Abstract
Flowers of the kiwifruit species Actinidia chinensis produce a mixture of sesquiterpenes derived from farnesyl diphosphate (FDP) and monoterpenes derived from geranyl diphosphate (GDP). The tertiary sesquiterpene alcohol (E)-nerolidol was the major emitted volatile detected by headspace analysis. Contrastingly, in solvent extracts of the flowers, unusually high amounts of (E,E)-farnesol were observed, as well as lesser amounts of (E)-nerolidol, various farnesol and farnesal isomers, and linalool. Using a genomics-based approach, a single gene (AcNES1) was identified in an A. chinensis expressed sequence tag library that had significant homology to known floral terpene synthase enzymes. In vitro characterization of recombinant AcNES1 revealed it was an enzyme that could catalyse the conversion of FDP and GDP to the respective (E)-nerolidol and linalool terpene alcohols. Enantiomeric analysis of both AcNES1 products in vitro and floral terpenes in planta showed that (S)-(E)-nerolidol was the predominant enantiomer. Real-time PCR analysis indicated peak expression of AcNES1 correlated with peak (E)-nerolidol, but not linalool accumulation in flowers. This result, together with subcellular protein localization to the cytoplasm, indicated that AcNES1 was acting as a (S)-(E)-nerolidol synthase in A. chinensis flowers. The synthesis of high (E,E)-farnesol levels appears to compete for the available pool of FDP utilized by AcNES1 for sesquiterpene biosynthesis and hence strongly influences the accumulation and emission of (E)-nerolidol in A. chinensis flowers.
Keywords: Actinidia, farnesol, floral volatiles, kiwifruit, nerolidol, terpene, terpene synthase
Introduction
Terpenes (also referred to as terpenoids or isoprenoids) represent the most abundant group of plant volatiles and are common components of floral scent and herbivore-induced volatile mixtures (Pichersky and Gershenzon, 2002; Shearer and Hampton, 2005; Dudareva and Pichersky, 2006). The volatility of plant terpenes enables them to act as chemical messengers that are received by organisms at different trophic levels. Terpenes not only underlie the complex communication system that exists between plants, pollinators, herbivores, and their parasites and predators (Unsicker et al., 2009; Dicke and Baldwin, 2010), but ultimately serve to increase plant fitness (Harborne, 1991).
The diversity of terpene compounds in nature derives from the C5 compound isopentenyl diphosphate (IDP) and its allylic isomer dimethylallyl diphosphate (DMADP). Successive condensations of IDP with DMADP that proceed via the action of prenyltransferase enzymes, result in the formation of linear elongated prenyldiphosphates, including the C10 monoterpene precursor geranyl diphosphate (GDP), the C15 sesquiterpene precursor farnesyl diphosphate and the C20 diterpene precursor geranylgeranyl diphosphate (GGDP). In higher plants, the synthesis of IDP is compartmentally segregated (Lichtenthaler et al., 1997) with the cytosolic mevalonate (MVA) pathway (Goodwin, 1977; Nes and McKean, 1977; Spurgeon and Porter, 1981), supplying IDP for sesquiterpenoid production via farnesyl diphosphate (FDP), while the plastid-located MVA-independent or 2-C-methyl-D-erythritol-4-phosphate pathway (Eisenreich et al., 1998; Lichtenthaler, 1999; Rohmer, 1999; Rodriguez-Concepcion and Boronat, 2002) produces IDP for mono- and di-terpenoid synthesis via GDP and GGDP, respectively. However, exchange of IDP, GDP, and/or FDP between the cytoplasmic and plastid compartments has also been observed (Adam and Zapp, 1998; Arigoni et al., 1999; Hemmerlin et al., 2003), while metabolic engineering in Arabidopsis, tobacco, and potato has shown the existence of low amounts of FDP in plastids and GDP in the cytosol (Aharoni et al., 2003, 2006; Wu et al., 2006).
Biosynthesis of terpenes is commonly restricted to specific plant tissues and specialized secretory structures contained within those tissues. Volatile terpenes are often emitted from specific floral tissues at particular times to attract pollinators. For example, Dudareva et al. (2003) demonstrated that the biosynthesis and emission of (E)-β-ocimene and β-myrcene in snapdragon (Antirrhinum majus) flowers correlated with specific expression patterns of the corresponding monoterpene synthase (TPS) genes in the upper and lower lobes of flower petals during floral development. The expression of these genes was also found to follow a weak diurnal cycle controlled by a circadian clock. Contrastingly, the expression of mono-TPS and sesqui-TPS enzymes in Arabidopsis flowers is limited to the stigma, anthers, nectaries, and sepals (Tholl et al., 2005). The expression profiles of these TPS enzymes suggested that the volatile terpenes released from Arabidopsis flowers might both function as short-range attractants of pollinating insects and also provide a measure of defence for floral tissues vulnerable to microbial pathogens or herbivores.
The catalytic conversion of linear mono-, sesqui-, and di-terpene prenyldiphosphate compounds to terpenes is carried out by the TPS enzyme family. TPS biochemistry is generally characterized by electrophilically initiated reactions which, with the assistance of divalent metal ions, drive carbocation formation via ionization of the diphosphate precursor (Croteau, 1987; Cane, 1990; Lesburg et al., 1998; Greenhagen and Chappell, 2001). Although TPS enzymes can produce a single compound with high regio- and stereospecificity (Croteau, 1987; Cane, 1990), they also show a propensity to generate multiple products from a single prenyldiphosphate substrate. In terms of floral terpene production, this is perhaps best illustrated in Arabidopsis, where only two enzymes were found to be responsible for the synthesis of the 20 identified sesquiterpenes (Tholl et al., 2005).
To date, floral TPS enzymes have been identified and characterized in many flowering plants including Arabidopsis, Clarkia, snapdragon (Dudareva et al., 1996; Dudareva et al., 2003; Tholl et al., 2005; Nagegowda et al., 2008), and, more recently, in Actinidia species (Nieuwenhuizen et al., 2009; Chen et al., 2010). Characterized Actinidia TPS enzymes include those responsible for the predominant sesquiterpene volatiles (E,E)-α-farnesene and a (+)-germacreneD, produced by male and female Actinidia deliciosa flowers (Nieuwenhuizen et al., 2009) and an (S)-linalool synthase, required for the production of floral lilac compounds in Actinidia arguta (Chen et al., 2010).
In this study, we describe the identification and functional characterization of the enzyme responsible for the production of (E)-nerolidol that constitutes the major floral volatile in the commercially important kiwifruit species Actinidia chinensis. We also provide evidence that the formation of the dephosphorylated FDP derivative (E,E)-farnesol influences the capacity of A. chinensis to synthesize floral sesquiterpenes.
Materials and methods
Chemicals and chemical synthesis
2-Methyl-but-3-en-2-ol, 3-methyl-but-2-enol, (E)-hex-2-enol, rac-linalool, rac-(E,Z)-nerolidol, and (E,E)-farnesol were obtained from the Aldrich Chemical Company. (S)-Linalool and geranyllinalool were obtained from Fluka. Geraniol was from Acros Organics and geranylgeraniol was obtained from the Sigma Chemical Company. (S)-(E)-nerolidol was obtained from neroli essential oil (Neroli, Citrus aurantium, Lotus Essential Oils, Auckland, New Zealand). The essential oil (0.25 ml) was partially purified by flash chromatography on silica gel (pentane:DCM:MeOH, 100:99:1) and (E)-nerolidol identified by GC-MS comparison with (E,Z)-nerolidol (Aldrich). Neroli contains exclusively (E)-nerolidol and predominantly (S)-(E)-nerolidol (Mondello et al., 2002). Enantioselective GC-MS of the partially purified neroli essential oil determined it to be 14:86 (R):(S)-(E)-nerolidol.
