Abstract
The influence of sulphur (S) depletion on the expression of genes related to S metabolism, and on metabolite and plant hormone contents was analysed in young and mature leaves, fine roots, xylem sap, and phloem exudates of poplar (Populus tremula×Populus alba) with special focus on early consequences. S depletion was applied by a gradual decrease of sulphate availability. The observed changes were correlated with sulphate contents. Based on the decrease in sulphate contents, two phases of S depletion could be distinguished that were denominated as ‘S limitation’ and ‘early S deficiency’. S limitation was characterized by improved sulphate uptake (enhanced root-specific sulphate transporter PtaSULTR1;2 expression) and reduction capacities (enhanced adenosine 5′-phosphosulphate (APS) reductase expression) and by enhanced remobilization of sulphate from the vacuole (enhanced putative vacuolar sulphate transporter PtaSULTR4;2 expression). During early S deficiency, whole plant distribution of S was impacted, as indicated by increasing expression of the phloem-localized sulphate transporter PtaSULTR1;1 and by decreasing glutathione contents in fine roots, young leaves, mature leaves, and phloem exudates. Furthermore, at ‘early S deficiency’, expression of microRNA395 (miR395), which targets transcripts of PtaATPS3/4 (ATP sulphurylase) for cleavage, increased. Changes in plant hormone contents were observed at ‘early S deficiency’ only. Thus, S depletion affects S and plant hormone metabolism of poplar during ‘S limitation’ and ‘early S deficiency’ in a time series of events. Despite these consequences, the impact of S depletion on growth of poplar plants appears to be less severe than in Brassicaceae such as Arabidopsis thaliana or Brassica sp.
Keywords: APS reductase, ATP sulphurylase, miR395, plant hormones, poplar, sulphate transporter (SULTR), sulphur deficiency
Introduction
Sulphur (S) is one of six macroelements which are essential for proper plant growth and development. It forms part of amino acids and proteins and plays an important role in redox control of cellular processes (reviewed in Foyer and Noctor, 2009) and in plant defence mechanisms (reviewed in Rausch and Wachter, 2005; Noctor, 2006). Plants take up S mainly in the form of sulphate from the soil. Within the plant, sulphate either remains in the roots or is transported via the xylem to the leaves, the main sites of sulphate reduction and assimilation (reviewed in Rennenberg, 1984; Brunold, 1990). Part of the sulphate from the leaves is redistributed back to the roots via the phloem (Hartmann et al., 2000; Herschbach and Rennenberg, 2001). Furthermore, sulphate is largely stored in the vacuoles (Leustek and Saito, 1999) and can be mobilized from these storage compartments if required (Bell et al., 1994, 1995). All these sulphate distribution processes within the plant are controlled by sulphate transporters (SULTRs) (reviewed in Buchner et al., 2004b; Hawkesford and De Kok, 2006). These processes require a complex regulation at the whole plant level and have to be coordinated with sulphate reduction and assimilation (reviewed in Kopriva and Rennenberg, 2004; Takahashi, 2010; Takahashi et al., 2011). Sulphate uptake and its distribution within the plant are thought to be driven by the demand for reduced S compounds (reviewed in Davidian and Kopriva, 2010). Sulphate itself, but also cysteine and/or glutathione (GSH), are supposed to serve as signals in this demand-driven regulation (Rennenberg et al., 1988, 1989; Herschbach and Rennenberg, 1991; Lappartient et al., 1999; Herschbach et al., 2000; Hartmann et al., 2004; van der Zalm et al., 2005). Beside these S compounds, other compounds such as O-acetylserine, the precursor of cysteine, and sugars also influence sulphate uptake and assimilation, emphasizing the strong regulatory connection with nitrogen and carbon metabolism (reviewed in Kopriva and Rennenberg, 2004). Moreover, plant hormones such as cytokinins and auxins, but also stress-related hormones such as abscisic acid (ABA) or jasmonate, are believed to be involved in the regulation of S metabolism (reviewed in Kopriva, 2006; Takahashi et al., 2011).
S depletion has been shown to induce enhanced sulphate uptake and assimilation capacity, which are strongly regulated at the transcriptional level (Bolchi et al., 1999; Lee and Leustek, 1999; Vidmar et al., 1999; Takahashi et al., 2000; Nikiforova et al., 2003; Buchner et al., 2004a, b; Nocito et al., 2006; Parmar et al., 2007). This response includes increased expression of several sulphate transporters and of enzymes involved in sulphate assimilation such as ATP sulphurylase (ATPS) and adenosine 5′-phosphosulphate (APS) reductase (APR) (Bolchi et al., 1999; Vidmar et al., 1999; Takahashi et al., 2000; Hirai et al., 2003; Nikiforova et al., 2003; Buchner et al., 2004a; Kataoka et al., 2004; Maruyama-Nakashita et al., 2004a, 2006; Parmar et al., 2007). Still considerable differences were observed between genes and species in their responsiveness to S depletion. In Arabidopsis and Brassica, the expression of SULTR1;1 and SULTR4;2 seems to be more susceptible to S depletion than the expression of SULTR1;2 and SULTR4;1 (Yoshimoto et al., 2002; Buchner et al., 2004a; Kataoka et al., 2004; Parmar et al., 2007; Barberon et al., 2008). Also the expression of different ATPS isoforms was regulated differently upon S depletion. Expression of the Arabidopsis ATPS AtAPS3 increased during S depletion (Liang et al., 2010; Kawashima et al., 2011), while results for AtAPS2, the putatively cytosolic isoform (Hatzfeld et al., 2000; Rotte and Leustek, 2000), were inconsistent between different studies (Logan et al., 1996; Takahashi et al., 1997; Kawashima et al., 2011). AtAPS1, AtAPS3, and AtAPS4 were shown to be post-transcriptionally regulated by microRNA395 (miR395), and the expression of miR395 was shown to be induced upon S depletion (Jones-Rhoades and Bartel, 2004; Kawashima et al., 2009, 2011; Liang et al., 2010). However, a different cellular or subcellular localization of miR395 and its targets can prevent the post-transcriptional regulation and might result in a discrepancy between gene expression and enzyme activity of AtAPS (Kawashima et al., 2009, 2011).
Beside these changes in gene expression, S depletion is also accompanied by changes in metabolite concentrations such as decreasing levels of glutathione which were shown to repress sulphate uptake and assimilation (Rennenberg et al., 1988, 1989; Herschbach and Rennenberg, 1991, 1994; Bolchi et al., 1999; Lappartient et al., 1999; Vidmar et al., 1999, 2000; Vauclare et al., 2002; Hartmann et al., 2004). Phenotypically, the first visible S depletion symptoms appear on young leaves which constitute the main sink organ for newly acquired sulphate (Burke et al., 1986; Anderson, 2005). Nutrient deficiency in general induces root growth (Lopez-Bucio et al., 2003) which might result in a rising sink capacity for sulphate in the roots. This enhanced sink capacity of the roots may decrease the transport of newly acquired sulphate via the xylem to the leaves and presumably causes the strong impact of S depletion on young leaves (Clarkson and Hawkesford, 1993; Anderson, 2005). In Brassica napus, the overall decreasing sulphate content within the plant during sulphate depletion is accompanied by a shift in the transport direction of remobilized sulphate from mature leaves in favour of the roots instead of young leaves (Abdallah et al., 2010). This is consistent with an enhanced sink capacity for sulphate of the roots and implies changes in activity and abundance of SULTRs involved in the plant-internal distribution of sulphate.
Plant hormones are also involved in the regulation of sulphate uptake and assimilation. The participation of cytokinins in regulation of S metabolism was demonstrated for AtSULTR1;1 and AtSULTR1;2. Expression of both genes was negatively affected by cytokinins in roots of Arabidopsis thaliana (Maruyama-Nakashita et al., 2004b; Werner et al., 2010). In contrast, Ohkama et al. (2002) showed a positive transcriptional regulation of AtAPR1 and AtSULTR2;2 by cytokinins in leaves of A. thaliana. The involvement of auxin in regulation of S metabolism was demonstrated by increasing expression and activity of nitrilase 3 (NIT3) upon S depletion in Arabidopsis (Kutz et al., 2002). This indicates an enhanced production of auxin upon degradation of glucosinolates, resulting in support of root growth and branching (Kutz et al., 2002). However, this auxin-dependent regulation relates to the glucosinolate pathway which exists almost exclusively in the plant family order of Brassicales (Halkier and Gershenzon, 2006).
Although the involvement of plant hormones such as cytokinins and auxins in regulation of S metabolism has been demonstrated, investigations revealing changes in the endogenous plant hormone contents during S depletion are extremely rare. The present study was aimed to characterize the consequences of S depletion in poplar, including endogenous changes of plant hormones parallel to the analyses of the expression of a broad spectrum of genes involved in S uptake and metabolism and of thiol and sugar contents. Poplar was chosen as the model species for trees (Cronk, 2005; Jansson and Douglas, 2007) which are assumed to possess a large S storage capacity (Rennenberg et al., 2007), and because of its commercial relevance in short rotation forestry for biomass/bioenergy production (Ceulemans and Deraedt, 1999; Mitchell et al., 1999). Such plantations might be the first ‘forests’ to be affected by the decreasing atmospheric S inputs (Schnug, 1991; McGrath and Zhao, 1996; Zhao et al., 1999). S depletion was induced by a gradual decrease of sulphate availability that allowed classification of S depletion responses into different phases in a time-resolved manner. To address the question of whole plant regulation in a comprehensive way, differences in the composition of S metabolites, sugars, and plant hormones were analysed not only in fine roots, young leaves, and mature leaves, but also in xylem sap and phloem exudates.
Materials and methods
Plant material
The experiment was carried out with the poplar hybrid Populus tremula×Populus alba, clone 717 1B4 (Institute National de la Recherche Agronomique, INRA, France). Plants were micropropagated by cuttings according to Strohm et al. (1995) and Noctor et al. (1996). Thirty-day-old poplar cuttings were transferred to pots of 1.0 l in size containing quartz sand (grain size 0.7–1.2 mm). The sand in each pot was pre-treated with 200 ml of 25% modified Hoagland solution (Hoagland and Arnon, 1950) [0.6 mM KNO3, 1.3 mM Ca(NO3)2·4H2O, 0.3 mM MgSO4·7H2O, 1.5 mM MgCl2·6H2O, 0.25 mM KH2PO4, 2.3 μM MnCl2·4H2O, 10 μM H3BO3, 0.08 μM CuCl2·4H2O, 0.2 μM ZnCl2, 0.2 μM Na2MoO4·4H2O, 0.04 μM CoCl2·6H2O, 22.5 μM Na2-EDTA, 22.5 μM FeCl2, pH 5.5] including 0.15% fungicide solution (Proplant, Dr. Stählern GmbH, Stade, Germany). Plants were cultivated in a greenhouse (26±5 °C) under long day conditions (described by Hartmann et al., 2000) and were watered with 25% modified Hoagland solution.
S depletion treatment
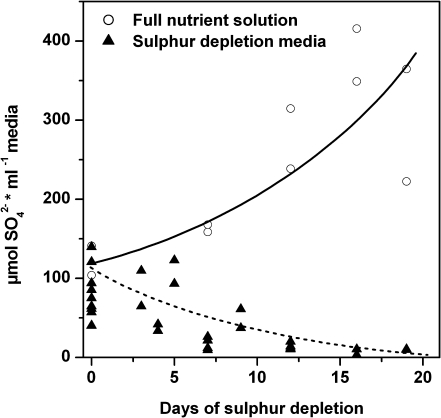
S depletion was started after 9 weeks of plant growth on sand by changing 25% modified Hoagland solution to S-free medium (25% modified Hoagland solution without MgSO4, but with 1.8 mM MgCl2·6H2O). Prior to the beginning of the depletion treatment, the sand in each pot was flushed three times with 200 ml of 1:10 diluted S-free medium to reduce the sulphate content in the sand. Controls were treated in the same way but were watered afterwards with full nutrient solution. S depletion was started time-shifted according to a time series of 0, 2, 5, 9, 14, and 21 d so that all plants were harvested on the same day. During the whole treatment, medium passing through the sand and flowing out of the pot was collected and analysed for sulphate content by anion exchange chromatography (described below). In each case the outflow from five plants standing together in one tray was mixed (one data point in Fig. 1). These measurements demonstrated that sulphate availability to the plants was reduced already by flushing the sand and further decreased with prolonged treatment with S-free medium (see Fig. 1).
Fig. 1.
