; there could still be other JA pathways with altered function. To investigate the connection between JA and antioxidants further, WT and mutants were treated with 0.5 mM MeJA for 8 h, and samples were taken for gene-expression analysis. Marker genes were selected to represent different classes of JA-responsive genes, VEGETATIVE STORAGE PROTEIN1 (VSP1), a marker gene used to test JA responses (Ellis and Turner, 2001), MDHAR3 encoding a protein central for the ascorbic acid–glutathione cycle and regulated by JA, SA, and ozone (Sasaki-Sekimoto et al., 2005; Wrzaczek et al., 2010), LIPOXYGENASE4 (LOX4)—a marker for JA biosynthesis and TYROSINE AMINOTRANSFERASE 3 (TAT3), a marker for wounding and JA (Yan et al., 2007). The several hundred-fold increased expression of VSP1 indicated effective induction of JA responsive signalling pathways (Fig. 5). MDHAR3 and TAT3 showed markedly higher MeJA-induced expression in vtc1 compared with WT, suggesting that this mutant could be primed to respond to JA. This effect was not seen for VSP1 or LOX4, suggesting that at least two parallel JA response pathways were acting in vtc1. NPR1 is traditionally considered a negative regulator of JA responses (Gfeller et al., 2010). MeJA induction of MDHAR3 and TAT3 was abolished in npr1 (Fig. 5), indicating that NPR1 also acted as a positive regulator for some JA-responsive genes.
Fig. 5.
MeJA-induced gene expression. Expression of marker genes in response to 8 h of 0.5 mM MeJA was analysed by qPCR in the antioxidant mutants. Transcript levels were normalized to the reference gene and expressed relative to mock treated Col-0. Values are the mean ±SE of four biological repeats. Statistical significance was calculated using Relative Expression Software Tool 2009 (*P <0.05; **P <0.01; ***P <0.001).
Regulation of glutathione and ascorbic acid biosynthesis, and the ascorbate–glutathione cycle at the gene-expression level
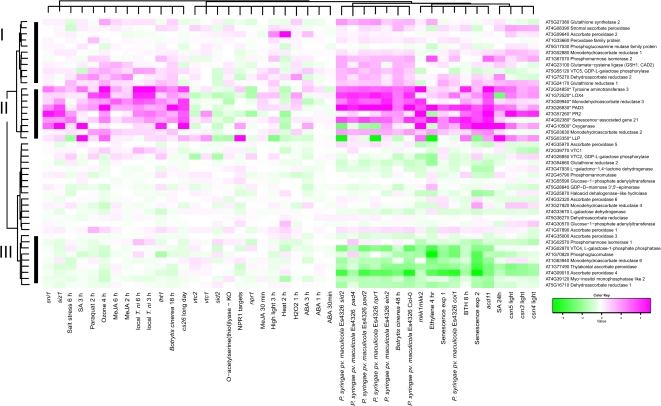
To further explore the interconnection between antioxidants, SA, JA, and stress, genes from the ascorbate–glutathione cycle and biosynthesis of ascorbic acid and glutathione were clustered using public array data from the Affymetrix ATH1 chip (Fig. 6). Experiments were selected to include hormone treatments, abiotic and biotic stress, senescence and mutants with constitutive activation of defence signalling (see Materials and methods for a complete description of experiments). The marker genes used in qPCR were also included in the analysis. All qPCR marker genes clustered together (cluster II) and were strongly induced by abiotic and biotic stress, in senescence and constitutive defence mutants, and by SA and the SA-analogue BTH (benzothiadiazole). The only genes from the ascorbate–glutathione cycle in this cluster were MDHAR2 and MDHAR3. Cluster I genes also had increased expression levels in response to biotic stress, some abiotic stresses (ozone and high light), and by SA, BTH, and ethylene. Genes of interest in this cluster include both steps of glutathione biosynthesis (GSH1, GSH2), GLUTATHIONE REDUCTASE1 and APX2. MeJA has previously been shown to increase GSH1 and GSH2 expression, which was also seen in cluster I (Xiang and Oliver, 1998). Additionaly ethylene increased expression of GSH1 and GSH2. Cluster III genes had decreased expression in response to biotic stress, senescence, BTH and ethylene. Whereas glutathione biosynthesis genes had increased expression by stress, the response of ascorbic acid biosynthesis genes was far more complex, probably related to several biosynthesis routes. In the main L-galactose pathway there are ten steps, where the last four steps are specific for ascorbic acid biosynthesis (Linster and Clarke, 2008). The first committed step catalysed by GDP-L-galactose phosphorylase, encoded by VTC2 and VTC5, only the latter gene appeared stress responsive with increased expression by biotic and abiotic stress. In contrast, the next step of the pathway (VTC4), had strongly decreased expression by biotic stress, ethylene and senescence and to a lesser extent by abiotic stress. The vtc1 and vtc2 mutants clustered together and had increased expression of SA marker genes, which was more prominent in vtc1. The knockout of a cytosolic O-acetylserine(thiol)lyase, which reduced cysteine and glutathione biosynthesis (López-Martín et al., 2008), had decreased expression of the SA qPCR marker genes, similar to the results from cad2 and pad2 (Fig. 3). Overall, the clustering reinforces that ROS, antioxidants, hormone and stress responses are highly interconnected, and by altering any of these, the output in gene expression will change.