8-Hydroxylinalool was synthesized according to a previously described method (Kreck et al., 2002). Prenyldiphosphate substrates were synthesized by phosphorylation of the appropriate alcohols (Keller and Thompson, 1993), viz. DMADP from 3-methyl-but-2-enol, linalyl diphosphate (LDP) from rac-linalool, GDP from geraniol, (E,E)-FDP (FDP) from (E,E)-farnesol, and GGDP from geranylgeraniol. The isotopically-labelled substrates, [C1–3H1]-FDP and [C1–3H1]-GDP were made by phosphorylation of tritium-labelled farnesol and geraniol, respectively (Green et al., 2007). The labelled alcohols were synthesized by MnO2 or Dess-Martin periodinane (Comeskey et al., 2004) oxidation of the appropriate alcohol to the aldehyde, then reduction (Croteau et al., 1994) with NaB3H4 (Amersham).
Headspace volatile trapping and solvent extractions
A. chinensis Planch. var. chinensis (‘Hort16A’) flowers were harvested from the Plant & Food Research Actinidia orchard in Te Puke, New Zealand. Headspace volatiles were collected on site from whole flowers according to Matich et al. (2003) with minor modifications. Five fully opened flowers were harvested in triplicate every 4 h from noon (12:00 h on 18 October 2007) until 08:00 the following day. The harvested flowers were placed in 50 ml Quickfit tubes and the flower volatiles trapped for 3 h in direct thermal desorption tubes (ATAS GL International, Eindhoven, The Netherlands) packed with 80 mg Chromosorb absorbent (Shimadzu Co, Kyoto, Japan), using purified air at a flow rate of 25 ml min−1.
Solvent extractions were carried out in triplicate on flowers samples (5 g) harvested at the equivalent time points to the headspace sampling. Flowers were transferred to 50 ml Quickfit tubes and extracted twice with 10 ml pentane:Et2O (1:1 v/v) for 30 min with gentle shaking. The two extractions were combined and stored overnight at –20 °C. The following day the upper solvent layer was carefully removed from the lower frozen water layer using a glass pipette and reduced to 2 ml under a gentle stream of N2. The concentrated extract was then passed through a column of anhydrous MgSO4 to remove any remaining water.
Qualitative and semi-quantitative analysis of floral compounds
Headspace volatiles were desorbed directly from the thermal desorption tubes with a temperature ramp of 45–175 °C at 16 °C s−1 and cryofocused on the front of the capillary column by a liquid nitrogen-cooled cryogenic trap at –110 °C. After cryofocussing, the trap temperature was ramped to 175 °C at 50 °C min−1 (Optic 3 thermal desorption system, ATAS GL). A 15:1 split was employed while the volatiles were transferred into the capillary column at a column flow of 1 ml min−1. The GC oven ramp was 35 °C for 2 min, 3 °C min−1 to 60 °C, 5 °C min−1 to 100 °C , 8 °C min−1 to 170 °C, 10 °C min−1 to 200 °C, and hold for 13 min. GC separations were on a 30 m × 0.25 mm i.d. × 0.25 μm film thickness DB-Wax (J & W Scientific, Folsom, CA, USA) capillary column in a HP6890 GC (Agilent Technologies) with helium as the carrier gas. The GC was coupled to a TOF-MS (Leco Pegasus III, St. Joseph, MI, USA). The ion source temperature was kept at 200 °C and ionization energy of 70 eV was used for electron impact ionization. The detector voltage was 1700 V, and ion spectra from 33 to 320 atomic mass units were collected with a data acquisition rate of 20 Hz. The total ion chromatograms were processed using the LECO ChromaTOF software.
Analysis of solvent extracted volatiles was carried out using the GC-MS system described above with the following modifications. Solvent extracts (1 μl) were injected into the GC with a 15:1 split and an injection port temperature of 200 °C. The GC oven ramp was 35 °C for 2 min, 3 °C min−1 to 60 °C, 5 °C min−1 to 100 °C, 8 °C min−1 to 190 °C, 10 °C min−1 to 230 °C, and hold for 15 min. The transfer line temperature was 220 °C.
Terpenes were identified using the following reference compounds: α-pinene, linalool, 1,8-cineole, and β-myrcene (Aldrich), limonene (BDH), β-pinene (K & K Laboratories), and sabinene (Phenomenex), Other compounds for which we did not have authentic standards (Tables 1 and 2) were identified by the ChromTOF software (version 2.3, Pegasus, Leco Australia) using the National Institute of Standards and Technology (NIST, version 2.0d, 2005) mass-spectral database, in combination with comparing the retention indices with those of a series of straight-chain hydrocarbon standards (C8–C23, 0.005 μl ml−1 for each hydrocarbon). Peaks were selected and integrated manually using the molecular ion and/or specific diagnostic ions of each compound. The terpenes were quantified by measuring m/z 93 peak areas against an average response factor for the m/z 93 peak areas of 1,8-cineole (0.0366 μl ml−1), linalool (0.0263 μl ml−1), and caryophyllene (0.03 μl ml−1), contained in an external standard. Calibration curves determined that the GC-MS system gave a linear response, with respect to analyte concentration, over the range of concentrations for which they were measured in the headspace and in the solvent extracts.
Table 1.
Actinidia chinensis flower semi-quantitative headspace terpene analysis
| Compound | Concentration | Fraction |
| Sesquiterpenes | ||
| (E)-Nerolidol | 7.50 | 29.90 |
| (E,E)-Farnesal | 1.30 | 5.18 |
| (E,E)-α-Farnesene | 1.15 | 4.58 |
| (Z,E)-Farnesal | 0.93 | 3.71 |
| (E,E)-Farnesol | 0.26 | 1.04 |
| (Z,E)-α-Farnesene | 0.14 | 0.56 |
| (Z,E)-Farnesol | 0.03 | 0.12 |
| Total | 11.31 | 45.1 |
| Monoterpenes | ||
| (E)-β-Ocimene | 1.55 | 6.18 |
| Linalool | 1.46 | 5.82 |
| α-Pinene | 1.40 | 5.58 |
| Sabinene | 1.07 | 4.27 |
| β-Pinene | 0.72 | 2.87 |
| 1,8-Cineole | 0.64 | 2.55 |
| (Z)-β-Ocimene | 0.51 | 2.03 |
| Limonene | 0.37 | 1.47 |
| β-Myrcene | 0.27 | 1.00 |
| α-Thujene | 0.22 | 0.88 |
| Total | 8.21 | 32.73 |
| Terpene metabolites | ||
| 6-Methyl-5-hepten-2-onea | 4.18 | 16.62 |
| (E)-Geranyl acetonea | 0.73 | 2.90 |
| 4,8-Dimethyl-1,3,7-nonatrieneb | 0.63 | 2.51 |
| (Z)-Geranyl acetoneb | 0.02 | 0.09 |
| Total | 5.56 | 22.17 |
Emission rates for individual terpenes (listed in order of concentration) are calculated on the average hourly emission over a 24 h day/night cycle and are presented as ng (g fresh weight)−1 h−1. Fractions are given as a percentage of total emitted terpenes. Sums for each terpene class are italicized.
Oxidative breakdown product (E,E)-α-farnesene (Anet, 1972).
Oxidative breakdown products of nerolidol (Donath and Boland, 1995; Boland et al., 1998).
Table 2.