Sulphate content in the flow-through solution during sulphur depletion treatment. Before starting sulphur depletion treatment, each plant pot was flushed three times with 1/10 diluted sulphur depletion medium (0 d). Solid (control) and dashed (sulphur depletion treatment) lines indicate approximated sulphate contents in the flow-through solution after watering the pots with full nutrient solution (filled triangle) or sulphur-free media (white circles), respectively. Each symbol represents one measurement which was collected as a mixture from the flow-through solution of five plants standing together in one tray.
Five replicates, each a pool of two plants, were harvested per every point of the time series (six different time points). For harvest, poplar plants were separated into (i) young leaves (third to sixth leaf counted from the first leaf with a clear petiole); (ii) mature leaves (ninth and 10th leaf); and (iii) fine roots with a diameter up to 1 mm. Fine roots were rinsed with tap water and dried with paper tissue. Additionally, xylem sap and phloem exudates were collected at each time point. Xylem sap was taken from the basal stem section of the poplar plants using the modification of the gas pressure technique of Scholander et al. (1965) described by Rennenberg et al. (1996). Phloem exudates were collected from bark pieces taken from the region around mature leaves (Rennenberg et al., 1996). For thiol measurements, the phloem exudation method described by Herschbach et al. (1998) was applied. Ion and carbohydrate analyses were performed with exudates collected as reported by Gessler et al. (2004). All samples were frozen in liquid nitrogen and stored at –80 °C until analysis.
Extraction of total RNA and cDNA preparation
All tissue samples were homogenized in liquid nitrogen. Total RNA was extracted from 90 mg of leaf tissue using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol or from 100 mg of ground fine roots using the method described by Kolosova et al. (2004). The purity and the amount of RNA were determined using a nano-spectrophotometer (NanoDrop® ND-1000, Peqlab, Erlangen, Germany) and the quality of the RNA was checked on a 1% agarose gel. To prevent contamination with genomic DNA, total RNA was treated with DNase I (Fermentas, GmbH, St.Leon-Rot, Germany) prior to cDNA synthesis. The reverse transcription reaction was performed with 1 μg of total RNA using a final concentration of 500 nM random primer R12 [5′-NNN NNN NNN NNN-3′] and Superscript II Reverse Transcriptase (Invitrogen, Karlsruhe, Germany) according to the manufacturer’s instructions. Samples without reverse transcriptase were prepared for each sample and used as a control.
Isolation and expression analyses of poplar gene sequences
The gene-specific primers used for isolation of partial gene sequences of PtaATPS1/2, PtaATPS3/4, PtaANT (Aintegumenta), and PtabTub (β-tubulin) were prepared according to sequence information in the Populus trichocarpa genome database (http://genome.jgi-psf.org/Poptr1/Poptr1.home.html, Tuskan et al., 2006) and are listed in Supplementary Table S1 available at JXB online. Four gene models were described in P. trichocarpa for PtcATPS (Table 1). By comparing the predicted amino acid sequence of these models with the protein sequences of A. thaliana, PtcATPS1 and PtcATPS2 were more similar to AtAPS2 while PtcATPS3 and PtcATPS4 were more closely related to AtAPS1, 3, and 4 (Table 1). Similarities were calculated by CLUSTALW (SDSC Biology Work-Bench 3.2, http://workbench.sdsc.edu/, Thompson et al., 1994) using pairwise alignment with the BLOSUM matrix and an open gap penality of 11 with a gap extension of 1. Because each two of the four PtcATPS gene sequences were highly similar (93% identity score for PtcATPS1 and PtcATPS2 and 92% between PtcATPS3 and PtcATPS4), primers were designed so that they fit to both of the closely related sequences. The corresponding gene sequences were named accordingly PtaATPS1/2 and PtaATPS3/4.
Table 1.
Sequence comparison between predicted protein ATP sulphurylase sequences of Populus trichocarpa and protein sequences of Arabidopsis thaliana
| AtAPS1 (At3g22890) | AtAPS2 (At1g19920) | AtAPS3 (At4g14680) | AtAPS4 (At5g43780) | |
| PtcATPS1 (protein ID: 754047) | 70% | 82% | 72% | 73% |
| PtcATPS2 (protein ID: 818792) | 71% | 83% | 73% | 74% |
| PtcATPS3 (protein ID: 720943) | 78% | 67% | 75% | 72% |
| PtcATPS4 (protein ID: 658460) | 77% | 66% | 76% | 73% |
Similarities were calculated by CLUSTALW (SDSC Biology Work-Bench 3.2, http://workbench.sdsc.edu/, Thompson et al., 1994) using pairwise alignment with the BLOSUM matrix and an open gap penality of 11 and a gap extension of 1.
The PCRs to isolate the gene sequences of P. tremula×P. alba were performed using a standard Taq-DNA Polymerase (Fermentas GmbH, St.Leon-Rot, Germany) according to the manufacturer’s instructions with cDNA from fine roots and leaves of P. tremula×P. alba. The amplified DNA segment was separated on a 0.8% agarose gel and subsequently ligated into the pCR2.1 vector (Invitrogen). The vector was transformed into Escherichia coli cells (INVF′α, Invitrogen) and the cloned gene segment was sequenced (Eurofins MWG Operon, Ebersberg, Germany) and verified by alignment with the corresponding gene sequences of P. trichocarpa (ALIGN function of SCSC Biology WorkBench, http://workbench.sdsc.edu/, Thompson et al., 1994). Sequences for sulphate transporter (SULTR) genes were taken from Dürr et al. (2010), the sequence for APR from Kopriva et al. (2004), and the sequence for elongation factor 1β (Ef1b) from Wildhagen et al. (2010).
Expression analyses were performed using quantitative real-time PCR (qRT-PCR). The set-up was performed as described by Wildhagen et al. (2010) with 2.5 μl (25 ng) of cDNA per reaction. Sequences of the oligonucleotide primers used for gene expression analysis with qRT-PCR and the resulting length of the PCR fragment are listed in Supplementary Table S2 at JXB online. The specificity of the primer pairs was tested by qRT-PCR, subsequent agarose gel electrophoresis of the amplified segments, and by sequencing of the amplification products.
Extraction of total RNA including small RNAs
Extraction of total RNA including the small RNA fraction was performed either using the TRIzol reagent (Invitrogen) according to the manufacturer’s instructions (leaf tissues) or using a modified method of Kolosova et al. (2004) and Chang et al. (1993) (fine roots). For the latter, 100 mg of ground plant tissue were mixed with 1 ml of extraction buffer [2% cetyltrimethylammonium bromide (CTAB), 2% (w/v) PVP K40, 100 mM TRIS-HCl, pH 8.0, 25 mM Na2EDTA, 2 M NaCl, 0.5 g l−1 spermidine, supplemented with 2% (v/v) 2-mercaptoethanol before use and pre-warmed at 65 °C] by vigorous shaking. After 10 min incubation at 65 °C in a water bath with shaking every 2 min, RNA was extracted twice with 1 ml of chloroform/isoamylalcohol (24:1, v/v) followed by centrifugation at 15 000 g for 15 min at 4 °C. RNA in the supernatant was precipitated by adding 1 ml of 100% ethanol containing 1/20 vol. of 1 M acetic acid and by incubation at –20 °C overnight. After centrifugation (15 000 g for 15 min at 4 °C), the pellet was washed three times with 75% ethanol and dissolved in nuclease-free water (Fermentas). RNA concentrations were determined using a nano-spectrophotometer (NanoDrop® ND-1000, Peqlab) and by comparison with control RNA of known concentration separated on an agarose gel. Separation on agarose gel was also used to check the quality of the RNA. Isolated RNA samples were stored at –80 °C.
Northern blot analysis for microRNAs
Aliquots of 15 μg (fine roots and young leaves) or 70 μg (young and mature leaves) of total RNA were separated in a 13% polyacrylamide gel containing 8.3 M urea in 1×TBE buffer and electroblotted onto nylon membranes (Hybond-NX for 15 μg of total RNA and Hybond-N+ for 70 μg of total RNA; Amersham, Freiburg, Germany) for 1 h at 400 mA. Oligonucleotides complementary to miRNA sequences were radiolabelled with [γ-32P]ATP using T4 polynucleotide kinase (Fermentas). The oligonucleotide probes used were 5′-GAGTTCCTCCAAACCCTTCAG-3′ (miR395) and 5′-ACGGGATTCTGCAATTCACAC-3′ (5S rRNA). The oligonucleotide sequence for detecting miR395 corresponds to the mature sequence of miR395a of P. trichocarpa (accession no. MIMAT0002031; taken from the microRNAs database ‘miRbase’ (http://www.mirbase.org). Blot hybridization was carried out in a buffer containing 0.05 M sodium phosphate (pH 7.2), 1 mM EDTA, 6× SSC, 1× Denhardt’s, and 5% SDS. Blots were washed 2–3 times with 2× SSC, 0.2% SDS, and once with 1× SSC, 0.1% SDS. Subsequently, blots were hybridized and washed at temperatures 10 °C below the Tm of the oligonucleotide.
Detection of RNA cleavage products by RNA ligase-mediated 5′ random amplification of cDNA ends (RLM 5′ RACE)
RLM 5′ RACE-PCR was performed with a modified GeneRacer Kit (Invitrogen) protocol (Llave et al., 2002). PCRs were performed using the GeneRacer forward primer 5′-CGACTGGAGCACGAGGACACTGA-3′ and the gene-specific reverse primer 5′-TTCTATCACCTCCAAGTCCCCTCCAATC-3′ followed by a PCR with the nested GeneRacer forward primer 5′-GGACACTGACATGGACTGAAGGAGTA-3′ and the nested gene-specific reverse primer 5′-CTTCAACGTAAGGTAAACCAGGGGCAGT-3′. Amplification products corresponding to the size of expected cleavage products were excised from the gel, cloned, and sequenced.
Determination of anions
Sulphate and phosphate contents were analysed using anion exchange chromatography as described by Herschbach et al. (2000). Aliquots of 50 mg of powdered tissue were added to 1 ml of deionized H2O containing 100 mg of polyvinylpolypyrrolidone (PVPP). The mixtures were shaken for 1 h at 4 °C, boiled for 10 min, and then centrifuged for 10 min at 15 000 g at 4 °C. The supernatants were diluted (1:1 for fine roots, 1:4 for leaves) before they were injected into the chromatography system DX-120 (Dionex, Idstein, Germany). A 300 μl aliquot of xylem sap or 1 ml of phloem exudates was mixed with 20 mg of PVPP and treated in the same way as tissue samples except for the boiling step. Both supernatants were subjected undiluted to anion exchange chromatography. The flow-through media collected from the pots after watering the plants were diluted at a ratio of 1:4 with deionized H2O and analysed directly. In each case, 50 μl were injected into the ion chromatography system.
Measurement of soluble carbohydrates
For analysis of soluble carbohydrates, tissue samples were prepared in the same way as described for anion analyses. Samples of xylem sap and phloem exudates were diluted prior to extraction (1:100 xylem sap, 1:20 phloem exudates). Soluble carbohydrates were separated, detected, and quantified by HPLC as described by Jaeger et al. (2009). Tissue samples were analysed (Dionex DX 500 HPLC system; Dionex, Sunnyvally, USA) after dilution (1:10 for fine roots, 1:40 for leaves), whereas xylem sap and phloem exudates were measured without further dilution. In each case, 50 μl of sample were injected into the HPLC system.
Determination of thiol contents
Thiol contents in tissue samples were measured as described by Strohm et al. (1995) and Herschbach et al. (2000) using 30 mg of powdered leaf tissue or 100 mg of powdered root tissue. Phloem exudates were analysed according to Herschbach et al. (2000), and thiol contents in xylem sap were analysed as described by Schupp et al. (1991) and Herschbach et al. (2000).
Plant hormone measurements
For plant hormone measurements, powdered tissue samples were lyophilized for 5 d (Freeze dryer: Alpha 2-4 with an LDC-1M modul for system control; Martin Christ, Germany). Freeze-dried tissue samples (each 10–15 mg) were analysed for plant hormone contents as described by Kojima et al. (2009). A 300 μl aliquot of xylem sap and 2 ml of phloem exudates were directly lyophilized and were subjected completely for plant hormone analyses.