Fig. 6.
Expression of ascorbate–glutathione cycle, ascorbic acid, and glutathione biosynthesis genes during abiotic and biotic stress, during hormone treatments, and in constitutive defence mutants. Bootstrapped Bayesian hierarchical clustering of genes is shown in plants subjected to stress treatments compared with normal growth conditions, or in mutant versus wild type. Magenta and green indicate increased and decreased expression compared with untreated or wild-type plants, respectively.
Developmental responses of antioxidant mutants
The gene-expression analysis (Figs 3, 5) indicated that low ascorbic acid or glutathione concentration altered both SA and JA signalling. Both of these hormones are important regulators of ROS induced cell death (Overmyer et al., 2003) which could contribute to increased cell death of vtc1, npr1 vtc1 and npr1 pad2 (Fig. 1). Plant hormones are not only regulators of stress responses; they are also intimately involved in plant development. To gain further insight into the consequence of low ascorbic acid or glutathione concentration the antioxidant mutants for three developmental responses, flower time, size and glucose inhibition of germination, were investigated, where low ascorbic acid or glutathione concentration have been shown to lead to altered responses (Ogawa et al., 2001; Pavet et al., 2005; Kotchoni et al., 2009).
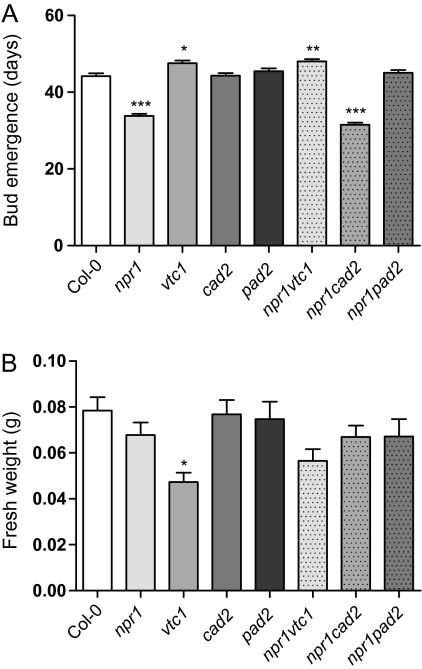
Mutants with low ascorbic acid concentration have been described as either early or late flowering in different studies, possibly a consequence of different light levels (Pavet et al., 2005; Kotchoni et al., 2009). Under the conditions of this experiment (12/12 h light/dark, 250 μmol photons m−2 s−1), vtc1 and npr1 vtc1 were to some extent late flowering (Fig. 7A). Low glutathione concentrations, either in the cad2 mutant or by treatment with a glutathione biosynthesis inhibitor, delayed flowering (Ogawa et al., 2001). Under our growth conditions, the npr1 and npr1 cad2 mutants were early flowering, but cad2, pad2 or npr1 pad2 were not, suggesting that a specific balance between glutathione concentration and NPR1 influences flowering.
Fig. 7.
Flower time and fresh weight of antioxidant mutants. (A) Flower time, determined as bud emergence in a short day (12/12 h light/dark). (B) Fresh weight, determined on 3-week-old plants. The data represent the means ±SE of four biological repeats. n=35 for flower time and n=54–59 for fresh weight. Mean values that differed significantly from the WT were identified with one-way ANOVA and Tukey’s post-hoc test (*P <0.05; **P <0.01; ***P <0.001).