Actinidia chinensis flower semi-quantitative solvent-extracted terpene analysis Terpenes are listed in order of concentration and are presented as ng (g fresh weight)−1 h−1. Fractions are given as a percentage of total accumulated terpenes.
| Compound | Concentration | Fraction |
| Sesquiterpenes | ||
| (E,E)-Farnesol | 18700 | 72.4 |
| (E,E)-Farnesal | 1600 | 6.2 |
| (Z,E)-Farnesol | 1070 | 4.1 |
| (E)-Nerolidol | 890 | 3.4 |
| (Z,E)-Farnesal | 820 | 3.2 |
| (E,Z)-Farnesol | 580 | 2.2 |
| Monoterpenes | ||
| Linalool | 1500 | 5.8 |
| Geraniol | 400 | 1.5 |
| Geranyl acetate | 280 | 1.1 |
Terpene glycoside extraction and analysis
Glycoside analysis was carried out according to previous methods (Young and Paterson, 1995) with minor modifications. Frozen flower tissue (2 g) was ground to a fine powder in liquid N2, and transferred to a 50 ml Falcon tube and resuspended in 40 ml of water. The sample was centrifuged at 2000 g for 10 min at room temperature and the supernatant passed through a 15 mm × 25 mm i.d. Amberlite XAD-2 column (Supelco, Bellefonte, PA) according to the manufacturer’s instructions. Bound glycosides were washed with 60 ml of water and the non-glycosylated compounds removed by the addition of Et2O (40 ml). The bound glycosides were eluted with methanol (40 ml) and evaporated to dryness in a rotary evaporator. The resulting glycoside pellet was resuspended in 2.5 ml of de-glycosylation buffer (200 mM Na2HPO4 (pH 5), 220 mM citric acid) and re-extracted three times with 2.5 ml Et2O. Enzymatic hydrolysis was carried out using Rapidase AR2000 (DSM Food Specialties, Delft, The Netherlands) which was dissolved in de-glycosylation buffer and used at a concentration of 2 mg ml−1. The hydrolysis sample was overlaid with 500 μl of Et2O and incubated for 24 h at 37 °C. Following the incubation, the sample was extracted a further three times with 1 ml of Et2O. Prior to GC-MS analysis the pooled Et2O extracts were passed through a column of anhydrous MgSO4 and reduced to 1.5 ml.
Quantification was carried out on the DB-Wax GC column on the Waters-Agilent GC-MS system described above. Linalool (0.0001 μl ml−1), 8-hydroxylinalool (0.00016 μl ml−1), synthesized in-house according to the methods of Kreck et al. (2002), (E,Z)-nerolidol (0.0002 μl ml−1), and (E,E)-farnesol (0.0002 μl ml−1) were used as external authentic standards. A spike of 2 μl of 1 μl ml−1 of tridecane (Koch-Light) in each of the 1.5 ml standards (to give 0.00133 μl ml−1) and c. 1.5 ml aglycone extracts was also used to correct for sample volumes and for GC-MS system responses, by correcting for the tridecane m/z 184 peak areas. Peak areas of the above terpenes were measured for m/z 93, and concentrations of the external standards were within an order of magnitude of those in the aglycone extracts, thus maintaining a linear response of the mass spectrometer over the concentrations of analytes in the different samples. Enantioselective GC-MS analysis of the linalool and (E)-nerolidol aglycones (data not shown) was performed on the Waters-Agilent GC-MS system and the β-Dex 325 GC column described above, using the oven temperature ramp employed for linalool.
Sequence analysis
Multiple amino acid sequence alignments of TPS genes were performed with Clustal X (Thompson et al., 1997), using default parameters, and were manually adjusted in GeneDoc (www.nrbsc.org/gfx/genedoc/). Prediction of the AcNES1 protein open reading frame (ORF) was carried out using the EMBOSS (http://www.ch.embnet.org/EMBOSS/index.html) translation tool Transeq (Rice et al., 2000). The bioinformatics tools ChloroP (Emanuelsson et al., 1999) and TargetP (Emanuelsson et al., 2000) available at http://www.cbs.dtu.dk/services/ were used to predict the intracellular targeting of AcNES1. Evolutionary relationships for TPS enzymes were inferred using the neighbour-joining method (Saitou and Nei, 1987). The evolutionary distances were computed using the Poisson correction method (Zuckerkandl and Pauling, 1965). Phylogenetic analyses were conducted in MEGA5.1 (Tamura et al., 2007). The TPS accession numbers are given in Supplementary Table S1, available at JXB online.
Production of AcNES1 protein
An AcNES1 fragment possessing 5′ EcoRI and 3′ XhoI endonuclease sites was amplified by PCR from a 1978-bp AcNES1 cDNA (GenBank accession JN242243) and cloned directly into the pET-30a expression vector (Novagen) as an EcoRI/XhoI fragment. Details of the primers are given in Supplementary Table S2. Recombinant proteins were expressed from the pET-30a plasmid following transformation into BL21-CodonPlus-RIL cells (Stratagene). Cultures (500 ml) were grown in ZYM-5052 autoinduction media (Studier, 2005) at 16 °C for 48–72 h at 300 rpm. Recombinant AcNES1 protein was extracted and purified according to previous methods (Green et al., 2007). Eluted recombinant proteins were analysed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and terpene synthase activity measured. Protein concentrations were determined by Experion (BioRad) automated electrophoresis analysis, according to the manufacturer’s instructions.
AcNES1 product analysis
AcNES1 solvent extraction assays for terpene product identification were carried out in 5 ml activity buffer (50 mM Bis-Tris propane (pH 7.5), 10 mM MgCl2, and 5 mM DTT), in 50 ml glass tubes typically containing 50–100 μg purified recombinant AcNES1 protein and 20 μM FDP or GDP precursor. Assays were overlaid with 5 ml pentane:Et2O (1:1 v/v) and incubated in a 30 °C water bath for 30 min. An additional 15 ml of solvent was added to each test tube and the tubes vortexed vigorously for 30 s to extract the volatiles. The solvent extracts were dried using MgSO4 and reduced in volume to 200 μl, under a stream of N2, before GC-MS analysis.
GC-MS separations were carried out on an Agilent 6890N GC coupled to a Waters GCT ToF mass spectrometer. Initial chromatographic separations of 1 μl samples were on a 20 m × 0.18 mm i.d. × 0.18 μm film thickness DB-Wax (Agilent) capillary column with a He flow of 0.9 ml min−1 and an injection port temperature of 220 °C. The oven temperature ramp was 1 min at 35 °C, 5 °C min−1 to 240 °C, and hold for 15 min. The retention time and mass spectrum agreed with those of the authentic compounds obtained commercially and synthesized in-house. Enantioselective GC-MS separations were performed on the above GC-MS system on a 30 m × 0.25 mm i.d. × 0.25 μm film thickness β-Dex 325 (Supelco) capillary column with a helium flow rate of 1 ml min−1. For analysis of AcNES1 products in vitro (after 1 μl, 1 min splitless injections), the oven temperature ramps were: 2-methyl-but-3-en-2-ol: 5 min at 28 °C and 5 °C min−1 to 230 °C: linalool: 1 min at 35 °C, 5 °C min−1 to 230 °C, and hold for 5 min; and (E)-nerolidol: 1 min at 50 °C, 3 °C min−1 to 130 °C, 7 °C min−1 to 230 °C, and hold for 10 min. For flower solvent extracts the oven temperature ramp for (E)-nerolidol and linalool was 1 min at 35 °C, 5 °C min−1 to 230 °C and hold for 5 min.