Statistical analyses
All statistical analyses were performed using the software R version 2.8.1 for windows 2000 (R Development Core Team). For testing statistical differences between groups, analysis of variance (ANOVA; R function ‘aov()’) was performed combined with the Tukey’s honest significant differences test (R function ‘TukeyHSD()’) as post-hoc test. Not normally distributed (tested either by Kolmogorov–Smirnov-test (R function ‘lillie.test()’) or by Shapiro–Wilk test (R function ‘shapiro.test()’)) or non-homogeneous data {tested using the Bartlett test (R function ‘bartlett.tes()’) for homogeneity of variances} were either transformed using an appropriate transformation or subjected to the non-parametrical Kruskal–Wallis rank sum test including the multiple comparison test after Kruskal–Wallis.
Bivariate Pearson correlation (for normally distributed data) or Spearman’s rank correlation analysis (for non-normally distributed data) were used to analyse correlations of transcript levels, thiol, sugar, or individual plant hormone contents with sulphate contents. The correlation coefficient ρ (Pearson) or ρs (Spearman) and the corresponding P-values were calculated by using the function ‘cor.test()’. Quantile–quantile plots (R function ‘qqnorm’) and appropriate statistical tests (Kolmogorov–Smirnov-test (R function ‘lillie.test()’) or Shapiro–Wilk test (R function ‘shapiro.test()’) were applied to test for normal distribution of the residuals. Furthermore, data were analysed for homogeneity of variance and linearity of the residuals (residuals plotted over fitted values) as well as for heteroscedasty (Goldfeld–Quandt test; R function ‘gqtest()’).
Accession numbers
Sequences can be found in the NCBI database (http://www.ncbi.nlm.nih.gov) under the following accession numbers for P. tremula×P. alba: DQ906929 (PtaSULTR1;1), DQ174472 (PtaSULTR1;2), DQ174474 (PtaSULTR2;2), DQ906930 (PtaSULTR4;1), DQ906935 (PtaSULTR4;2), HM853668 (PtaATPS1/2), HM853669 (PtaATPS3/4), AY353089 (PtaAPR), FJ372570 (PtaEf1b), HM853671 (PtabTub), and HM853670 (PtaANT).
Results
Changes in anion, thiols, and carbohydrate contents
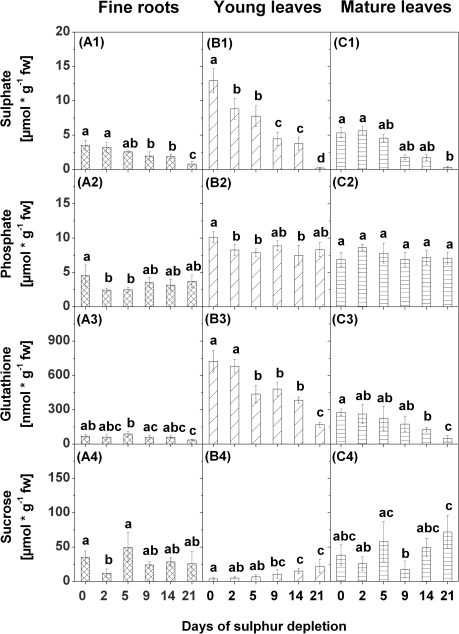
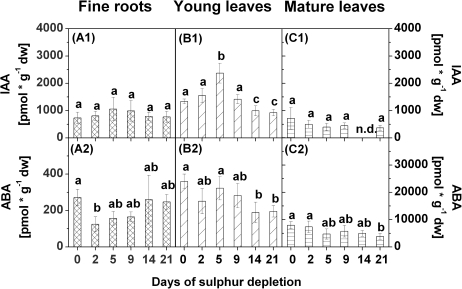
Sulphate contents used as an indicator of the S status in the plants decreased successively in fine roots, young leaves, and mature leaves in response to S depletion (Fig. 2A1, B1, C1). The strongest decrease was found in young leaves, where sulphate contents declined significantly already after 2 d of S depletion. After 9 d of S depletion, the sulphate content in fine roots, and young and mature leaves was almost halved compared with the control (0 d of S depletion). After 21 d of S depletion, sulphate contents in young and mature leaves were close to zero, whereas considerable amounts of sulphate were still present in fine roots.
Fig. 2.
Sulphate (1), phosphate (2), glutathione (3), and sucrose (4) contents in fine roots (A), young leaves (B), and mature leaves (C) of poplars treated with sulphur depletion and of control plants (day 0). Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue. fw, fresh weight
Phosphate contents were determined to obtain an indication of the general nutrient status of the plant. Phosphate contents varied in response to S depletion in fine roots and young leaves, but not in mature leaves (Fig. 2A2, B2, C2). After 2 d and 5 d of S depletion, phosphate contents decreased in fine roots, and after 2, 5, and 14 d in young leaves. None of these changes coincided with the successive decline of the sulphate contents.
Contents of GSH declined in fine roots, and in young and mature leaves with decreasing sulphate contents (Fig. 2A3, B3, C3). The strongest decline in GSH content was observed in young leaves where a first significant reduction was already found after 5 d of S depletion and a second after 21 d. In fine roots and mature leaves, a significant decrease of the GSH content was first observed after 21 d of S depletion. Comparable changes were not observed for cysteine or γ-EC contents (Supplementary Fig. S1A1-2, B1-2, C1-2 at JXB online).
The sugars glucose, fructose, and sucrose were determined because of their regulatory influence on sulphate metabolism (Kopriva et al., 1999; Hesse et al., 2003; Kopriva and Rennenberg, 2004). Sucrose contents in fine roots did not change in response to S depletion except for a transient decline after 2 d of S depletion (Fig. 2A4) that coincided with the decrease in the phosphate, but not the sulphate content. In contrast, sucrose contents in young leaves increased with decreasing sulphate contents (Fig. 2B4). A first significant increase was found after 9 d, and a second after 14 d and 21 d of S depletion. In mature leaves, a clear tendency of changes in sucrose contents was not observed during S depletion (Fig. 2C4). Glucose and fructose contents remained unaffected in roots, except for a peak value after 5 d of S depletion (Supplementary Fig. S1A3–4 at JXB online). In young and mature leaves, the contents of these sugars fluctuated strongly during the treatment but declined after 21 d of S depletion (Supplementary Fig. S1B3–4, C3–4).
Gene expression of sulphur-metabolism related genes
Expression of five SULTR genes, i.e. the root-localized PtaSULTR1;2, the phloem-specific PtaSULTR1;1, the two vacuolar sulphate transporter genes PtaSULTR4;1 and PtaSUTR4;2, and the leaf-localized sulphate transporter PtaSULTR2;2, was analysed (see Supplementary fig. 1 of Dürr et al., 2010). Furthermore, expression of genes encoding enzymes involved in sulphate reduction, i.e. the ATP sulphurylase genes PtaATPS1/2 and PtaATPS3/4 and the APS reductase gene PtaAPR, was examined.
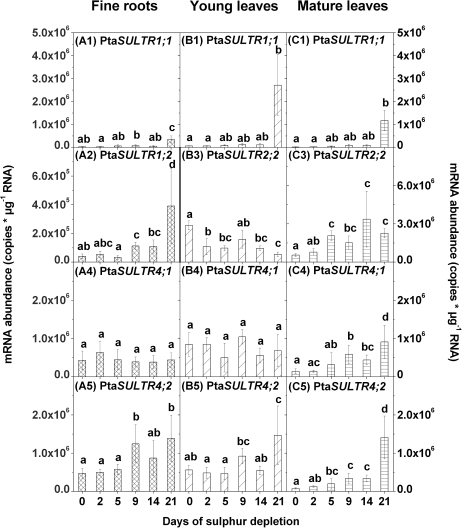
In all tissues, mRNA levels of the group 1 sulphate transporter gene, PtaSULTR1;1, increased first after 21 d of S depletion (Fig. 3A1, B1, C1) when almost no sulphate was left (see Fig. 2A1, B1, C1). In contrast, expression of the root-specific PtaSULTR1;2 (see Supplementary fig. 1 of Dürr et al., 2010) increased in fine roots already after 9 d and further after 21 d of S depletion (Fig. 3A2). This pattern coincides with the first and the second decrease of the root sulphate content.
Fig. 3.
Changes in gene expression of sulphate transporter genes in fine roots (A), young leaves (B), and mature leaves (C) due to sulphur depletion treatment. Controls are represented by day 0. Expression of PtaSULTR1;2 (2) was analysed in fine roots only (left y-axis), expression of PtaSULTR2;2 (3) solely in leaf tissues (right y-axis), and expression of PtaSULTR1;1 (1), PtaSULTR4;1 (4), and PtaSULTR4;2 (5) was measured in fine roots, young leaves, and mature leaves. mRNA abundance was related to total RNA. Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue.
Transcripts of the leaf-localized PtaSULTR2;2 (Supplementary fig. 1 of Dürr et al., 2010) reacted in the opposite way in young and mature leaves (Fig. 3B3, C3). While transcript levels of PtaSULTR2;2 were reduced after 2, 5, 14, and 21 d of S depletion in young leaves, levels in mature leaves increased with decreasing sulphate contents, with the first significant increase after 5 d of S depletion.
Expression of the putatively vacuolar sulphate transporter PtaSULTR4;1 reacted differently to S depletion in fine roots, and mature and young leaves. In fine roots and young leaves, transcript levels of PtaSULTR4;1 remained unaffected during the whole period of S depletion (Fig. 3A4, B4). In mature leaves, transcript levels of PtaSULTR4;1 continuously increased, with a first significant increase after 5 d and a second increase after 21 d of S depletion (Fig. 3C4). Expression of PtaSULTR4;2 was significantly enhanced in all tissues after 9 d of S depletion when the sulphate content was almost halved (Fig. 3A5, B5, C5). In both leaf types, but not in the roots, PtaSULTR4;2 transcripts were further enhanced after 21 d of S depletion.
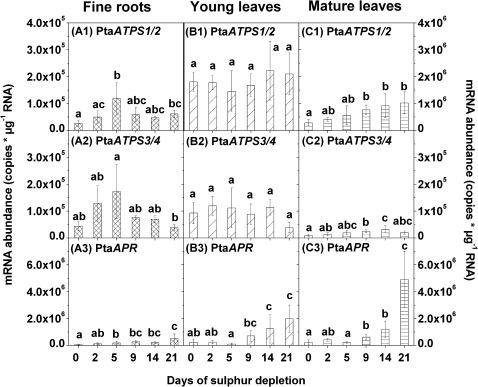
As found for the expression of the putative vacuolar SULTR, transcript levels of PtaATPS1/2 and PtaATPS3/4 also reacted differently to S depletion in fine roots, and young and mature leaves. Expression of PtaATPS1/2 increased in fine roots after 5 d and 21 d, and in mature leaves already after 9 d of S depletion (Fig. 4A1, C1). No changes were observed in young leaves (Fig. 4B1). In contrast, transcript levels of PtaATPS3/4 increased continuously in mature leaves for up to 14 d of S depletion (Fig. 4C2). In fine roots, a peak was detected after 5 d of S depletion (Fig. 4A2) similar to expression of PtaATPS1/2 (see Fig. 4A1), while PtaATPS3/4 expression did not change significantly in young leaves (Fig. 4B2).
Fig. 4.
Changes in gene expression of ATP sulphurylase 1/2 (PtaATPS1/2) (1), 3/4 (PtaATPS3/4) (2), and APS reductase (PtaAPR) (3) in fine roots (A), young leaves (B), and mature leaves (C) due to sulphur depletion treatment. Controls are represented by day 0. Expression levels of PtaATPS1/2 are related to different y-axes (A to the left y-axis B and C to the right y-axis). mRNA abundance was related to total RNA. Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue.
PtaAPR transcript levels increased in fine roots, and young and mature leaves continuously with decreasing sulphate contents (Fig. 4A3, B3, C3). The first increase of PtaAPR transcripts appeared in fine roots after 5 d, and in mature and young leaves after 9 d of S depletion. After 21 d, transcript levels of PtaAPR increased further in all three tissues.
Gene expression data were not normalized by using expression of reference genes, but transcripts of three reference genes, namely PtaEF1b, PtabTub, and PtaANT, were analysed (Supplementary Fig. S2 at JXB online). No changes in gene expression of these genes were observed in fine roots and young leaves. In mature leaves, the expression patterns of all three reference genes were different from each other.