The vtc1 mutant was smaller than WT (Fig. 7B) and has smaller cells (Pavet et al., 2005). The small size could be a result of a lower amount of precursors available for cell-wall biosynthesis (the ascorbic acid biosynthesis pathway shares intermediates with cell-wall polysaccharide synthesis), or due to constitutive activation of SA-dependent defences (Pavet et al., 2005). When compared with vtc1, npr1 vtc1 was larger, indicating that constitutive activation of SA defences through NPR1 in vtc1, at least partially, decreases its size.
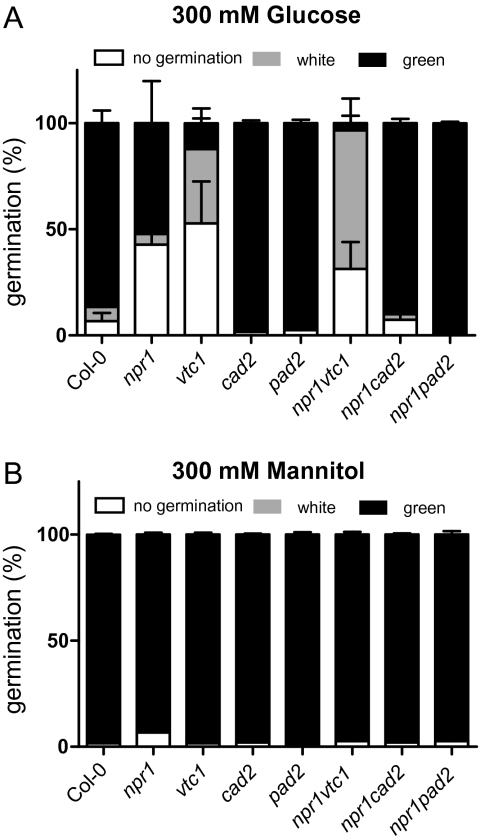
High glucose concentrations reduce germination and growth of Arabidopsis seeds on MS medium. A complex network of signals is involved in this response, including general metabolism and the plant hormones abscisic acid and ethylene (Ramon et al., 2008). After 7 d of growth on 300 mM glucose, three different classes of seedlings were observed: seeds that did not germinate, white seedlings, and green seedlings (Fig. 8A). The growth of npr1, vtc1, and particularly npr1 vtc1 was inhibited by glucose. Interestingly, growth of npr1 cad2 and npr1 pad2 double mutants was not inhibited as in npr1, indicating that all three components ascorbic acid, glutathione, and NPR1 were involved in sugar signalling. No major changes were observed on growth in the osmotic stress control mannitol (Fig. 8B).
Fig. 8.
Sugar inhibition of germination. The germination frequency of seeds from antioxidant mutants grown on MS media supplemented with 300 mM glucose (A) or 300 m M mannitol (B) for 7 d. Seeds were scored in three different categories: no germination, white seedlings, and green seedlings. Values are the mean ±SE of three biological repeats. n=150–300.
Discussion
The correct integration of internal developmental signals with external cues from the environment is essential for plant survival. ROS are signalling intermediates for both development and abiotic and biotic stress responses. Ozone exposure leads to a controlled production of ROS in the apoplast, similar to the ROS burst produced in the hypersensitive response elicited in plants in response to pathogen infection (Wohlgemuth et al., 2002; Kangasjärvi et al., 2005; Ahlfors et al., 2009). Thus, ozone can be used to study the role of apoplastic ROS as signalling molecules without the added complexity of any other manipulation of the plant or infection with pathogens. Plants with low ascorbic acid concentrations are ozone-sensitive, which traditionally has been considered to be a consequence of decreased ROS detoxification (Conklin and Barth, 2004), but it is likely that ascorbic acid concentration of a specific compartment, for example, the apoplast (Yoshida et al., 2006), could be more important than the plant’s total ascorbic acid concentration or total antioxidant capacity. A model for ROS-induced cell death has previously been suggested where initial ROS production activates biosynthesis of SA, JA, and ethylene. Subsequently, the balance of these hormones determines the extent of cell death (Overmyer et al., 2003). The vtc1 mutant accumulates high concentrations of SA and SA glucoside, indicating a primed state that is able to respond faster to stress (Mukherjee et al., 2010). Thus, a pulse of apoplastic ROS could tip the balance of signalling pathways towards cell death in vtc1. This process appears to be under partial control of NPR1, since cell death was further significantly increased in npr1 vtc1. In contrast when the npr1 mutation was introduced into the ozone-sensitive mutant rcd1, it led to increased ozone tolerance (Overmyer et al., 2005). Thus, NPR1 appears to be at the intersection of several signalling pathways, and even in response to a single stress (i.e. ozone), absence of NPR1 can promote either cell death or survival.