Enzyme kinetic analysis
Quadruplicate assays (50 μl) containing 50 mM BIS-TRIS propane (pH 7.5) and 150 nmoles AcNES1 protein were initiated by the addition of [C1–3H1]-FDP (4.0 GBq mmol−1) or [C1–3H1]-GDP (6.2 GBq mmol−1) precursors and incubated for 20 s at room temperature. Reactions were stopped with the addition of three volumes of 0.1M KOH/0.2 M EDTA solution and labelled products extracted with 0.5 ml pentane:Et2O (1:1, v/v) and an aliquot taken for scintillation analysis. FDP and GDP kinetic determinations were carried out in the presence of 20 mM MgCl2 or 20 mM MnCl2 in separate assays. Cofactor determinations for Mg2+ and Mn2+ were carried out in the presence of 10 μM [C1–3H1]-FDP. Control assays using boiled enzyme were used to determine background radioactive counts. Kinetic constants were calculated from Bq data by non-linear regression of the Michaelis–Menten equation using the Origin 7.5 (Microcal Software. Northampton, MA, USA) graphics package. Data were calculated from three independent experiments.
Alternative substrate analysis
Relative velocity data (Vrel) for AcNES1 was also determined using prenyldiphosphates (50 μM) that were not tritium labelled, using semi-quantitative GC-MS. Assays were set up in the same manner as the 5 ml solvent extraction assay described above with the following exceptions: the duration of incubation was increased to 1 h and the enzyme concentration was decreased to 55 nmoles. For semi-quantitative GC-MS, sample peak areas were measured relative to authentic compounds (external standards) with tetradecane used as an internal standard in all samples to correct for day-to-day variation in the sensitivity of the GC-MS system. Enzyme activities were linear with time and enzyme concentration in all cases and were measured at least twice.
Real-time gene expression analysis
RNA was extracted from flowers, flower parts, fruit, and leaves according to Nieuwenhuizen et al. (2007) and treated with 10 U of DNaseI (Roche Applied Science, Mannheim, Germany) before cDNA synthesis. First-strand cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche) according to the manufacturer’s instructions and diluted 50-fold before use. Relative quantitation real-time gene expression analysis of targets and the housekeeping gene elongation factor 1α were performed (four technical replicates) on a LightCycler 480 platform using the LightCycler 480 SYBR Green master mix and results were analysed using the LightCycler 480 software (Roche). The analysis was carried out using the –ΔCp method according to Montefiori et al., (2011), enabling a comparison of the level of expression of multiple target genes normalized to a common reference gene considered stable and unchanging in the different samples. The thermal programme was 5 min at 95 °C and 40 cycles of 10 s at 95 °C, 10 s at 60 °C, and 20 s at 72 °C, followed by melting curve analysis at 95 °C 5 s and 65 °C 60 s, and then ramping at 0.18 °C s−1 to 95 °C. Primers for real-time gene expression analysis are listed in Supplementary Table S2.
Transient expression and subcellular targeting
In planta transient expression was carried out according to the methods of Nieuwenhuizen et al. (2009) utilizing the Agrobacterium tumefaciens strain GV3101 harbouring the pHEX2 binary vector (Hellens et al., 2005). For transient expression vector construction, AcNES1 was amplified by PCR using universal primers (Hellens et al., 2005) and cloned into the pHEX2 binary vector.
For the AcNES1 subcellular targeting analysis, an in-frame N-terminal green fluorescent protein (GFP) fusion was constructed according to the methods of Nieuwenhuizen et al. (2009). Primers for the AcNES1 PCR amplification step are listed in Supplementary Table S2. The amplified DNA was digested with BamHI and XbaI and cloned into the p326-SGFP vector (Lee et al., 2001), culminating in the p326-AcNES1/SGFP vector.
Results
Volatile emission and accumulation in A. chinensis flowers
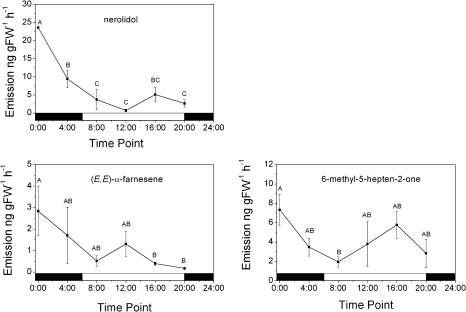
The emission of floral terpenes by A. chinensis was investigated using a combination of dynamic headspace sampling and solvent extraction followed by GC-MS analysis. Volatile sampling, which was carried out over the course of a 24 h day/night cycle, revealed a mixture of monoterpenes, sesquiterpenes, and various terpene derivatives to be present in the headspace of the flowers (Table 1). (E)-nerolidol emission, calculated on a ng (g fresh weight)−1 h−1 (gFW−1 h−1) basis over a day/night cycle, constituted ∼30% of the total emitted terpenes and was produced at an average rate of 7.5 ng gFW−1 h−1. The (E,E)-α-farnesene oxidation product, 6-methyl-5-hepten-2-one (Anet, 1972), was the second most prevalent terpene compound, with an emission rate of ∼4.18 ng gFW−1 h−1, while (E,E)-α-farnesene emission was the next highest, at ∼1.15 ng gFW−1 h−1. Lower amounts of the terpene metabolites (E)- and (Z)-geranyl acetone and 4,8-dimethyl−1,3,7-nonatriene, which were assumed to be oxidative degradation products of (E)-nerolidol (Donath and Boland, 1995; Boland et al., 1998), were also present. The emission profiles for the main floral sesquiterpenes (Fig. 1) showed that peak (E)-nerolidol emission occurred at midnight (0:00 h) then declined over the next 8 h where it remained low until evening. Although there was a suggestion of smaller day-time (E)-nerolidol emission peak at 16:00 h, the emission rates between noon and 20:00 h were not significantly different. The emission profile for (E,E)-α-farnesene and its oxidation product 6-methyl-5-hepten-2-one remained relatively constant, although levels did appear to gradually decline from midnight onwards. Generally, the concentrations of the different monoterpenes (Supplementary Fig. S1) were low (< 3 ng gFW−1 h−1) and showed little variation over the course of the day/night cycle, although cumulative amounts of monoterpenes and sesquiterpenes (predominantly (E)-nerolidol) were similar (Table 1).
Fig. 1.
Rates of sesquiterpene and homoterpene release from A. chinensis flowers during a day/night cycle. Terpene volatiles were trapped from triplicate flower samples by dynamic headspace sampling at 4-h intervals and analysed by GC-MS. Data are presented as mean ± SEM (n = 3). Means labelled without a common letter are significantly different (P < 0.05) based on LSD of one-way ANOVA.
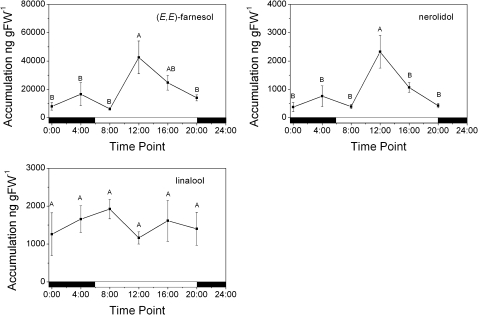
Solvent extractions of flowers taken at the equivalent time points to the above headspace analysis were analysed for the presence of accumulated volatiles. In contrast to the emitted volatiles, the profile of accumulated volatiles was significantly less complex and primarily consisted of sesquiterpenes (Table 2). On a whole-flower fresh-weight basis, the dephosphorylated FDP derivative (E,E)-farnesol was by far the most prevalent compound occurring at ∼18,700 ng gFW−1, or ∼72%, of the total extracted terpene volatiles. The accumulated terpenes, presumed to be produced by TPS enzymes, were dominated by linalool and (E)-nerolidol, with ∼1500 and ∼890 ng gFW−1 respectively. Time point analysis of these terpenes (Fig. 2) showed that (E,E)-farnesol and (E)-nerolidol were largely accumulated during the day, with peak concentrations occurring at noon. Notably, the highest concentrations of accumulated (E)-nerolidol corresponded to the lowest rate of emitted (E)-nerolidol and vice versa, while linalool accumulation remained relatively constant over the course of the day/night cycle.
Fig. 2.