The role of miR395 during S depletion
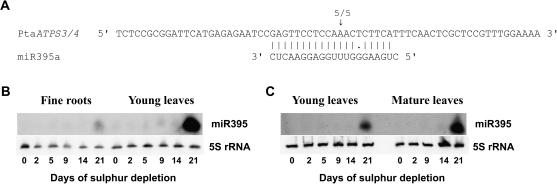
In previous studies, miR395 was shown to regulate gene expression of AtAPS1 and AtAPS4 (Jones-Rhoades and Bartel, 2004; Maruyama-Nakashita et al., 2006; Kawashima et al., 2009, 2011), and its expression was demonstrated to be induced under S depletion (Jones-Rhoades and Bartel, 2004; Kawashima et al., 2009, 2011). In poplar, PtaATPS3 and PtaATPS4 were predicted to be targets of miR395 (data not shown), and miR395-dependent cleavage products of PtaATPS3/4 were obtained by 5′ RACE-PCR (Fig. 5A). Therefore, expression of miR395a was examined in fine roots, young leaves, and mature leaves upon S depletion. In all tissues, expression of miR395a was detected first after 21 d of S depletion (Fig. 5B, C). The strongest expression of miR395a was found in mature leaves followed by young leaves (Fig. 5C). In fine roots, expression of miR395a could only be demonstrated with a highly sensitive method, but even then the expression of miR395a was very weak compared with the expression in young leaves (Fig. 5B).
Fig. 5.
(A) Validation of PtaATPS3/4 as the target of miR395. Validation was carried out by rapid amplification of 5′ cDNA ends (5′ RACE). RNA from mature leaves after 0 d or 21 d of sulphur depletion treatment and a primer pair for PtaATPS3/4 products were used (see materials and methods). Each sample derived from two plants pooled together. Arrows indicate the 5′ ends of cleavage products mapped inside the displayed sequence including the number of cloned and sequenced RACE products with the respective 5′ end. Target sequences are shown on top of the miR395a sequence. (B, C) Expression of miR395a in fine roots, young leaves, and mature leaves of poplar due to sulphur depletion treatment. Northern blot analysis was carried out with 15 μg of total RNA from fine roots and young leaves using a high-sensitive membrane (Amersham-Hybond-NX) (B) or with 70 μg of total RNA from young leaves and mature leaves using a less sensitive membrane (Amersham-Hybond-N+) (C). All tissues derived from pooled samples of two poplar plants. 5S rRNA was used as loading control.
Responses of plant hormones to S depletion
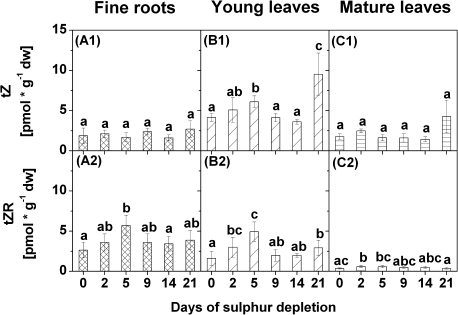
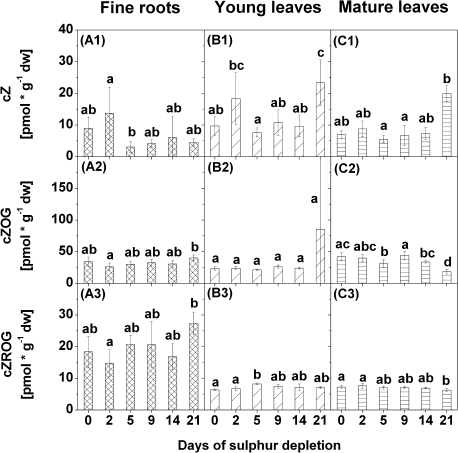
To examine the importance of plant hormonal regulation during S depletion in poplar, internal cytokinin, auxin, gibberellic acid, and ABA contents were measured in fine roots, young leaves, and mature leaves (Figs 6–9, for details see Supplementary Tables S3, S4, and S5 at JXB online). Differences in response to S depletion were found in contents of trans-zeatins (tZs), cis-zeatins (cZs), and dihydrozeatins (Figs 6–8). Two peak values were detected for contents of tZ and of tZ riboside (tZR) in young leaves after 5 d and 21 d of S depletion (Fig. 6B1–2). In fine roots, contents of tZR were increased after 5 d of S depletion only, while they were elevated after 2 d in mature leaves (Fig. 6A2). Among the cZs, contents of cZ increased in young leaves after 21 d of S depletion (Fig. 7B1) while contents of cZ riboside O-β-glucoside (cZROG) were increased after 5 d (Fig. 7B3). In mature leaves, contents of cZ O-β-glucoside (cZOG) and cZROG decreased after 21 d of S depletion (Fig. 7C2–3). Differences in contents of cZ, cZOG, and cZROG were also found in fine roots (Fig. 7A1–3) but not in relation to the control values. Significant differences exist for cZ between 2 d and 5 d of S depletion and for cZOG and cZROG between 2 d and 21 d of S depletion.
Fig. 6.
Contents of trans-zeatin (tZ) (1) and of tZ riboside (tZR) (2) in fine roots (A), young leaves (B), and mature leaves (C) of poplars treated with sulphur depletion and of control plants (day 0). Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue. dw, dry weight
Fig. 7.
Contents of cis-zeatin (cZ) (1), of cZ O-β-glucoside (cZOG) (2), and of cZ riboside O-β-glucoside (cZROG) (3) in fine roots (A), young leaves (B), and mature leaves (C) of poplars treated with sulphur depletion and of control plants (day 0). Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue. dw, dry weight
Fig. 8.
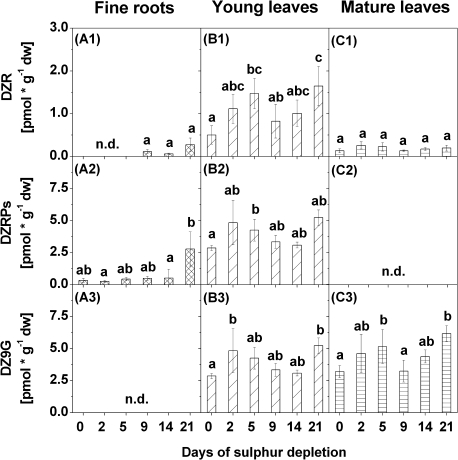
Contents of dihydrozeatin riboside (DZR) (1), dihydrozeatin riboside phosphates (DZRPs) (2), and dihydrozeatin-9-N-glucoside (DZ9G) (3) in fine roots (A), young leaves (B), and mature leaves (C) of poplars treated with sulphur depletion and of control plants (day 0). Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue. dw, dry weight; n.d., not detected
Fig. 9.
Contents of indole-3-acetic acid (IAA) (1) and of abscisic acid (ABA) (2) in fine roots (A), young leaves (B), and mature leaves (C) of poplars treated with sulphur depletion and of control plants (day 0). Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue. dw, dry weight; n.d., not detected
Several dihydrozeatins were increased in fine roots, young leaves, and mature leaves after 21 d of S depletion. However, the composition of individual dihydrozeatins differed greatly between tissues. In fine roots, dihydrozeatin riboside (DZR) and DZR phosphates (DZRPs) were detected, but DZR was found only at the end of the S depletion treatment (Fig. 8A1–2). Contents of both dihydrozeatins increased with decreasing sulphate contents, although this increase was not statistically significant. Both dihydrozeatins were also increased in young leaves, as was dihydrozeatin 9-N-glucoside (DZ9G) (Fig. 8B1–3). However, contents of all three dihydrozeatins fluctuated strongly during S depletion treatment in young leaves, with peak values after 5 d (DZR and DZRPs) or after 2 d (DZ9G). A comparable pattern was observed for DZ9G in mature leaves where contents of DZ9G were elevated after 5 d and after 21 d of S depletion (Fig. 8C3). Contents of DZR did not change in mature leaves during S depletion (Fig. 8C1), and DZRPs were not detected at all (Fig. 8C2).
Contents of the auxin indole acetic acid (IAA) did not change in fine roots in response to S depletion (Fig. 9A1), but tended to decrease with decreasing sulphate contents in mature leaves (Fig. 9C1). In young leaves, IAA contents first increased until 5 d of S depletion and then decreased to values lower than found in the controls after 14 d and 21 d of S depletion (Fig. 9B1). Contents of ABA decreased in young and mature leaves after 14 d or 21 d of S depletion, respectively (Fig. 9B2, C2). In fine roots, ABA contents were reduced after 2 d of S depletion, but then returned to the level of the controls (Fig. 9A2).
Analyses of xylem sap and phloem exudates — indications of a whole plant regulation
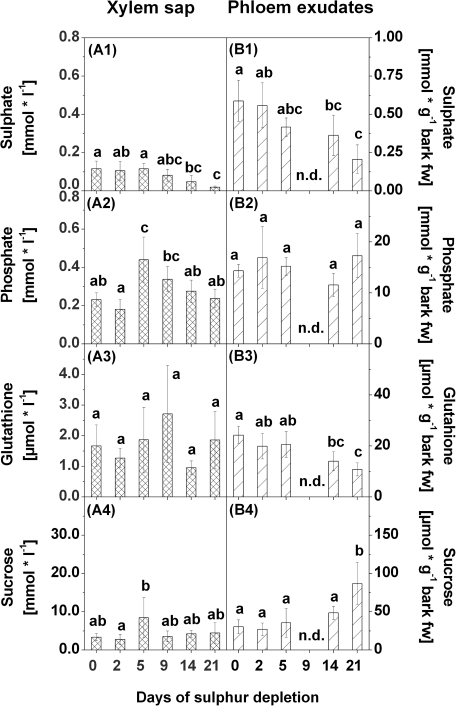
Sulphate, phosphate, and metabolite contents as well as plant hormone contents were also analysed in xylem sap and phloem exudates. Sulphate contents in xylem sap and in phloem exudates decreased continuously upon S depletion. A first significant decrease was observed after 14 d of S depletion (Fig. 10A1, B1). Phosphate contents in xylem sap were enhanced after 5 d and 9 d of S depletion, but subsequently decreased again to the level of the controls (Fig. 10A2). In phloem exudates, no differences in phosphate contents were found in response to S depletion (Fig. 10B2).
Fig. 10.
Sulphate (1), phosphate (2), glutathione (3), and sucrose (4) contents in xylem sap (A) and phloem exudates (B) of poplars treated with sulphur depletion and of control plants (day 0). Data presented are the means ±SD of five replicates where each replicate consisted of two pooled plants. Lower case letters indicate significant differences at P < 0.05 between different time points within one tissue. Contents were related to litres of xylem sap or bark fresh weight (fw) of the bark piece used for phloem exudation, respectively. n.d., not detected
Contents of GSH did not change in xylem sap due to S depletion (Fig. 10A3), whereas GSH contents in phloem exudates were diminished with decreasing sulphate contents (Fig. 10B3). This reduction became significant after 14 d of S depletion.
In xylem sap, sucrose contents were more or less unaffected, except for a slight increase after 5 d of S depletion (Fig. 10A4). In contrast, sucrose contents in phloem exudates increased after 21 d of S depletion (Fig. 10B4).
For contents of cytokinins, auxins, gibberellic acids, and ABA, no significant differences were found either in xylem sap or in phloem exudates in response to S depletion (see Supplementary Table S6 and S7 at JXB online).
Correlation analyses
To underline the connection between decreasing sulphate contents and changes in the transcript levels, metabolite and plant hormone contents, correlation analyses were performed. In fine roots, young leaves, and mature leaves, transcript levels of PtaSULTR1;1, PtaSULTR4;2, and PtaAPR were strongly negatively correlated with sulphate contents (Table 2). Further strong negative correlations with sulphate contents were found for transcript levels of PtaSULTR1;2 in fine roots and for PtaSULTR4;1, PtaATPS1/2 and PtaATPS3/4 in mature leaves. Transcript levels of PtaSULTR2;2 were positively correlated with sulphate contents in young leaves and negatively in mature leaves. Although GSH contents decreased in all tissues with decreasing sulphate contents, a positive correlation with sulphate contents was found only in young and mature leaves, but not in fine roots. Moreover, sucrose contents in young leaves correlated negatively with sulphate contents. Among the different plant hormones, negative correlations with sulphate contents were found for DZR contents in fine roots and in young leaves. A positive correlation with sulphate contents was detected for contents of cZROG in mature leaves, for IAA in young leaves, and for ABA in both leaf types.
Table 2.