To gain further insight into which pathways could be altered in mutants with low ascorbic acid or glutathione concentrations, a gene-expression analysis was performed using a set of stress-related marker genes. Constitutive activation of defences in vtc1 has been proposed to be regulated by NPR1 based on the movement of GFP-NPR1 into the nucleus of vtc1 (Pavet et al., 2005). High expression of SA related marker genes in vtc1 was fully suppressed by npr1 (Fig. 3), and the concentration of SA glucoside was reduced in vtc1 npr1 and double mutants affecting SA accumulation vtc1 pad4 and vtc1 eds5 (Mukherjee et al., 2010), strongly suggesting that primed defence responses in vtc1 are indeed regulated though SA and NPR1. In contrast, SA marker genes had a lower expression in cad2 and pad2; thus, these mutants resemble npr1 (Fig. 3). Low expression of SA marker genes has also been observed in plants treated with a glutathione biosynthesis inhibitor (Colville and Smirnoff, 2008) and was also seen in a mutant for cytosolic O-acetylserine(thiol)lyase with reduced cysteine and glutathione biosynthesis (Fig. 6). This suggests that glutathione itself could act as a signalling molecule, and less glutathione in cad2 or pad2 might impact directly on defence responses (Ball et al., 2004; Senda and Ogawa, 2004). The ratio of reduced (GSH) to oxidized (GSSG) glutathione may also be an important factor, feeding GSH but not GSSG to leaves induced expression of PR-1 (Gomez et al., 2004). Consistent with this, a gr1 mutant (which lacks GLUTATHIONE REDUCTASE1, and thus has increased concentrations of GSSG) had lower SA concentrations and decreased PR-1 expression in a catalase-deficient mutant background (Mhamdi et al., 2010). NPR1, as well as one of its interacting transcription factors, TGA1, is under redox control (Despres et al., 2003; Mou et al., 2003); thus the low expression of SA marker genes in cad2 and pad2 could be a result of low glutathione concentrations, or the redox balance in these mutants could lead to increased sequestering of NPR1 in inactive oligomer complexes (Mou et al., 2003).
JA regulates expression of multiple genes in ascorbic acid and glutathione biosynthesis and ROS detoxification (Xiang and Oliver, 1998; Sasaki-Sekimoto et al., 2005; Suza et al., 2010). In the MeJA root inhibition assay, cad2 and pad2 had WT responses indicating a normal response to MeJA (Fig. 4). The poor growth of vtc1 roots makes it difficult to use this assay to estimate its JA response. However, a complex response was seen for the mutants in gene-expression analysis. At least two separate JA signalling pathways were active based on MeJA hyperinduction of MDHAR3 and TAT3 in vtc1 and, in contrast, normal induction of VSP1 and LOX4 (Fig. 5). This contrasting gene-expression response illustrates that to fully explore SA and JA responses, the selection of marker genes should be extended to include additional genes other than only the classical PR-1 (SA marker) and VSP1 (JA marker). NPR1 is a required signalling component for many SA responses and is usually described as a negative regulator of JA signalling (Gfeller et al., 2010). The use of separate JA marker genes showed that NPR1 can act as a positive regulator of at least one JA signalling pathway; MeJA-induction of MDHAR3 and TAT3 was impaired in npr1 (Fig. 5). Interestingly, in systemic induced resistance NPR1 also acts as a positive regulator downstream from JA (Ramirez et al., 2010), indicating that NPR1 is recruited to multiple steps in SA and JA signalling.
Antagonistic interaction among plant hormones is a common phenomenon and is frequently observed between SA and JA where NPR is a central regulator (Spoel et al., 2003). The JA-responsive gene PDF1.2 is highly sensitive to SA inhibition, and this antagonistic action has been proposed to act through redox changes in glutathione, as application of a glutathione biosynthesis inhibitor removes the antagonistic action of SA on JA (Koornneef et al., 2008). A critical role for the GSH:GSSG ratio in expression of JA-related genes was seen in the effect of the gr1 mutation, which leads to repression of JA-responsive genes including LOX and TAT3 (Mhamdi et al., 2010). Interestingly, in cad2 and pad2, the PDF1.2 expression was significantly higher than in WT (Fig. 3), thus further supporting a role for glutathione in regulating JA responses.