Rates of sesquiterpene and monoterpene accumulation in A. chinensis flowers during a day/night cycle. Terpene volatiles were solvent extracted at 4-h intervals over the course of 24 h. Data are presented as mean ± SEM (n = 3). Means labelled without a common letter are significantly different (P < 0.05) based on LSD of one-way ANOVA.
Volatile glycosylation in A. chinensis flowers
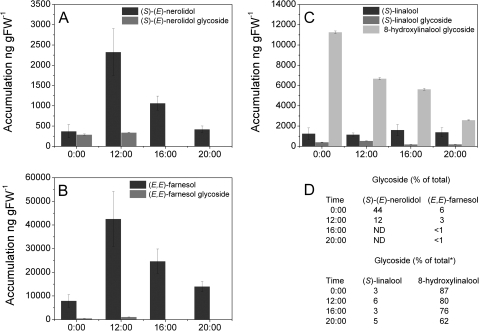
Differences in the accumulation and emission patterns for (E)-nerolidol and (E,E)-farnesol in A. chinensis flowers suggested that these compounds might be sequestered in non-volatile glycosylated form for later release. Floral glycosides were extracted from A. chinensis and analysed by GC-MS after enzymatic hydrolysis (Fig. 3). This analysis showed that glycosylated forms of (E)-nerolidol, (E,E)-farnesol, and linalool were present in the flowers, as well as high levels of glycosylated 8-hydroxylinalool. Glycosylated (E)-nerolidol was detected at midnight, at ∼290 ng gFW−1 (or ∼44% of the total (E)-nerolidol pool), and increased to 340 ng gFW−1 at noon (although this level now only equated to ∼12% of the total (E)-nerolidol pool). There was no glycosylated (E)-nerolidol detected following the period of peak (E)-nerolidol accumulation at noon (Fig. 2), suggesting that between noon and 16:00 h the sequestered (E)-nerolidol had been remobilized and released from the flower. The pattern of (E,E)-farnesol glycosylation (Fig. 3B) was similar to that of (E)-nerolidol, although it only comprised a minor component (∼0.1–6%) of the total (E,E)-farnesol pool. Glycosylated linalool accounted for between ∼2.5% and 6.5% of the total linalool pool with peak accumulation occurring between midnight and noon. Unexpectedly, 8-hydroxylinalool (Fig. 3C), was by far the most predominant A. chinensis glycosylated floral terpene, with levels declining from ∼87% (∼11,300 ng gFW−1) of the total linalool pool at midnight to 62 % (∼2600 ng gFW−1) at 20:00 h.
Fig. 3.
Analysis of glycosylated terpenes in A. chinensis flowers. Glycosylated terpenes were extracted from flower tissue harvested at four time points during a day/night cycle and enzymically hydrolysed to release the corresponding aglycones. (A–C) Levels of glycosylated (S)-(E)-nerolidol (A), (E,E)-farnesol (B), and (S)-linalool (C) shown in comparison with non-glycosylated levels taken from Fig. 2. (D) Glycosylated terpenes given as a percentage of total terpenes. * Total linalool includes the 8-hydroxylinalool component. ND, not detected. Data are presented as mean ± SEM (n = 3).
Identification of terpene synthase-like sequences from A. chinensis flowers
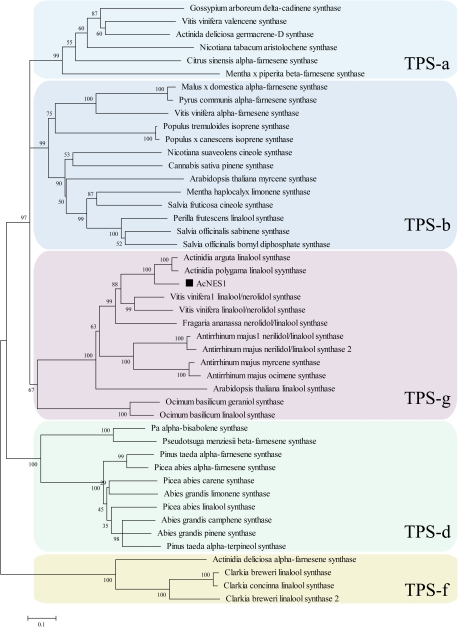
A homology-based gene mining approach was taken to identify putative TPS enzymes that could account for the production of the terpene compounds identified in A. chinensis flowers. A search of an Actinidia expressed sequence tag database (Crowhurst et al., 2008) identified a single contig from floral tissue with sequence homology to known floral TPS sequences (Supplementary Fig. S2). This transcript represented a full-length cDNA (termed AcNES1) of 1978 nucleotides encoding a predicted protein of 573 amino acids and ∼65.4 kDa. AcNES1 was not predicted to contain a plastid-targeting peptide according to the ChloroP and TargetP prediction programmes. Phylogenetic analysis showed that AcNES1 clustered in a well-supported manner with the TPS enzymes in the TPS-g subgroup (Bohlmann et al., 1998; Dudareva et al., 2003) (Fig. 4). The TPS-g enzymes are characterized by the absence of an N-terminal RRX8W motif, which is reported to be essential for cyclic monoterpene production in the angiosperm TPS-b and gymnosperm TPS-d enzyme subgroups (Savage et al., 1994). AcNES1 was most similar to the Actinidia polygama and A. arguta linalool synthases (Chen et al., 2010), with ∼78% amino acid identity.
Fig. 4.
Phylogenetic analysis of selected plant terpene synthase (TPS) enzymes. The clusters correspond to the previously recognized TPS subfamilies (TPS-a -TPS-g) (Bohlmann et al., 1998; Dudareva et al., 2003). Terpene synthases from the TPS-e and TPS-c subgroups, which are involved in plant primary metabolism, were not included in this analysis. AcNES1 is indicated by a filled square. Protein alignments were conducted using the ClustalX algorithm and trees were inferred using the neighbour-joining method. N = 10,000 replicates for bootstrapping. Accession numbers for each enzyme are given in Supplementary Table S1.
Enzymatic properties of AcNES1
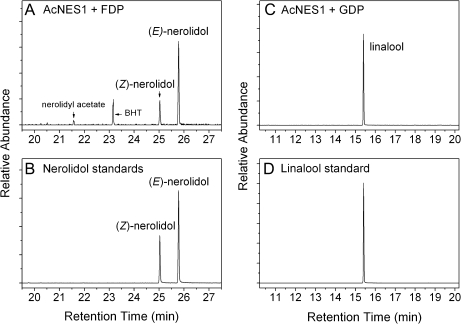
Purified recombinant AcNES1 protein was analysed for activity with GDP and FDP precursors in separate solvent extraction assays (Fig. 5). GC-MS analysis of the pentane/Et2O extracted volatiles showed that AcNES1 could catalyse the conversion of both FDP and GDP precursors to the respective terpene alcohols (E)-nerolidol and linalool. The smaller amount of (Z)-nerolidol produced by AcNES1 was assumed to derive from (Z,E)-FDP contamination of the (E,E)-FDP used. Kinetic evaluation of recombinant AcNES1 enzyme (Table 3) showed that it had a higher binding affinity for FDP compared with GDP (Km values of 0.80 and 1.89 μM respectively) and an approximate four-fold increase in catalytic efficiency (kcat/Km) in the presence of FDP (300 s−1 mM−1) compared with GDP (69 s−1 mM−1). AcNES1 also showed an approximate 8-fold increase in its affinity for Mn2+ (Km ≈ 14.2 μM compared with Mg2+ (Km ≈ 117 μM) as the divalent metal cofactor. However, in the presence of Mn2+ AcNES1, activity was only ∼22% of that observed with FDP and Mg2+ together and ∼54% of that observed with GDP and Mg2+ together. The kinetics for substrate and divalent metal ion preference are similar to those observed for other TPS-g enzymes (Nagegowda et al., 2008; Chen et al., 2010).