Correlation analysis of glutathione contents, sucrose contents, mRNA abundance, and of plant hormone contents with the sulphate content in fine roots, young leaves, and mature leaves
| Fine roots |
Young leaves |
Mature leaves |
|||||||
| ρ | P-value | n | ρ or ρs | P-value | n | ρs | P-value | n | |
| PtaSULTR1;1 | –0.81 | <0.0001 | 30 | –0.85* | <0.0001 | 30 | –0.91* | <0.0001 | 30 |
| PtaSULTR1;2 | –0.77 | <0.0001 | 30 | ||||||
| PtaSULTR2;2 | 0.68 | <0.0001 | 30 | –0.66* | 0.0001 | 30 | |||
| PtaSULTR4;1 | –0.75* | <0.0001 | 30 | ||||||
| PtaSULTR4;2 | –0.69 | <0.0001 | 30 | –0.68 | <0.0001 | 30 | –0.85* | <0.0001 | 30 |
| PtaATPS1/2 | –0.70* | <0.0001 | 30 | ||||||
| PtaATPS3/4 | –0.52* | 0.0035 | 30 | ||||||
| PtaAPR | –0.79 | <0.0001 | 30 | –0.78 | <0.0001 | 30 | –0.73* | <0.0001 | 30 |
| Glutathione | 0.89 | <0.0001 | 30 | 0.74* | <0.0001 | 30 | |||
| Sucrose | –0.75 | <0.0001 | 30 | ||||||
| cZROG | 0.57* | 0.0011 | 30 | ||||||
| DZR | –0.75 | 0.0047 | 12 | –0.54 | 0.0019 | 30 | |||
| IAA | 0.59 | 0.0006 | 30 | ||||||
| ABA | 0.65 | 0.0001 | 30 | 0.52* | 0.0033 | 30 | |||
Only correlation coefficients with ρ or ρs >0.5 or ρ or ρs less than –0.5 and corresponding P-values <0.01 are presented.
ρ, Pearson correlation coefficient, ρs. Spearmans rank correlation coefficient (indicated by *), n, number of replicates.
No significant correlations with sulphate contents were found in xylem sap. In phloem exudates, a positive correlation with sulphate contents was found for GSH contents (ρ=0.63, P=0.0007), and sucrose contents correlated negatively with sulphate contents (ρ= –0.53, P7=0.0061).
Discussion
Different responses upon ‘S limitation’ and ‘early S deficiency’
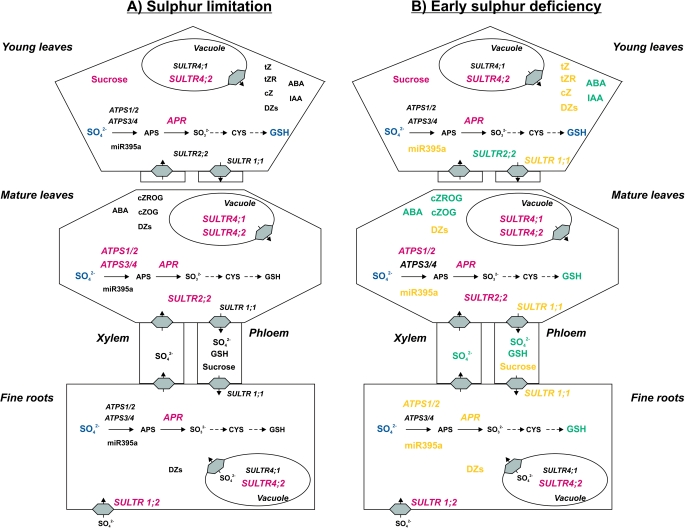
The present experiment investigated early events of S depletion at a high temporal resolution in different plant tissues and the transport tissues xylem and phloem. A broad spectrum of genes of S metabolism (sulphate transporters and genes of sulphate assimilation), of metabolites, and of plant hormone contents was analysed and correlated with the sulphate content. The observed changes in response to S depletion can be separated into two phases, denominated as ‘S limitation’ and ‘early S deficiency’.
S limitation was defined by halved sulphate contents compared with the controls (0 d of S depletion). This corresponds in fine roots, mature leaves, and young leaves to day 9 of S depletion (although sulphate contents in young leaves were significantly reduced already before). S limitation was accompanied by increasing transcript levels of PtaSULTR1;2 in fine roots and of PtaSULTR4;2 and PtaAPR in fine roots, mature leaves, and young leaves (Fig. 11A). In addition, increasing transcript levels of PtaSULTR2;2, PtaSULTR4;1, PtaATPS1/2, and PtaATPS3/4 in mature leaves as well as a decline in GSH and an increase in sucrose contents in young leaves were identified as tissue-specific responses to S limitation (Fig. 11A). The peak expression of PtaATPS1/2 and PtaATPS3/4 in fine roots after 5 d of S depletion could not be related to the decreasing sulphate contents and was therefore not regarded as an S limitation response. Further experiments have to show if those peaks in expression of the PtaATPS genes are of importance for the regulation of S metabolism during S depletion. In young leaves, transcript levels of PtaSULTR2;2 were also reduced before 9 d of S depletion but increased again at day 9. Therefore, this reaction in young leaves could not clearly be defined as a response to S limitation.
Fig. 11.
Schematic overview of the changes in sulphur metabolism of poplar during sulphur limitation (A) and during early sulphur deficiency (B). Changes in expression of sulphur-metabolism related genes, metabolite or plant hormone contents during sulphur limitation are labelled in red (increase) or blue (decrease). Additional changes appearing first during early sulphur deficiency are marked in yellow (increase) or blue green (decrease). ABA, abscisic acid; APS, adenosine 5′-phosphosulphate; CYS, cysteine; cZ, cis-zeatin; cZOG, cZ O-β-glucoside; cZROG, cZ riboside O-β-glucoside; DZs, dihydrozeatins; GSH, glutathione; IAA, indole-3-acetic-acid; tZ, trans-zeatin; tZR, tZ riboside. For abbreviations of genes (in italics) see text.
As known from several other studies (Smith et al., 1997; Takahashi et al., 1997; Vidmar et al., 2000; Buchner et al., 2004a; Koralewska et al., 2007, 2009; Barberon et al., 2008), transcripts of group 1 SULTR genes responsible for sulphate uptake and of APR strongly react to S depletion. This is consistent with the findings in poplar. The up-regulation of the corresponding SULTR from poplar, PtaSULTR1;2 (Supplementary fig. 1 of Dürr et al., 2010), and of PtaAPR indicates an improvement in the sulphate uptake capacity in fine roots and in the sulphate reduction capacity in all tissues already during S limitation. The elevated expression of PtaSULTR4;2 is likely to be an indication of increased sulphate re-mobilization from the vacuolar sulphate pool (Kataoka et al., 2004; Zuber et al., 2010) as observed in Brassica and Arabidopsis (Buchner et al., 2004a; Kataoka et al., 2004; Parmar et al., 2007; Abdallah et al., 2010). This assumption is supported by the finding that high transcript abundance of PtaSULTR4;2 correlated with low sulphate contents in leaves during seasonal growth (Dürr et al., 2010). Up-regulation of SULTR4;2 induced by S depletion occurred earlier and was stronger than that of SUTLR4;1 in mature leaves. It can be assigned to tissue-specific differences in the responsiveness of vacuolar SULTR genes as observed in Arabidopsis and Brassica (Kataoka et al., 2004; Dubousset et al., 2009). BnSULTR4;1 expression was induced only in mature leaves but not in young leaves (Dubousset et al., 2009). In addition, PtaSULTR4;1 expression in poplar is more likely to be controlled by leaf development than by the sulphate content itself as no correlation with the sulphate content was observed during seasonal growth (Dürr et al., 2010).
The second denominated phase was characterized by an almost depleted soluble sulphate pool. This phase is reached in all tissues after 21 d of S depletion. As basic plant characteristics such as tree height, growth increment, and chlorophyll contents (Supplementary Fig. S3, S4 at JXB online) were not affected by 21 d of S depletion, the observed changes were denoted as early S deficiency responses. The effect of prolonged S depletion was much stronger in young and mature leaves than in fine roots. Nevertheless, the most dominant responses to S limitation, the increasing transcript levels of PtaSULTR1;2, PtaSULTR4;2, and PtaAPR, were further enhanced in all tissues upon early S deficiency and this was accompanied by increasing transcript levels of the phloem-specific PtaSULTR1;1 (Fig. 11B). In addition, enhanced expression of PtaATPS1/2 in fine roots and decreased expression of the leaf-localized PtaSULTR2;2 (Supplementary fig. 1 of Dürr et al., 2010) in young leaves were detected as tissue-specific responses. At the metabolite level, GSH contents decreased further in young leaves and were also reduced in fine roots and mature leaves (Fig. 11B). In both leaf types, a strong correlation was found between sulphate and GSH contents. All these reactions are generally described as specific reactions to S depletion (Smith et al., 1997; Lappartient and Touraine 1997; Vidmar et al., 1999; Hirai et al., 2003; Maruyama-Nakashita et al., 2003; Nikiforova et al., 2003; Hopkins et al., 2005; Koralewska et al., 2007, 2009; Parmar et al., 2007; Barberon et al., 2008). Only responses of the PtaSULTR2;2 in leaves seem controversial, but might be under developmental control. This is indicated by up-regulation of PtaSULTR2;2 transcripts in mature leaves and down-regulation in young leaves upon S depletion. Controversial reactions of SULTR2;2 expression upon S depletion were also observed with herbaceous plants. SULTR2;2 expression is induced in leaves of A. thaliana and B. napus (Takahashi et al., 2000; Parmar et al., 2007) but not in Brassica oleracea (Buchner et al., 2004a).
Increasing miR395 expression is related to early S deficiency
During early S deficiency, post-transcriptional regulation of PtaATPS3/4 via miR395a might be relevant in all analysed plant tissues. When sulphate nearly disappeared in leaves, miR395a expression increased. In fine roots, where the decrease in sulphate was not as strong, induction of miR395a was very low. PtaATPS3/4 has been identified as an in vivo target of miR395a by 5′ RACE. In mature leaves, transcript levels of PtaATPS3/4 were still higher than in the controls after 21 d of S depletion. In Arabidopsis, miR395 targeted AtAPS1, AtAPS3, AtAPS4, and AtSULTR2;1 (Jones-Rhoades and Bartel, 2004; Jones-Rhoades et al., 2006; Kawashima et al., 2009), but the cell type-specific expression pattern was different for miR395 and AtSULTR2;1 in roots (Kawashima et al., 2009; Liang et al., 2010). Moreover, post-transcriptional regulation via miR395 seems to affect primarily transcripts of AtAPS4 and only to a lesser extent transcripts of AtAPS1 and AtAPS3 (Liang et al., 2010; Kawashima et al., 2011). Thus, different sensitivities of PtaATPS3 and PtaATPS4 transcripts for post-transcriptional regulation via miR395a cannot be excluded in the present study with poplar. Sensitivity might depend on different local expression patterns and can explain the higher levels of PtaATPS3/4 in mature leaves after 21 d of S depletion compared with the control levels. Cell-specific localization via either in situ hybridization or the use of reporter gene constructs will help to address this question in future studies.
In fine roots and mature leaves, the increase in PtaATPS1/2 transcript levels could support sulphate activation for further use in the sulphate reduction pathway during S deficiency. This could be of particular importance if the subcellular localization of PtaATPS1/2 differs from that of PtaATPS3/4. The predicted amino acid sequences of the two ATPS isoforms of P. trichocarpa, PtcATPS1 and PtcATPS2, were most similar to the protein sequence of AtAPS2 that is proposed to be the only cytosolic ATPS in A. thaliana (Hatzfeld et al., 2000). Bioinformatic prediction of the subcellular localization of PtcATPS1 and PtcATPS2 from P. trichocarpa using the program TargetP (www.cbs.dtu.dk/services/TargetP/; Emanuelsson et al., 2007) proposed a cytosolic localization for these PtcATPS sequences (data not shown). The predicted amino acid sequences of PtcATPS3 and PtcATPS4 were more closely related to the chloroplastic AtAPS1, AtAPS3, and AtAPS4 and were predicted to contain a putative chloroplastic transit peptide in the N-terminus of their amino acid sequences (data not shown). Thus, it appears likely that sulphate activation in the cytosol by PtaATPS1/2 may become more important when sulphate supply is reduced due to decreasing influx but counteracted by a higher sulphate efflux from the vacuole. This view is consistent with the observation that sulphate fluxes into thiols and proteins were reduced in aps1 mutants and APS4-RNAi Arabidopsis plants under S depletion (Kawashima et al., 2011). Furthermore, AtAPS3 transcripts increased upon S depletion in roots and shoots of Arabidopsis so that sulphate activation can be maintained at S deficiency by AtAPS3 (Liang et al., 2010).