ROS detoxification via the ascorbate–glutathione cycle is a multi-gene network where many enzymes are encoded by multiple genes (Mittler et al., 2011). To gain further insight into how this network at the gene-expression level responds to stress, ROS, and plant hormones, array data from selected experiments were analysed by the Bayesian hierarchical clustering method (Fig. 6). The list of genes used in the analysis included ascorbate–glutathione cycle, ascorbic acid and glutathione biosynthesis genes, and the marker genes used in qPCR analysis. Genes with very high induced expression by multiple stresses and hormones (cluster II) were all the qPCR marker genes and two genes from the ascorbate–glutathione cycle MDHAR2 and MDHAR3 (the latter is the qPCR marker gene in Figs 3 and 5). Genes with increased expression (cluster I) included both steps of glutathione biosynthesis (GSH1 and GSH2) and one of two genes encoding GDP-L-galactose phosphorylase (VTC5). Genes with reduced expression included several ascorbic acid biosynthesis genes VTC1, VTC2, VTC4, GDP-Man-3,5-epimerase, and Phosphomannomutase (PMM). In addition to JA and SA, also ethylene was found to induce GSH1 and GSH2 expression. The similar gene-expression profiles of several biotic stresses (P. syringae ES4326 and B. cinerea), senescence, ethylene, the SA analogue benzothiadiazole S-methyl ester (BTH), and mutants undergoing spontaneous cell death acd11 and mkk1mkk2 (Gao et al., 2008; Qiu et al., 2008; Palma et al., 2010), indicate that these treatments elicit signalling pathways that eventually converge at the promoters of genes in biosynthesis of ascorbic acid and glutathione, and in the ascorbate–glutathione cycle. Identification of these transcription factors could help unravel the ROS-antioxidant signalling network.
For many stress-related phenotypes, low ascorbic acid concentration and low glutathione concentration lead to opposite phenotypes and gene-expression profiles (Table 1, Fig. 3). This opposite effect was clearly evident in the sugar inhibition of seed germination, especially in the npr1 background (Fig. 8). The npr1 mutant had decreased germination, which was enhanced in the double mutant npr1 vtc1 and was suppressed in npr1 cad2 and npr1 pad2, again indicating that glutathione or the redox balance influences NPR1 signalling.
For some defence-related phenotypes differing glutathione concentrations lead to phenotypic variation, exemplified by different gene-expression patterns in rax1 and cad2 (Ball et al., 2004). In our data, the most striking difference between cad2 and pad2 was observed in the flowering time of the double mutants with npr1, where npr1 cad2 was early flowering, but npr1 pad2 was not. Since the difference in glutathione concentration of cad2 and pad2 is rather minor (20 vs 30% of WT; Parisy et al., 2007), this indicates that very small changes in redox balance can induce major changes in developmental fate. That these small changes can have such a dramatic effect could also explain the differences in phenotypes between different laboratories; even a slight change in growth condition could alter the balance of a redox signalling pathway.
Both ROS and antioxidants are clearly important in plant development and stress responses, and can be used by the plant as signalling molecules (Foyer and Noctor, 2005a, b; Potters et al., 2010). However, testing the role of the ‘cellular redox state’ or ‘oxidative signalling’ represents a fundamental challenge. The diverse chemical nature of various ROS, redox-active components, and ROS scavenging enzymes makes it difficult to measure more than a few representative species per sample. To probe the complexity of oxidative signalling, the application of new tools could provide novel insights, including in vivo probes for ROS and redox measurements, e.g. HyPer (Costa et al., 2010) and redox-sensitive GFP (Meyer et al., 2007), measurements of antioxidant concentrations in specific subcellular compartments (Queval et al., 2011), mutants with specific defects in redox balance or ROS signalling, double mutants to position the order of redox events in a signalling pathway, and a broader range of marker genes in gene-expression analysis reflecting the full range of hormone signalling, including responses to SA and JA.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table 1. Primer sequences and amplification efficiency used in real-time quantitative PCR.