Fig. 5.
AcNES1 GC-MS analysis. (A) Pentane/Et2O samples extracted from assays containing purified recombinant AcNES1 in the presence of FDP. (B) Authentic (Z)- and (E)-nerolidol standards. (C) Linalool produced by AcNES1 from GDP. (D) Authentic linalool standard. The butylhydroxytoluene contaminant seen in (A) is derived from the diethyl ether used in the terpene volatile extraction.
Table 3.
Kinetic profiles of recombinant AcNES1 protein Recombinant AcNES1 enzyme was obtained using a combination of Ni2+ affinity and gel filtration chromatography. Kinetic parameters are given for both farnesyl diphosphate (FDP) and geranyl diphosphate (GDP) (0.25–100 μM) in the presence of 20 mM MgCl2. FDP and GDP kinetic determinations in the presence of MnCl2 were not calculated due to the low activity rates of AcNES1 with the Mn2+ cation. Kinetic parameters are also given for Mg2+ (0.05–20 mM) and Mn2+ (0.01–2.5 mM) in the presence of 10 μM FDP. Km, Michaelis constant. All values represent mean ± SE (n = 3). AcNES1 turnover rates (kcat) were calculated under conditions of saturating substrate and divalent metal ion concentrations. Relative velocity (Vrel) data were determined from kcat values. NA, not applicable.
| Km (μM) | kcat (s−1) | kcat/Km (s−1 mM−1) | Vrel (%) | |
| FDP (Mg2+) | 0.80 ± 0.26 | 0.24 ± 0.013 | 300 | 100 |
| Mg2+ (FDP) | 117 ± 37 | |||
| Mn2+ (FDP) | 14.2 ± 2.70 | 0.053 ± 0.005 | 22 | |
| GDP (Mg2+) | 1.89 ± 0.20 | 0.13 ± 0.002 | 69 | 54 |
| GDP (Mn2+) | NA | 0.03 ± 0.003 | 12 |
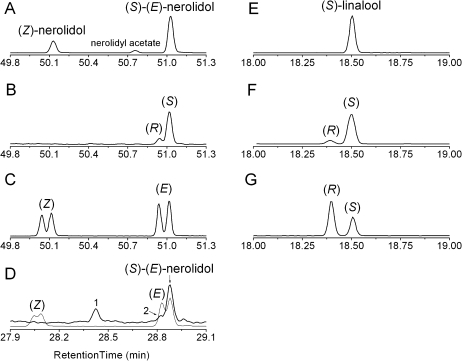
Enantiomeric analysis of terpene compounds from AcNES1 and A. chinensis flowers
Enantioselective GC-MS analysis of (E)-nerolidol and linalool produced by AcNES1 determined that (S)-(E)-nerolidol and (S)-linalool (Fig. 6) were the predominant enantiomers. AcNES1 was also observed to produce (S)-linalool (Fig. 6F) from racemic LDP, while the small amount of (R)-linalool observed was assumed to have derived from LDP breakdown. This assumption was based on the fact that other racemic monoterpene products were seen in this analysis (data not shown). Equivalent analysis of an A. chinensis whole-flower extract (Fig. 6D) also showed that (S)-(E)-nerolidol was the predominant enantiomer and hence supports the assumption that AcNES1 is likely to be responsible for floral (S)-(E)-nerolidol biosynthesis in A. chinensis. In contrast to the (S)-linalool produced by AcNES1, the predominant linalool enantiomer in A. chinensis flowers, as previously shown by Matich et al. (2010) was (R)-linalool.
Fig. 6.
Enantioselective analysis of AcNES1 terpene synthase activity. (A) GC-MS profile (m/z 93) for AcNES1 activity in the presence of FDP. (B) Profile (m/z 93) for a neroli standard showing (R)-(E)-nerolidol and (S)-(E)-nerolidol peaks. (C) Total ion current GC-MS profile for nerolidol standard (Aldrich) showing (Z)-nerolidol and (E)-nerolidol peaks. (D) Comparative GC-MS profile for whole-flower extract (black line) with nerolidol standard (grey line); a non-terpene peak (1) and small (R)-(E)-nerolidol peak (2) are also indicated. (E) AcNES1 with GDP. (F) Racemic linalyl diphosphate. (G) Control GC-MS trace (m/z 93) for a racemic and (R)-linalool standard. A faster ramp rate was used in (D).
Alternative substrate usage by AcNES1
The sequence homology of AcNES1 to TPS-g nerolidol/linalool and linalool/nerolidol/geranyllinalool synthases from grape (Martin et al., 2010) provided a rational basis for testing additional prenyldiphosphate substrates ranging from C5 to C20. This analysis (summarized in Table 4) showed that AcNES1 was able to catalyse the conversion of GGDP (C20) to the corresponding terpene alcohol geranyllinalool at ∼10% of the optimized rate for (E)-nerolidol from FDP but was unable to convert the C5 hemiterpene (isoprene) precursor DMADP to its corresponding alcohol. This in vitro plasticity for GDP, FDP and GGDP prenyldiphosphate usage in AcNES1 is mirrored in the activity of the three grape TPS-g sesqui-TPS enzymes (Martin et al., 2010) and again demonstrates an inherent capacity for TPS enzymes to evolve different product and substrate specificities.
Table 4.
Semi-quantitative GC-MS analysis of AcNES1 alternate substrate usage FDP, Farnesyl diphosphate; GDP, geranyl diphosphate; GGDP, geranylgeranyl diphosphate; LDP, linalyl diphosphate.
| Relative velocity (Vrel %) |
||||
| Product (%) | LDP | GDP | FDP | GGDP |
| (R)-Linalool | 0.7 | |||
| (S)-Linalool | 2.2 | 58.4 | ||
| (S)-(E)-Nerolidol | 100a | |||
| Geranyl linalool | 10.3 | |||
100% = kcat ∼0.127 s−1.
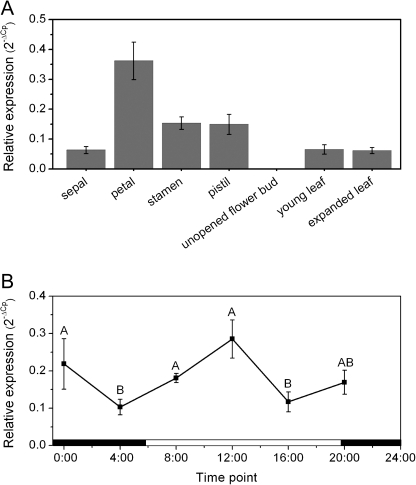
Gene expression analysis
Relative gene expression analysis was carried out to investigate the spatial and temporal regulation patterns of AcNES1 expression in A. chinensis (Fig. 7). The highest AcNES1 expression was observed in the petals of fully open flowers, with lower expression in the stamens and pistils (Fig. 7A). AcNES1 expression was not observed in unopened flowers. Over the course of a day/night cycle, AcNES1 expression followed a weak diurnal rhythm with peak expression levels occurring between 20:00–0:00 h and 8:00–12:00 h (Fig. 7B).
Fig. 7.
Relative gene expression analysis of AcNES1 in A. chinensis. AcNES1 expression levels were determined in floral and vegetative tissue (A) and in whole flowers during a 24 h day/night cycle (B). The data were analysed using the target/reference ratio calculated with the LightCycler 480 software, enabling a comparison of the level of expression of different gene family members compared to the reference gene EF1α, considered stable and unchanging in the different tissues during development. Primer efficiencies were between 1.96 and 2.0. Data are presented as mean ± SEM (n = 4). Means labelled without a common letter are significantly different (P < 0.05).