Changes in plant hormone levels are related to early S deficiency
In the present experiment, early responses to S deficiency are not only connected to almost extinguished sulphate contents, decreasing GSH contents, and increasing transcript levels of genes related to S metabolism. In leaves, early S deficiency is also accompanied by changes in cytokinin contents, whereas none of the changes in fine roots could be related to one of the two phases of S depletion. The active cytokinin forms tZ, tZR and cZ (Sakakibara, 2006) increased in young leaves during early S deficiency (Fig. 11B). The peaks of tZ and tZR contents after 5 d of S depletion were not regarded as S limitation responses because of the subsequent decrease in the contents of these cytokinins (Fig. 11A). In mature leaves, the contents of the storage forms cZOG and cZROG which can be activated by conversion into cZ (Sakakibara, 2006) decreased (Fig. 11B). This may indicate cytokinin activation of cZs in mature leaves, although the increase of cZ contents itself was not significant. In contrast, the increase of cZ in young leaves cannot be attributed to cytokinin activation because cZOG and cZROG contents did not change in the phase of early S deficiency.
cZs are assumed to possess less physiological activity than tZs (Schmitz et al., 1972; Kamínek et al., 1987). However, Gajdošová et al. (2011) hypothesized that cZs are of special importance under growth-limiting conditions for sustaining basic cytokinin signalling. Increasing active cZ during early S deficiency may support this hypothesis. Overexpression of a cytokinin oxidases (CKX) let to increasing transcript levels of AtSULTR1;2 in Arabidopsis roots (Werner et al., 2010). As active cytokinin contents were drastically diminished in these Arabidopsis plants, it was hypothesized that active cytokinins block the up-regulation of SULTR expression in roots under normal S conditions. Only if active cytokinins decline due to inactivation/degradation repression of SULTR transcription upon S depletion thought to be abolished. This is consistent with findings from Maruyama-Nakashita et al. (2004b). The authors prevented up-regulation of AtSULTR1;1 and AtSULTR1;2 in Arabidopsis roots by application of benzyladenine, an artificial active cytokinin. When tZ or tZR was applied exogenously to Arabidopsis plants, increasing AtAPR and AtSULTR2;2 transcript levels were observed in rosette leaves independent of the S supply (Ohkama et al., 2002). This positive regulation of S-responsive genes by cytokinins is consistent with the increasing tZ and tZR contents in young leaves of poplar during early S deficiency. However, Ohkama et al. (2002) hypothesized that exogenous cytokinin operates indirectly via sucrose in rosette leaves because they found no increasing cytokinin contents during S depletion. Such a positive effect of sucrose has already been described for APR (Kopriva et al., 1999, 2002) and, thus, is consistent with increasing PtaAPR transcript levels and sucrose contents in young poplar leaves in the present study.
Furthermore, an increase of dihydrozeatins was found in all tissues studied, namely fine roots, mature leaves, and young leaves, upon early S deficiency. Dihydrozeatins are biologically very stable cytokinins because of their insensitivity to CKX and because their contents in plant tissues are relatively low compared with tZs and isopentenyladenines (Sakakibara, 2006; Hirose et al., 2008; see also Supplementary Tables S3, S4, and S5 at JXB online). Therefore, they are thought to be rather inactive (Armstrong, 1994; Sakakibara, 2006). Nevertheless, increasing DZR contents in fine roots and young leaves strongly correlate with the decline in sulphate contents. Cytokinin ribosides such as DZR are more active than O- and N-glucosides (Sakakibara, 2006). As recently reported for cZs (Gajdošová et al., 2011), Mok and Mok (2001) proposed that the conversion into dihydrozeatins might help to stabilize the physiological activity of cytokinins. The maintenance of basic cytokinin functions during adaptation of plant metabolism to decreasing S availability could be an explanation for the increasing dihydrozeatin contents observed in all tissues during early S deficiency.
Considering other plant hormones, almost consistent reactions were observed in mature and young poplar leaves upon early S deficiency. In both tissues, ABA and IAA contents declined in parallel with decreasing sulphate contents. Increasing amounts of ABA are often related to environmental stress, for example to drought or salt stress (Raghavendra et al., 2010). In the present experiment, the observed decline in ABA during early S deficiency is assumed to be part of adaptive processes rather than stress reactions. Therefore, a stress-related role for ABA in this phase might not be relevant. As ABA is believed to have a positive effect on leaf growth of poplar (Arend et al., 2009), decreasing ABA contents might accommodate adaptation of growth processes to early S deficiency.
Auxin assists in tissue development and growth (Vieten et al., 2007), and its decline in young leaves may indicate growth retardation. However, an effect of S depletion on the growth of poplar was not observed (Supplementary Fig. S3 at JXB online). In Arabidopsis up-regulation of auxin correlates with the nitrilase3 (NIT3) gene that is part of the auxin synthesis pathway from glucosinolate degradation and is increased during S depletion (Grsic et al., 1999; Bak et al., 2001; Kutz et al., 2002; Nikiforova et al., 2003). As poplar does not contain glucosinolates, this pathway does not exist, and changes in auxin contents in young poplar leaves at early S deficiency must account for other, so far unidentified processes.
Responses to early S deficiency at the whole plant level
It has been reported that S remobilization from old and mature leaves during S depletion is negligible (Adiputra and Anderson, 1995). Already Bell et al. (1994, 1995) demonstrated that sulphate efflux from the vacuole during S stress is scarce, but possible during vegetative growth. In the present experiment with poplar, the increasing expression of the putative tonoplast-localized SULTR, PtaSULTR4;2, clearly indicates enhanced sulphate remobilization already during S limitation from both leaf and root vacuoles. Comparable results were obtained with vegetative B. napus (Dubousset et al., 2009; Abdallah et al., 2010) and wheat (Shinmachi et al., 2010). Increasing sulphate remobilization from the vacuole in the roots under S limitation counteracts sulphate storage within the vacuole and can maintain sulphate availability for loading into the xylem (Kataoka et al., 2004). This might be the reason for the almost unaffected sulphate contents in xylem sap for up to 9 d of S depletion in poplar.
Another route for the distribution of sulphate remobilized from the vacuole in the plant can be sulphate loading into the phloem. This process requires expression of the SULTR involved in phloem loading, especially in mature leaves (Dürr et al., 2010) that are thought to be sources for the sulphate transported into developing leaves of the apex and into roots in poplar (Hartmann et al., 2000). In the present study, expression of the phloem-localized PtaSULTR1;1 increased in leaves during early S deficiency and, thus, could suggest an improved capacity for phloem loading of sulphate for its transport to developing leaves and roots. Also in wheat, S depletion is accompanied by increased expression of TaeSULTR4;1 and TaeSULTR1;1 in the wheat shoot (Buchner et al., 2010), and the expression pattern of TaeSULTR1;1 is comparable with the expression pattern of PtaSULTR1;1 in poplar (Dürr et al., 2010). These results again indicate an enhanced phloem loading capacity of sulphate in combination with increased sulphate efflux from the vacuole (Buchner et al., 2010). Together these processes are expected to be responsible for a steady sulphate supply of sink tissues such as developing grains in wheat as total S in grains did not change significantly upon S depletion (Shinmachi et al., 2010). Still S remobilization from roots and leaves in poplar is not sufficient to maintain the sulphate content in young, developing leaves upon S depletion. The reduced sulphate and GSH contents in phloem exudates during early S deficiency indicate insufficient re-mobilization of S. Young poplar plants show continuous growth and, thus, developed new leaves throughout the S depletion treatment of 21 d. Since developing leaves are the main sinks for sulphate from mature leaves (Anderson and Anderson, 1996a, b, 1997), it can be assumed that the remobilized sulphate from vacuoles is completely transported to the developing leaves around the apex to maintain shoot growth. Consequently, vacuolar storage of sulphate in young leaves cannot be established.
Moreover, nutrient deficiency generally induces enhanced root growth (López-Bucio et al., 2003). Consequently, root sink strength for nutrients and carbon which are delivered from the shoot via phloem transport increases. In Brassica, sulphate remobilized from the vacuole of mature leaves was mainly found in roots and supported root growth upon S depletion (Abdallah et al., 2010). As the sulphate pool of fine roots of poplar was considerably less affected than the sulphate pool in young and mature leaves at early S deficiency, these results are consistent. Therefore, it can also be assumed that in poplar remobilization of sulphate in mature leaves predominantly promoted sulphate contents of the roots. This assumption is supported by increased sucrose contents in phloem exudates indicating higher carbon flow to the roots for growth.
The reduced sulphate and GSH pools in fine roots, leaves, and phloem exudates, however, clearly indicate reduced S availability and S cycling within the poplar plants at early S deficiency (Hartmann et al., 2000; Herschbach et al., 2011). Still GSH remained unaffected in the xylem sap. Whether increased sulphate reduction in the roots, indicated by the increased PtaAPR and PtaATPS1/2 transcript levels, supports the supply of reduced S to the shoot under S depletion remains to be investigated in future studies. In conclusion, S depletion clearly affects S metabolism of poplar on the transcriptional and metabolite level, including changes of endogenous plant hormones in a time series of events, but its impact on plant growth seems to be less severe than in herbaceous plants such as Arabidopsis or Brassica.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Cysteine (1), γ-glutamyl-cysteine (γ-EC) (2), glucose (3), and fructose (4) contents in fine roots (A), young leaves (B), and mature leaves (C) of poplar treated with sulphur depletion and of control plants (day 0).
Figure S2. Changes in gene expression of three reference genes, i.e. elongation factor 1β (Ef1b) (1), β-tubulin (bTub) (2), and Aintegumenta (ANT) (3), in fine roots (A), young leaves (B), and mature leaves (C) due to sulphur depletion treatment.
Figure S3. Leaf number, plant height, and plant growth of poplar treated with sulphur depletion and of control plants (day 0).
Figure S4. Contents of chlorophyll a (1), chlorophyll b (2), and carotinoids (3) in young (A) and mature leaves (B) of poplar treated with sulphur depletion and of control plants (day 0).
Table S1. Sequences of oligonucleotide primer pairs used for cloning the gene sequences of PtaATPS1/2, PtaATPS3/4, PtabTub, and PtaANT.
Table S2. Oligonucleotide primer sequences used for qRT-PCR analysis and resulting PCR fragment lengths.
Table S3. Contents of all analysed plant hormones in fine roots of poplar treated with sulphur depletion.
Table S4. Contents of all analysed plant hormones in young leaves of poplar treated with sulphur depletion.
Table S5. Contents of all analysed plant hormones in mature leaves of poplar treated with sulphur depletion.
Table S6. Contents of all analysed plant hormones in xylem sap of poplars treated with sulphur depletion.
Table S7. Contents of all analysed plant hormones in phloem exudates of poplars treated with sulphur depletion.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft within the graduate school ‘Signal systems in plant model organisms’ (GRK 1305) to A.H.