Acknowledgments
We thank Tuomas Puukko for technical assistance, Leena Laakso for plant care, Shaman Narayanasamy for assistance with analysis of public array data, Jarkko Salojärvi for statistical advice, and Michael Wrzaczek, Hannes Kollist, and Kirk Overmyer for comments on the manuscript. The work was supported by the Academy of Finland Centre of Excellence programme (2006–2011) to JK and Academy of Finland (decision no 135751 and 140981) and by the EU through the European Social Fund (Mobilitas Top Researchers grant MTT9) to MB.
Glossary
Abbreviations
- AAPH
2,2′-azobis-2-methyl-propanimidamide
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- JA
jasmonic acid
- MeJA
methyl jasmonate
- ORAC
oxygen radical absorbance capacity
- ROS
reactive oxygen species
- SA
salicylic acid
- WT
wild type
References
- Ahlfors R, Brosché M, Kollist H, Kangasjärvi J. Nitric oxide modulates ozone-induced cell death, hormone biosynthesis and gene expression in Arabidopsis thaliana. The Plant Journal. 2009;58:1–12. doi: 10.1111/j.1365-313X.2008.03756.x. [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta—General Subjects. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Ball L, Accotto GP, Bechtold U, et al. Evidence for a direct link between glutathione biosynthesis and stress defense gene expression in Arabidopsis. The Plant Cell. 2004;16:2448–2462. doi: 10.1105/tpc.104.022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Gouzd ZA, Steele HP, Imperio RM. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. Journal of Experimental Botany. 2010;61:379–394. doi: 10.1093/jxb/erp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Moeder W, Klessig DF, Conklin PL. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiology. 2004;134:1784–1792. doi: 10.1104/pp.103.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz F, Hothorn T, Westfall P. Multiple comparisons using R. Boca Raton, FL: CRC Press; 2010. [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in gamma-glutamylcysteine synthetase. The Plant Journal. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Colville L, Smirnoff N. Antioxidant status, peroxidase activity, and PR protein transcript levels in ascorbate-deficient Arabidopsis thaliana vtc mutants. Journal of Experimental Botany. 2008;59:3857–3868. doi: 10.1093/jxb/ern229. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant, Cell and Environment. 2004;27:959–970. [Google Scholar]
- Conklin PL, Saracco SA, Norris SR, Last RL. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, et al. H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. The Plant Journal. 2010;62:760–772. doi: 10.1111/j.1365-313X.2010.04190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despres C, Chubak C, Rochon A, Clark R, Bethune T, Desveaux D, Fobert PR. The Arabidopsis NPR1 disease resistance protein is a novel cofactor that confers redox regulation of DNA binding activity to the basic domain/leucine zipper transcription factor TGA1. The Plant Cell. 2003;15:2181–2191. doi: 10.1105/tpc.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N. Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. The Plant Journal. 2007;52:673–689. doi: 10.1111/j.1365-313X.2007.03266.x. [DOI] [PubMed] [Google Scholar]
- Ellis C, Turner JG. The Arabidopsis mutant cev1 has constitutively active jasmonate and ethylene signal pathways and enhanced resistance to pathogens. The Plant Cell. 2001;13:1025–1033. doi: 10.1105/tpc.13.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C, Turner JG. A conditionally fertile coi1 allele indicates cross-talk between plant hormone signalling pathways in Arabidopsis thaliana seeds and young seedlings. Planta. 2002;215:549–556. doi: 10.1007/s00425-002-0787-4. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant, Cell and Environment. 2005a;28:1056–1071. [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. The Plant Cell. 2005b;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxidants and Redox Signaling. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inze D, Mittler R, Van Breusegem F. Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiology. 2006;141:436–445. doi: 10.1104/pp.106.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Research. 2008;18:1190–1198. doi: 10.1038/cr.2008.300. [DOI] [PubMed] [Google Scholar]
- Gfeller A, Liechti R, Farmer EE. Arabidopsis jasmonate signaling pathway. Science Signaling. 2010;3 doi: 10.1126/scisignal.3109cm4. cm4. [DOI] [PubMed] [Google Scholar]
- Gillespie KM, Chae JM, Ainsworth EA. Rapid measurement of total antioxidant capacity in plants. Nature Protocols. 2007;2:867–870. doi: 10.1038/nprot.2007.100. [DOI] [PubMed] [Google Scholar]