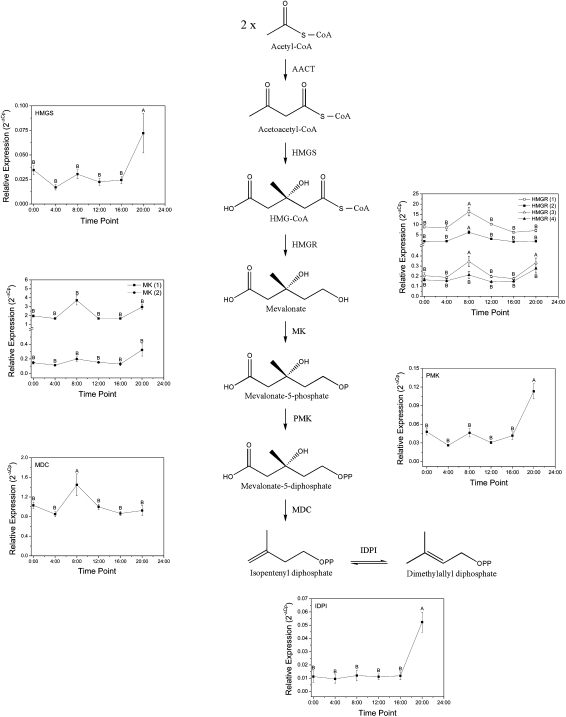
Gene expression analysis was also carried out to determine how genes in the MVA pathway might modulate FDP flux and consequently sesquiterpene emission and/or accumulation in A. chinensis flowers (Fig. 8). The profiles of three family members encoding the key regulatory enzyme HMG-CoA reductase (HMGR) (Maurey et al., 1986; Gondet et al., 1992; Hemmerlin et al., 2003; Hey et al., 2006; Muñoz-Bertomeu et al., 2007) and the mevalonate-5-diphosphate decarboxylase gene (MDC) peaked in expression at 08:00 h. This peak in expression preceded maximal (E,E)-farnesol and (E)-nerolidol accumulation at noon (Fig. 2), and suggests a link between FDP substrate flux and sesquiterpene accumulation in A. chinensis flowers. Interestingly, the reduction of floral HMGR gene expression between 08:00 h and noon also coincided with increasing (E,E)-farnesol concentrations (Figs. 8 and 2, respectively). Expression analysis for the other MVA genes (Fig. 8) showed that peak expression for the 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS), phosphomevalonate kinase (PMK), and isopentenyldiphosphate isomerase (IDPI) genes all occurred at 20:00 h.
Fig. 8.
Relative expression analysis of mevalonate (MVA) pathway genes in A. chinensis flowers. All genes were identified from an in-house Actinidia expressed sequence tag database collection (Crowhurst et al., 2008). The indicated enzymes are acetyl-CoA/acetyl-CoA C-acetyltransferase (AACT), 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), mevalonate kinase (MK), phosphomevalonate kinase (PMK), mevalonate-5-diphosphate decarboxylase (MDC), and isopentenyl diphosphate isomerase (IDPI). Expression levels of the MVA pathway genes were determined using the primers listed in Table S2. Primer efficiencies were between 1.94 and 2.0. MVA expression levels were compared to the reference gene EF1α, as indicated in Fig. 7. Data are presented as mean ± SEM (n = 4). Means labelled without a common letter are significantly different (P < 0.05).
Subcellular localization and in planta activity of AcNES1
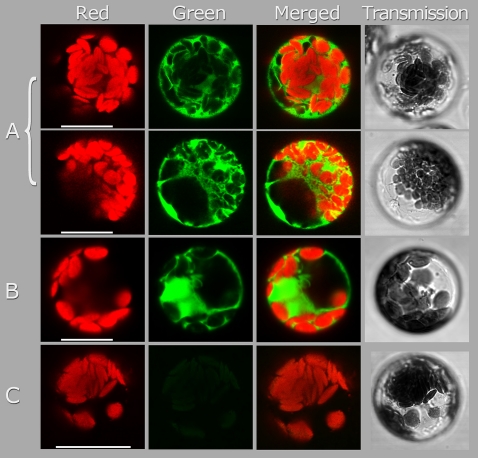
The subcellular localization of AcNES1 was investigated using an AcNES1-GFP fusion protein in Arabidopsis protoplasts. Confocal laser scanning microscopy (Fig. 9) revealed a diffuse GFP fluorescence pattern for AcNES1-GFP protein distribution exclusively present in the cytosol. Similar fluorescence patterns were observed for a control GFP expression product, while no fluorescence was observed in untransfected protoplasts. These results are in agreement with the lack of a predicted AcNES1 plastid-targeting signal (ChloroP) and confirm that this enzyme is located in the cytosol.
Fig. 9.
Subcellular localization of AcNES1 in Arabidopsis leaf protoplasts. The full-length coding region of AcNES1 was fused to the GFP reporter gene in the vector p326S-GFP to produce the p326-AcNES1/SGFP construct. The fusion construct were then transferred into Arabidopsis protoplasts and analysed by confocal laser scanning microscopy for GFP expression. The red columns shows chlorophyll autofluorescence, the green column shown GFP fluorescence, the merged column shows combined chlorophyll autofluorescence and GFP fluorescence, and the transmission column shows light-microscopy images of the intact protoplasts. (A) p326-AcNES1/SGFP, in duplicate from two independent experiments. (B) Control p326-SGFP construct (expressed in the cytosol). (C) No-DNA control. Bars, 25 μm.
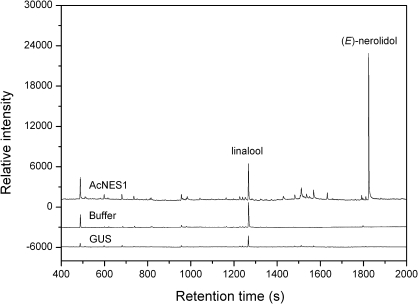
A. tumefaciens-mediated transient expression (Hellens et al., 2005) in Nicotiana benthamiana leaves was used to confirm that AcNES1 acted as a (E)-nerolidol synthase in planta. N. benthamiana leaves infiltrated with a CaMV 35S-driven binary AcNES1 vector construct produced significant amounts of nerolidol (Fig. 10). (E)-nerolidol was not produced in control N. benthamiana leaves that had been infiltrated with buffer or a GUS-containing binary vector. Small amounts of linalool were also identified in both the control and AcNES1 infiltrated leaves and were therefore assumed to be derived from an endogenous linalool synthase.
Fig. 10.
Transient expression of AcNES1. A full-length AcNES1 cDNA was cloned into the binary vector pHEX2 and transformed into A. tumefaciens. Bacterial cell suspensions were infiltrated into the adaxial side of young N. benthamiana leaves (in triplicate). Dynamic headspace sampling of volatiles from detached leaves was carried out 14 days after infiltration. Control leaves were infiltrated with buffer-only or with a vector expressing the GUS reporter gene (pHEX2-GUS). Volatiles transiently expressed in N. benthamiana are shown after infiltration with AcNES1 (upper trace), buffer-only (middle trace), and pHEX2-GUS (lower trace).
Discussion
Terpene emission by A. chinensis flowers is dominated by the tertiary sesquiterpene alcohol (E)-nerolidol. (E)-nerolidol is a common volatile constituent of plants and nerolidol synthases have been characterized in a number of plant species including strawberry (Aharoni et al., 2004), grape (Martin et al., 2010), snapdragon (Nagegowda et al., 2008), and maize (Schnee et al., 2002). (E)-nerolidol is likely to play a role in attracting pollinators or seed dispersal agents and is also produced by plants in response to insect herbivory (Bouwmeester et al., 1999; Degenhardt and Gershenzon, 2000; Danner et al., 2011). Our data shows that, in A. chinensis, (E)-nerolidol mostly accumulates during the day, while its release occurs mostly during the night (Figs. 1 and 2, respectively). This not only contrasts to the emission of (E)-nerolidol in snapdragon flowers (Dudareva et al., 2005) and (E,E)-farnesene and germacrene-D in A. deliciosa (Nieuwenhuizen et al., 2009), which occurs during the day, but also suggests a possible role in attracting night-time insect pollinators.