References
- Abdallah M, Dubousset L, Meuriot F, Etienne P, Avice J-C, Ourry A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. Journal of Experimental Botany. 2010;61:2635–2646. doi: 10.1093/jxb/erq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adiputra IGK, Anderson JW. Effect of sulphur nutrition on redistribution of sulphur in vegetative barley. Physiologia Plantarum. 1995;95:643–650. [Google Scholar]
- Anderson JW. Regulation of sulphur distribution and redistribution in grain plants. In: Saito K, De Kok LJ, Stulen I, Hawkesford MJ, Schnug E, Sirko A, Rennenberg H, editors. Sulfur transport and assimilation in plants in the postgenomic era. Leiden, The Netherlands: Backhys Publishers; 2005. pp. 23–31. [Google Scholar]
- Anderson S, Anderson JW. Distribution and redistribution of sulphur supplied as [35S]sulphate to roots during vegetative growth of soybean. Plant Physiology. 1996a;110:1151–1157. doi: 10.1104/pp.110.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Anderson JW. Effect of sulphur nutrition on the redistribution of sulphur in vegetative soybean plants. Plant Physiology. 1996b;112:623–631. doi: 10.1104/pp.112.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Anderson JW. Allocation of S in generative growth of soybean. Plant Physiology. 1997;114:687–693. doi: 10.1104/pp.114.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend M, Schnitzler JP, Ehlting B, Hänsch R, Lange T, Rennenberg H, Himmelbach A, Grill E, Fromm J. Expression of the Arabidopsis mutant abi1 alters abscisic acid sensitivity, stomatal development, and growth morphology in Gray poplars. Plant Physiology. 2009;151:2110–2119. doi: 10.1104/pp.109.144956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. Cytokinin oxidase and the regulation of cytokinin degradation. In: Mok DWS, Mok MC, editors. Cytokinin: chemistry, activity, and function. Boca Raton, FL: CRC Press; 1994. pp. 139–154. [Google Scholar]
- Bak S, Tax FE, Feldmann KA, Galbraith DW, Feyereisen R. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in Arabidopsis. The Plant Cell. 2001;13:101–112. doi: 10.1105/tpc.13.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Berthomieu P, Clairotte M, Shibagaki N, Davidian J, Gosti F. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytologist. 2008;180:608–619. doi: 10.1111/j.1469-8137.2008.02604.x. [DOI] [PubMed] [Google Scholar]
- Bell CI, Clarkson DT, Cram WJ. Sulphate supply and its regulation of transport in roots of a tropical legume Macroptilium atropurpureum cv. Sirato. Journal of Experimental Botany. 1995;46:65–71. [Google Scholar]
- Bell CI, Cram WJ, Clarkson DT. Compartmental analysis of 35SO42– exchange kinetics in roots and leaves of a tropical legume Macroptilium atropurpureum cv. Siratro. Journal of Experimental Botany. 1994;45:879–886. [Google Scholar]
- Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S. Coordinate modulation of maize sulphate permease and ATP sulfurylase mRNAs in response to variations in sulphur nutritional status: stereospecific down-regulation by L-cysteine. Plant Molecular Biology. 1999;39:527–537. doi: 10.1023/a:1006148815106. [DOI] [PubMed] [Google Scholar]
- Brunold C. Reduction of sulphate to sulphide. In: Rennenberg H, Brunold C, De Kok L, Stulen I, editors. Sulphur nutrition and sulphur assimilation in higher plants. The Hague, The Netherlands: SPB Academic Publishing; 1990. pp. 13–31. [Google Scholar]
- Buchner P, Parmar S, Kriegel A, Carpentier M, Hawkesford MJ. The sulphate transporter family in wheat: tissue-specific gene expression in relation to nutrition. Molecular Plant. 2010;3:374–389. doi: 10.1093/mp/ssp119. [DOI] [PubMed] [Google Scholar]
- Buchner P, Stuiver CEE, Westerman S, Wirtz M, Hell R, Hawkesford MJ, De Kok LJ. Regulation of sulphate uptake and expression of sulphate transporter genes in Brassica oleracea as affected by atmospheric H2S and pedospheric sulphate nutrition. Plant Physiology. 2004a;136:3396–3408. doi: 10.1104/pp.104.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner P, Takahashi H, Hawkesford MJ. Plant sulphate transporters: co-ordination of uptake, intracellular and long-distance transport. Journal of Experimental Botany. 2004b;55:1765–1773. doi: 10.1093/jxb/erh206. [DOI] [PubMed] [Google Scholar]
- Burke J, Holloway P, Dalling M. The effect of sulphur deficiency on the organization and photosynthetic capability of wheat leaves. Journal of Plant Physiology. 1986;125:371–375. [Google Scholar]
- Ceulemans R, Deraedt W. Production physiology and growth potential of poplars under short-rotation forestry culture. Forest Ecology and Management. 1999;121:9–23. [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter. 1993;11:113–116. [Google Scholar]
- Clarkson DT, Hawkesford MJ. Molecular biological approaches to plant nutrition. Plant and Soil. 1993;155–156:21–31. [Google Scholar]
- Cronk QCB. Plant eco–devo: the potential of poplar as a model organism. New Phytologist. 2005;166:39–48. doi: 10.1111/j.1469-8137.2005.01369.x. [DOI] [PubMed] [Google Scholar]
- Davidian J-C, Kopriva S. Regulation of sulphate uptake and assimilation—the same or not the same? Molecular Plant. 2010;3:314–325. doi: 10.1093/mp/ssq001. [DOI] [PubMed] [Google Scholar]
- Dubousset L, Abdallah M, Desfeux AS, et al. Remobilization of leaf S compounds and senescence in response to restricted sulphate supply during the vegetative stage of oilseed rape are affected by mineral N availability. Journal of Experimental Botany. 2009;60:3239–3253. doi: 10.1093/jxb/erp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr J, Bücking H, Susanne Mult, Wildhagen H, Palme K, Rennenberg H, Ditengou F, Herschbach C. Seasonal and cell type specific expression of sulphate transporters in the phloem of Populus reveals tree specific characteristics for SO42– storage and mobilization. Plant Molecular Biology. 2010;72:499–517. doi: 10.1007/s11103-009-9587-6. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H. Locating proteins in the cell using TargetP, SignalP and related tools. Nature Protocols. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signalling, acclimation, and implications. Antioxidants and Redox Signalling. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Gajdošová S, Spíchal L, Kamínek M, et al. Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. Journal of Experimental Botany. 2011;62:2827–2840. doi: 10.1093/jxb/erq457. [DOI] [PubMed] [Google Scholar]
- Gessler A, Rennenberg H, Keitel C. Stable isotope composition of organic compounds transported in the phloem of European beech—evaluation of different methods of phloem sap collection and assessment of gradients in carbon isotope composition during leaf-to-stem transport. Plant Biology. 2004;6:721–729. doi: 10.1055/s-2004-830350. [DOI] [PubMed] [Google Scholar]
- Grsic S, Kirchheim B, Pieper K, Fritsch M, Hilgenberg W, Ludwig-Müller J. Induction of auxin biosynthetic enzymes by jasmonic acid and in clubroot diseased Chinese cabbage plants. Physiologia Plantarum. 1999;105:521–531. [Google Scholar]
- Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annual Review of Plant Biology. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Honicke P, Wirtz M, Hell R, Rennenberg H, Kopriva S. Regulation of sulphate assimilation by glutathione in poplars (Populus tremula×P. alba) of wild type and overexpressing γ-glutamylcysteine synthetase in the cytosol. Journal of Experimental Botany. 2004;55:837–845. doi: 10.1093/jxb/erh094. [DOI] [PubMed] [Google Scholar]
- Hartmann T, Mult S, Suter M, Rennenberg H, Herschbach C. Leaf age-dependent differences in sulphur assimilation and allocation in poplar (Populus tremula×P. alba) leaves. Journal of Experimental Botany. 2000;51:1077–1088. doi: 10.1093/jexbot/51.347.1077. [DOI] [PubMed] [Google Scholar]
- Hatzfeld Y, Lee S, Lee M, Leustek T, Saito K. Functional characterization of a gene encoding a fourth ATP sulfurylase isoform from Arabidopsis thaliana. Gene. 2000;248:51–58. doi: 10.1016/s0378-1119(00)00132-3. [DOI] [PubMed] [Google Scholar]
- Hawkesford MJ, De Kok LJ. Managing sulphur metabolism in plants. Plant, Cell and Environment. 2006;29:382–395. doi: 10.1111/j.1365-3040.2005.01470.x. [DOI] [PubMed] [Google Scholar]
- Herschbach C, Gessler A, Rennenberg H. Long-distance transport and plant internal cycling of N- and S-compounds. Progess in Botany. 2011;73 (in press) [Google Scholar]
- Herschbach C, Jouanin L, Rennenberg H. Overexpression of γ-glutamylcysteine synthetase, but not of glutathione synthetase, elevates glutathione allocation in the phloem of transgenic poplar trees. Plant and Cell Physiology. 1998;39:447–451. [Google Scholar]
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on sulphate influx, xylem loading and exudation in excised tobacco roots. Journal of Experimental Botany. 1991;42:1021–1029. [Google Scholar]
- Herschbach C, Rennenberg H. Influence of glutathione (GSH) on net uptake of sulphate and sulphate transport in tobacco plants. Journal of Experimental Botany. 1994;45:1069–1076. [Google Scholar]
- Herschbach C, Rennenberg H. Sulphur nutrition of deciduous trees. Naturwissenschaften. 2001;88:25–36. doi: 10.1007/s001140000200. [DOI] [PubMed] [Google Scholar]
- Herschbach C, van der Zalm E, Schneider A, Jouanin L, De Kok LJ, Rennenberg H. Regulation of sulphur nutrition in wild-type and transgenic poplar over-expressing γ-glutamylcysteine synthetase in the cytosol as affected by atmospheric H2S. Plant Physiology. 2000;124:461–474. doi: 10.1104/pp.124.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse H, Trachsel N, Suter M, Kopriva S, von Ballmoos P, Rennenberg H, Brunold C. Effect of glucose on assimilatory sulphate reduction in Arabidopsis thaliana roots. Journal of Experimental Botany. 2003;54:1701–1709. doi: 10.1093/jxb/erg177. [DOI] [PubMed] [Google Scholar]
- Hirai MY, Fujiwara T, Awazuhara M, Kimura T, Noji M, Saito K. Global expression profiling of sulphur-starved Arabidopsis by DNA macroarray reveals the role of O-acetyl-L-serine as a general regulator of gene expression in response to sulphur nutrition. The Plant Journal. 2003;33:651–663. doi: 10.1046/j.1365-313x.2003.01658.x. [DOI] [PubMed] [Google Scholar]
- Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. Regulation of cytokinin biosynthesis, compartmentalization and translocation. Journal of Experimental Botany. 2008;59:75–83. doi: 10.1093/jxb/erm157. [DOI] [PubMed] [Google Scholar]
- Hoagland D, Arnon D. The water-culture method for growing plants without soil. California Agricultural Experiment Station Circular 347. 1950 [Google Scholar]
- Hopkins L, Parmar S, Blaszczyk A, Hesse H, Hoefgen R, Hawkesford MJ. O-Acetylserine and the regulation of expression of genes encoding components for sulphate uptake and assimilation in potato. Plant Physiology. 2005;138:433–440. doi: 10.1104/pp.104.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger C, Gessler A, Biller S, Rennenberg H, Kreuzwieser J. Differences in C metabolism of ash species and provenances as a consequence of root oxygen deprivation by waterlogging. Journal of Experimental Botany. 2009;60:4335–4345. doi: 10.1093/jxb/erp268. [DOI] [PubMed] [Google Scholar]
- Jansson S, Douglas CJ. Populus: a model system for plant biology. Annual Review of Plant Biology. 2007;58:435–458. doi: 10.1146/annurev.arplant.58.032806.103956. [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Molecular Cell. 2004;14:787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAs and their regulatory roles in plants. Annual Review of Plant Biology. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- Kamínek M, Vaněk T, Motyka V. Cytokinin activities of N6-benzyladenosine derivatives hydroxylated on the side-chain phenyl ring. Journal of Plant Growth Regulation. 1987;6:113–120. [Google Scholar]
- Kataoka T, Watanabe-Takahashi A, Hayashi N, Ohnishi M, Mimura T, Buchner P, Hawkesford MJ, Yamaya T, Takahashi H. Vacuolar sulphate transporters are essential determinants controlling internal distribution of sulphate in Arabidopsis. The Plant Cell. 2004;16:2693–2704. doi: 10.1105/tpc.104.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima CG, Matthewman CA, Huang S, et al. Interplay of SLIM1 and miR395 in the regulation of sulphate assimilation in Arabidopsis. The Plant Journal. 2011;66:863–876. doi: 10.1111/j.1365-313X.2011.04547.x. [DOI] [PubMed] [Google Scholar]
- Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, Dalmay T. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. The Plant Journal. 2009;57:313–321. doi: 10.1111/j.1365-313X.2008.03690.x. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, et al. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography–tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant and Cell Physiology. 2009;50:1201–1214. doi: 10.1093/pcp/pcp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova N, Miller B, Ralph S, Ellis BE, Douglas C, Ritland K, Bohlmann J. Isolation of high-quality RNA from gymnosperm and angiosperm trees. BioTechniques. 2004;36:821–824. doi: 10.2144/04365ST06. [DOI] [PubMed] [Google Scholar]