- Gomez LD, Noctor G, Knight MR, Foyer CH. Regulation of calcium signalling and gene expression by glutathione. Journal of Experimental Botany. 2004;55:1851–1859. doi: 10.1093/jxb/erh202. [DOI] [PubMed] [Google Scholar]
- Jaspers P, Kangasjärvi J. Reactive oxygen species in abiotic stress signaling. Physiologia Plantarum. 2010;138:405–413. doi: 10.1111/j.1399-3054.2009.01321.x. [DOI] [PubMed] [Google Scholar]
- Kangasjärvi J, Jaspers P, Kollist H. Signalling and cell death in ozone-exposed plants. Plant Cell and Environment. 2005;28:1021–1036. [Google Scholar]
- Koornneef A, Leon-Reyes A, Ritsema T, Verhage A, Den Otter FC, Van Loon LC, Pieterse CMJ. Kinetics of salicylate-mediated suppression of jasmonate signaling reveal a role for redox modulation. Plant Physiology. 2008;147:1358–1368. doi: 10.1104/pp.108.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiology. 2009;149:803–815. doi: 10.1104/pp.108.132324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Clarke SG. L-Ascorbate biosynthesis in higher plants: the role of VTC2. Trends in Plant Science. 2008;13:567–573. doi: 10.1016/j.tplants.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Martín MC, Becana M, Romero LC, Gotor C. Knocking out cytosolic cysteine synthesis compromises the antioxidant capacity of the cytosol to maintain discrete concentrations of hydrogen peroxide in. Arabidopsis. Plant Physiology. 2008;147:562–572. doi: 10.1104/pp.108.117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo A, Funck D, Muhlenbock P, Kular B, Mullineaux PM, Karpinski S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. Journal of Experimental Botany. 2006;57:1795–1807. doi: 10.1093/jxb/erj196. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. The Plant Journal. 2007;52:973–986. doi: 10.1111/j.1365-313X.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Hager J, Chaouch S, et al. Arabidopsis glutathione reductase1 plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiology. 2010;153:1144–1160. doi: 10.1104/pp.110.153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F. ROS signaling: the new wave? Trends in Plant Science. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Mou Z, Fan WH, Dong XN. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell. 2003;113:935–944. doi: 10.1016/s0092-8674(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Mukherjee M, Larrimore KE, Ahmed NJ, Bedick TS, Barghouthi NT, Traw MB, Barth C. Ascorbic acid deficiency in Arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene. Molecular Plant–Microbe Interactions. 2010;23:340–351. doi: 10.1094/MPMI-23-3-0340. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Tasaka Y, Mino M, Tanaka Y, Iwabuchi M. Association of glutathione with flowering in Arabidopsis thaliana. Plant and Cell Physiology. 2001;42:524–530. doi: 10.1093/pcp/pce065. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Brosché M, Kangasjärvi J. Reactive oxygen species and hormonal control of cell death. Trends in Plant Science. 2003;8:335–342. doi: 10.1016/S1360-1385(03)00135-3. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Brosché M, Pellinen R, et al. Ozone-induced programmed cell death in the Arabidopsis radical-induced cell death1 mutant. Plant Physiology. 2005;137:1092–1104. doi: 10.1104/pp.104.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Kangasjärvi J. Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. The Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma K, Thorgrimsen S, Malinovsky FG, Fiil BK, Nielsen HB, Brodersen P, Hofius D, Petersen M, Mundy J. Autoimmunity in Arabidopsis acd11 is mediated by epigenetic regulation of an immune receptor. PLoS Pathogens. 2010 doi: 10.1371/journal.ppat.1001137. 6, e1001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisy V, Poinssot B, Owsianowski L, Buchala A, Glazebrook J, Mauch F. Identification of PAD2 as a gamma-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. The Plant Journal. 2007;49:159–172. doi: 10.1111/j.1365-313X.2006.02938.x. [DOI] [PubMed] [Google Scholar]
- Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, Noctor G, Foyer CH. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. The Plant Cell. 2003;15:939–951. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiology. 2005;139:1291–1303. doi: 10.1104/pp.105.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, Horemans N, Bellone S, Caubergs RJ, Trost P, Guisez Y, Asard H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiology. 2004;134:1479–1487. doi: 10.1104/pp.103.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, Horemans N, Jansen MAK. The cellular redox state in plant stress biology—a charging concept. Plant Physiology and Biochemistry. 2010;48:292–300. doi: 10.1016/j.plaphy.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Qiu J-L, Zhou L, Yun B-W, Nielsen HB, Fiil BK, Petersen K, MacKinlay J, Loake GJ, Mundy J, Morris PC. Arabidopsis mitogen-activated protein kinase kinases MKK1 and MKK2 have overlapping functions in defense signaling mediated by MEKK1, MPK4, and MKS1. Plant Physiology. 2008;148:212–222. doi: 10.1104/pp.108.120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queval G, Jaillard D, Zechmann B, Noctor G. Increased intracellular H2O2 availability preferentially drives glutathione accumulation in vacuoles and chloroplasts. Plant, Cell and Environment. 2011;34:21–32. doi: 10.1111/j.1365-3040.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- Ramirez V, Van der Ent S, Garcia-Andrade J, Coego A, Pieterse CMJ, Vera P. OCP3 is an important modulator of NPR1-mediated jasmonic acid-dependent induced defenses in Arabidopsis. BMC Plant Biology. 2010;10:199. doi: 10.1186/1471-2229-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon M, Rolland F, Sheen J. Sugar sensing and signaling. The Arabidopsis Book. 2008;6:1–22. doi: 10.1199/tab.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhier N, Lemaire SD, Jacquot JP. The role of glutathione in photosynthetic organisms: Emerging functions for glutaredoxins and glutathionylation. Annual Review of Plant Biology. 2008;59:143–166. doi: 10.1146/annurev.arplant.59.032607.092811. [DOI] [PubMed] [Google Scholar]
- Sasaki-Sekimoto Y, Taki N, Obayashi T, et al. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. The Plant Journal. 2005;44:653–668. doi: 10.1111/j.1365-313X.2005.02560.x. [DOI] [PubMed] [Google Scholar]
- Senda K, Ogawa K. Induction of PR-1 accumulation accompanied by runaway cell death in the lsd1 mutant of Arabidopsis is dependent on glutathione levels but independent of the redox state of glutathione. Plant and Cell Physiology. 2004;45:1578–1585. doi: 10.1093/pcp/pch179. [DOI] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SMC, et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant Cell. 2003;15:760–770. doi: 10.1105/tpc.009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suza WP, Avila CA, Carruthers K, Kulkarni S, Goggin FL, Lorence A. Exploring the impact of wounding and jasmonates on ascorbate metabolism. Plant Physiology and Biochemistry. 2010;48:337–350. doi: 10.1016/j.plaphy.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou ZL, Song JQ, Wang C, Zuo JR, Dong XN. Plant immunity requires conformational charges of NPR1 via S-nitrosylation and thioredoxins. Science. 2008;321:952–956. doi: 10.1126/science.1156970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA. ROS in biotic interactions. Physiologia Plantarum. 2010;138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH. Low ascorbic acid in the vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiology. 2001;127:426–435. [PMC free article] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in Initiation and maintenance of cell division during postembryonic root development. The Plant Cell. 2000;12:97–109. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos PD, Dong YP, Ziegler K, Markovic J, Pallardo FV, Pellny TK, Verrier PJ, Foyer CH. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. The Plant Journal. 2010;64:825–838. doi: 10.1111/j.1365-313X.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Sandermann H, Langebartels C. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant, Cell and Environment. 2002;25:717–726. [Google Scholar]
- Wrzaczek M, Brosché M, Salojärvi J, Kangasjärvi S, Idänheimo N, Mersmann S, Robatzek S, Karpinski S, Karpinska B, Kangasjärvi J. Transcriptional regulation of the CRK/DUF26 group of Receptor-like protein kinases by ozone and plant hormones in Arabidopsis. BMC Plant Biology. 2010;10:95. doi: 10.1186/1471-2229-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang CB, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. The Plant Cell. 1998;10:1539–1550. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YX, Stolz S, Chetelat A, Reymond P, Pagni M, Dubugnon L, Farmer EE. A downstream mediator in the growth repression limb of the jasmonate pathway. The Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Tamaoki M, Shikano T, et al. Cytosolic dehydroascorbate reductase is important for ozone tolerance in Arabidopsis thaliana. Plant and Cell Physiology. 2006;47:304–308. doi: 10.1093/pcp/pci246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.