- Kopriva S. Regulation of sulphate assimilation in Arabidopsis and beyond. Annals of Botany. 2006;97:479–495. doi: 10.1093/aob/mcl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva S, Büchert T, Fritz G, et al. The presence of an iron–sulphur cluster in adenosine 5′-phosphosulphate reductase separates organisms utilizing adenosine 5′-phosphosulphate and phosphoadenosine 5′-phosphosulphate for sulphate assimilation. Journal of Biological Chemistry. 2002;277:21786–21791. doi: 10.1074/jbc.M202152200. [DOI] [PubMed] [Google Scholar]
- Kopriva S, Hartmann T, Massaro G, Hönicke P, Rennenberg H. Regulation of sulphate assimilation by nitrogen and sulphur nutrition in poplar trees. Trees – Structure and Function. 2004;18:320–326. [Google Scholar]
- Kopriva S, Muheim R, Koprivova A, Trachsel N, Catalano C, Suter M, Brunold C. Light regulation of assimilatory sulphate reduction in Arabidopsis thaliana. The Plant Journal. 1999;20:37–44. doi: 10.1046/j.1365-313x.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- Kopriva S, Rennenberg H. Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolism. Journal of Experimental Botany. 2004;55:1831–1842. doi: 10.1093/jxb/erh203. [DOI] [PubMed] [Google Scholar]
- Koralewska A, Buchner P, Stuiver CEE, Posthumus FS, Kopriva S, Hawkesford MJ, De Kok LJ. Expression and activity of sulphate transporters and APS reductase in curly kale in response to sulphate deprivation and re-supply. Journal of Plant Physiology. 2009;166:168–179. doi: 10.1016/j.jplph.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Koralewska A, Posthumus FS, Stuiver CEE, Buchner P, Hawkesford MJ, De Kok LJ. The characteristic high sulphate content in Brassica oleracea is controlled by the expression and activity of sulphate transporters. Plant Biology. 2007;9:654–661. doi: 10.1055/s-2007-965438. [DOI] [PubMed] [Google Scholar]
- Kutz A, Müller A, Hennig P, Kaiser WM, Piotrowski M, Weiler EW. A role for nitrilase 3 in the regulation of root morphology in sulphur-starving Arabidopsis thaliana. The Plant Journal. 2002;30:95–106. doi: 10.1046/j.1365-313x.2002.01271.x. [DOI] [PubMed] [Google Scholar]
- Lappartient AG, Touraine B. Glutathione-mediated regulation of ATP sulfurylase activity, SO42– uptake, and oxidative stress response in intact Canola roots. Plant Physiology. 1997;114:177–183. doi: 10.1104/pp.114.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine B. Inter-organ signalling in plants: regulation of ATP sulfurylase and sulphate transporter genes expression in roots mediated by phloem-translocated compound. The Plant Journal. 1999;18:89–95. doi: 10.1046/j.1365-313x.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Leustek T. The affect of cadmium on sulphate assimilation enzymes in Brassica juncea. Plant Science. 1999;141:201–207. [Google Scholar]
- Leustek T, Saito K. Sulphate transport and assimilation in plants. Plant Physiology. 1999;120:637–644. doi: 10.1104/pp.120.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Yang F, Yu D. MicroRNA395 mediates regulation of sulphate accumulation and allocation in Arabidopsis thaliana. The Plant Journal. 2010;62:1046–1057. doi: 10.1111/j.1365-313X.2010.04216.x. [DOI] [PubMed] [Google Scholar]
- Llave C, Xie Z, Kasschau KD, Carrington JC. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science. 2002;297:2053–2056. doi: 10.1126/science.1076311. [DOI] [PubMed] [Google Scholar]
- Logan HM, Cathala N, Grignon C, Davidian JC. Cloning of a cDNA encoded by a member of the Arabidopsis thaliana ATP sulfurylase multigene family. Journal of Biological Chemistry. 1996;271:12227–12233. doi: 10.1074/jbc.271.21.12227. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Inoue E, Watanabe-Takahashi A, Yamaya T, Takahashi H. Transcriptome profiling of sulphur-responsive genes in Arabidopsis reveals global effects of sulphur nutrition on multiple metabolic pathways. Plant Physiology. 2003;132:597–605. doi: 10.1104/pp.102.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H. Arabidopsis SLIM1 is a central transcriptional regulator of plant sulphur response and metabolism. The Plant Cell. 2006;18:3235–3251. doi: 10.1105/tpc.106.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, Yamaya T, Takahashi H. Induction of SULTR1;1 sulfate transporter in Arabidopsis roots involves protein phosphorylation/dephosphorylation circuit for transcriptional regulation. Plant and Cell Physiology. 2004a;45:340–345. doi: 10.1093/pcp/pch029. [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Nakamura Y, Yamaya T, Takahashi H. A novel regulatory pathway of sulphate uptake in Arabidopsis roots: implication of CRE1/WOL/AHK4-mediated cytokinin-dependent regulation. The Plant Journal. 2004b;38:779–789. doi: 10.1111/j.1365-313X.2004.02079.x. [DOI] [PubMed] [Google Scholar]
- McGrath SP, Zhao FJ. Sulphur uptake, yield responses and the interactions between nitrogen and sulphur in winter oilseed rape (Brassica napus) Journal of Agricultural Science. 1996;126:53–62. [Google Scholar]
- Mitchell CP, Stevens EA, Watters MP. Short-rotation forestry—operations, productivity and costs based on experience gained in the UK. Forest Ecology and Management. 1999;121:123–136. [Google Scholar]
- Mok D, Mok M. Cytokinin metabolism and action. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- Nikiforova V, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R. Transcriptome analysis of sulphur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. The Plant Journal. 2003;33:633–650. doi: 10.1046/j.1365-313x.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian JC, Sacchi GA. Heavy metal stress and sulphate uptake in maize roots. Plant Physioliogy. 2006;141:1138–1148. doi: 10.1104/pp.105.076240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G. Metabolic signalling in defence and stress: the central role of soluble redox couples. Plant, Cell and Environment. 2006;29:409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert KJ, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar overexpressing [gamma]-glutamylcysteine synthetase. Plant Physiology. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkama N, Takei K, Sakakibara H, Hayashi H, Yoneyama T, Fujiwara T. Regulation of sulphur-responsive gene expression by exogenously applied cytokinins in Arabidopsis thaliana. Plant and Cell Physiology. 2002;43:1493–1501. doi: 10.1093/pcp/pcf183. [DOI] [PubMed] [Google Scholar]
- Parmar S, Buchner P, Hawkesford MJ. Leaf developmental stage affects sulphate depletion and specific sulphate transporter expression during sulphur deprivation in Brassica napus L. Plant Biology. 2007;9:647–653. doi: 10.1055/s-2007-965428. [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Gonugunta VK, Christmann A, Grill E. ABA perception and signalling. Trends in Plant Science. 2010;15:395–401. doi: 10.1016/j.tplants.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Rausch T, Wachter A. Sulphur metabolism: a versatile platform for launching defence operations. Trends in Plant Science. 2005;10:503–509. doi: 10.1016/j.tplants.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Rennenberg H. The fate of excess sulphur in higher plants. Annual Review of Plant Physiology. 1984;35:121–153. [Google Scholar]
- Rennenberg H, Herschbach C, Haberer K, Kopriva S. Sulphur metabolism in plants: are trees different? Plant Biology. 2007;9:620–637. doi: 10.1055/s-2007-965248. [DOI] [PubMed] [Google Scholar]
- Rennenberg H, Kemper O, Thoene B. Recovery of sulphate transport into heterotrophic tobacco cells from inhibition by reduced glutathione. Physiologia Plantarum. 1989;76:271–276. [Google Scholar]
- Rennenberg H, Polle A, Martini N, Thoene B. Interaction of sulphate and glutathione transport in cultured tobacco cells. Planta. 1988;176:68–74. doi: 10.1007/BF00392481. [DOI] [PubMed] [Google Scholar]
- Rennenberg H, Schneider S, Weber P. Analysis of uptake and allocation of nitrogen and sulphur compounds by trees in the field. Journal of Experimental Botany. 1996;47:1491–1498. [Google Scholar]
- Rotte C, Leustek T. Differential subcellular localization and expression of ATP sulfurylase and 5′-adenylylsulphate reductase during ontogenesis of Arabidopsis leaves indicates that cytosolic and plastid forms of ATP sulfurylase may have specialized functions. Plant Physiology. 2000;124:715–724. doi: 10.1104/pp.124.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- Schmitz RY, Skoog F, Playtis AJ, Leonard NJ. Cytokinins: synthesis and biological activity of geometric and position isomers of zeatin. Plant Physiology. 1972;50:702–705. doi: 10.1104/pp.50.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnug E. Sulphur nutritional status of European crops and consequences for agriculture. Sulphur in Agriculture. 1991;15:7–12. [Google Scholar]
- Scholander P, Hammel H, Bradstreet E, Hemmingsen E. Sap pressure in vascular plants: negative hydrostatic pressure can be measured in plants. Science. 1965;148:339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]
- Schupp R, Glavac V, Rennenberg H. Thiol composition of xylem sap of beech trees. Phytochemistry. 1991;30:1415–1418. [Google Scholar]
- Shinmachi F, Buchner P, Stroud JL, Parmar S, Zhao FJ, McGrath SP, Hawkesford MJ. Influence of sulphur deficiency on the expression of specific sulphate transporters and the distribution of sulphur, selenium, and molybdenum in wheat. Plant Physiology. 2010;153:327–336. doi: 10.1104/pp.110.153759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Vanden Berg PJ, Belcher AR, Warrilow AG. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. The Plant Journal. 1997;12:875–884. doi: 10.1046/j.1365-313x.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of glutathione synthesis in leaves of transgenic poplar (Populus tremula×P. alba) overexpressing glutathione synthetase. The Plant Journal. 1995;7:141–145. [Google Scholar]
- Takahashi H. Regulation of sulphate transport and assimilation in plants. International Review of Cell and Molecular Biology. 2010;281:129–159. doi: 10.1016/S1937-6448(10)81004-4. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kopriva S, Giordano M, Saito K, Hell R. Sulphur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annual Review of Plant Biology. 2011;62:157–84. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Watanabe-Takahashi A, Smith Frank W, Blake-Kalff M, Hawkesford MJ, Saito K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. The Plant Journal. 2000;23:171–182. doi: 10.1046/j.1365-313x.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, Engler J de A, Engler G, Van Montagu M, Saito K. Regulation of sulphur assimilation in higher plants: a sulphate transporter induced in sulphate-starved roots plays a central role in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 1997;94:11102–11107. doi: 10.1073/pnas.94.20.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuskan GA, Di Fazio S, Jansson S, et al. The genome of Black Cottonwood, Populus trichocarpa (Torr. & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- van der Zalm E, Schneider A, Rennenberg H. Regulation of sulphate uptake and xylem loading of poplar roots (Populus tremula×P. alba) Trees – Structure and Function. 2005;19:204–212. [Google Scholar]
- Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, von Ballmoos P, Krähenbühl U, den Camp RO, Brunold C. Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulphate reductase is more susceptible than ATP sulphurylase to negative control by thiols. The Plant Journal. 2002;31:729–740. doi: 10.1046/j.1365-313x.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- Vidmar JJ, Schjoerring JK, Touraine B, Glass AD. Regulation of the hvst1 gene encoding a high-affinity sulfate transporter from Hordeum vulgare. Plant Molecular Biology. 1999;40:883–892. doi: 10.1023/a:1006230131841. [DOI] [PubMed] [Google Scholar]
- Vidmar JJ, Tagmount A, Cathala N, Touraine B, Davidian JCE. Cloning and characterization of a root specific high-affinity sulphate transporter from Arabidopsis thaliana. FEBS Letters. 2000;475:65–69. doi: 10.1016/s0014-5793(00)01615-x. [DOI] [PubMed] [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J. Molecular and cellular aspects of auxin-transport-mediated development. Trends in Plant Science. 2007;12:160–168. doi: 10.1016/j.tplants.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. The Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildhagen H, Dürr J, Ehlting B, Rennenberg H. Seasonal nitrogen cycling in the bark of field-grown Grey poplar is correlated with meteorological factors and gene expression of bark storage proteins. Tree Physiology. 2010;30:1096–1110. doi: 10.1093/treephys/tpq018. [DOI] [PubMed] [Google Scholar]
- Yoshimoto N, Takahashi H, Smith Frank W, Yamaya T, Saito K. Two distinct high-affinity sulphate transporters with different inducibilities mediate uptake of sulphate in Arabidopsis roots. The Plant Journal. 2002;29:465–473. doi: 10.1046/j.0960-7412.2001.01231.x. [DOI] [PubMed] [Google Scholar]
- Zhao FJ, Hawkesford MJ, McGrath SP. Sulphur assimilation and effects on yield and quality of wheat. Journal of Cereal Science. 1999;30:1–17. [Google Scholar]
- Zuber H, Davidian J-C, Aubert G, et al. The seed composition of Arabidopsis mutants for the group 3 sulphate transporters indicates a role in sulphate translocation within developing seeds. Plant Physiology. 2010;154:913–926. doi: 10.1104/pp.110.162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.