One feature that differentiates A. chinensis floral terpene metabolism from that of other kiwifruit species is the low rates of floral terpene emission. Peak (E)-nerolidol emission in A. chinensis is more than 3-fold lower than the equivalent (E,E)-α-farnesene emission in A. deliciosa (i.e. ∼24 v. 76 ng gFW−1 h−1, respectively) (Nieuwenhuizen et al., 2009), while total monoterpene emissions in A. chinensis (∼8.2 ng gFW−1 h−1) equate to only ∼9% of the linalool emission in A. arguta (Chen et al., 2010). Although the level of accumulated linalool in A. chinensis flowers appears to be higher than that observed for A. arguta (1.5 μg gFW−1 versus 0.1 μg gFW−1 respectively), linalool turnover in A. arguta flowers is likely to be significantly higher. The linalool produced in A. arguta flowers has many highly abundant biosynthetic derivatives (Chen et al., 2010), which would be expected to deplete the pool of accumulated linalool.
Terpene volatile sequestration in A. chinensis flowers
The apparent disconnect between the accumulation and emission of (E)-nerolidol suggested that sequestration might be involved in regulating levels of terpene volatiles in A. chinensis flowers. However, our analysis showed that the glycosylation profile cannot account for the difference in the timing of (E)-nerolidol production and release. A significant proportion of the total (E)-nerolidol pool (44%) was glycosylated at midnight (Fig. 3A and D), which corresponded to the time of peak of (E)-nerolidol emission (Fig. 1) rather than accumulation as might be expected. Moreover, during the peak accumulation of (E)-nerolidol at noon and then during its steady decline from noon to 20:00 h, glycosylated (E)-nerolidol was not detected.
Interestingly, glycosylation is likely to be impacting on floral linalool emission in A. chinensis. Significant amounts of linalool are produced (Fig. 3C and D); however, the majority is either glycosylated directly or enzymatically converted to 8-hydroxylinalool and then glycosylated. The presence of 8-hydroxylinalool has also been observed as an unexpected consequence of over-expressing a Clarkia (S)-linalool synthase in tomato (Lewinsohn et al., 2001) and was put down to the presence of a hydroxylase able to act on (S)-linalool.
The function of AcNES1 in A. chinensis flowers
Subcellular protein localization can control terpene biosynthesis in planta by modulating terpene precursor accessibility. Although it is generally accepted that GDP and FDP biosynthesis are segregated to plastidial and cytosolic compartments, respectively, low concentrations of plastidial FDP and cytosolic GDP have been identified in Arabidopsis, tobacco, and potato (Aharoni et al., 2003, 2006; Wu et al., 2006). It is therefore possible that an enzyme that shows bifunctional behaviour in vitro can also exhibit the same behaviour in planta. This appears to be true for the bifunctional, cytosolically targeted FaNES1 enzyme in strawberry, which is reported to synthesize both (E)-nerolidol and linalool in cultivated strawberry fruit (Aharoni et al., 2004). However in Antirrhinum majus flowers, the bifunctional cytosolically-targeted AmNES/LIS1 enzyme has been shown to produce (E)-nerolidol exclusively from cytoplasmic FDP pools, whilst the chloroplast-targeted AmNES/LIS2 enzyme exclusively produces linalool from plastid (specifically leucoplasts) GDP pools (Nagegowda et al., 2008). The cytosolically targeted AcNES1 enzyme from A. chinensis flowers is also likely to act exclusively as a (E)-nerolidol synthase, as N. benthamiana leaves transiently over-expressing AcNES1 (Fig. 10) produced only (E)-nerolidol. Two additional results support the contention that AcNES1 acts as an (S)-(E)-nerolidol synthase and not a linalool synthase in A. chinensis flowers. The (S)-(E)-nerolidol enantiomer produced by AcNES1 matches the predominant floral (E)-nerolidol enantiomer (Fig. 6A and D, respectively) and AcNES1 produces (S)-linalool from GDP, not (R)-linalool as found in flowers.
Regulation of terpene biosynthesis in A. chinensis flowers
An unusual feature of A. chinensis flowers is the high amounts of (E,E)-farnesol that they accumulate (Table 2) compared with flowers from other species, including other kiwifruit (Matich et al., 2003; Crowhurst et al., 2008). It is also notable that the delicate floral tissue is not adversely affected by (E,E)-farnesol concentrations that can reach 42,600 ng gFW−1 (or ∼190 μM), considering that 25 μM is reported to be toxic to tobacco BY-2 cells (Hemmerlin and Bach, 2000). The accumulation of (E,E)-farnesol in A. chinensis flowers suggests that farnesyl pyrophosphatase activity (Nah et al., 2001) is elevated and/or that the ability to regenerate FDP through the successive phosphorylation of farnesol to farnesyl phosphate and farnesyl phosphate to FDP (Thai et al., 1999; Hemmerlin and Bach, 2000; Hemmerlin et al., 2006; Fitzpatrick et al., 2011) is decreased. However, the later explanation seems less plausible given that there is significant turnover of the accumulated farnesol occurring (Fig. 2). The uptake of farnesol by plant cells and incorporation into sterols, proteins, and ubiquinone is likely to account for most of this turnover (Thai et al., 1999; Hemmerlin and Bach, 2000; Hartmann and Bach, 2001), although 7–10% can be accounted for by the conversion of farnesol to farnesal (Supplementary Fig. S3) and up to ∼6% is glycosylated (Fig. 3D). Although reasons for the high levels of (E,E)-farnesol accumulation remain to be determined, it is likely that the consequence of this accumulation will be a depleted pool of FDP available to AcNES1 and hence a diminished capacity for floral sesquiterpene production in A. chinensis.
Our work also shows that the regulation of FDP flux in A. chinensis flowers and hence sesquiterpene production is likely to be modulated by a number of different MVA pathway genes (Fig. 8). Specifically, the peak levels of HMGR RNA expression (Fig. 8) were found to correlate with the day-time accumulation profile of (E)-nerolidol and (E,E)-farnesol (Fig. 2). Moreover, the inverse correlation that exists between the day-time increases in (E,E)-farnesol accumulation and decreases in HMGR expression levels (Figs. 2 and 8 respectively) indicates that high (E,E)-farnesol concentrations may down-regulate floral HMGR expression and/or HMGR enzyme activity. While farnesol treatment has been shown to increase both HMGR mRNA and apparent enzyme activity levels in tobacco cells (Hemmerlin and Bach, 2000) it has also been shown to down-regulate HMGR enzyme activity in yeast as a consequence of induced structural changes following farnesol binding (Shearer and Hampton, 2005).
In this study we have identified a sesqui-TPS gene that accounts for (E)-nerolidol production in A. chinensis flowers. Moreover, we have provided evidence to suggest that elevated (E,E)-farnesol production in A. chinensis flowers reduces their capacity for sesquiterpene biosynthesis. In the future it will be interesting to establish the reasons why such high amounts of the MVA pathway carbon flux ends up as (E,E)-farnesol and how the enzymes involved in the dephosphorylation of FDP, its salvage from (E,E)-farnesol, and subsequent reincorporation into primary metabolism are influencing the availability of a precursor crucial for secondary plant metabolic processes.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Rates of monoterpene release from A. chinensis flowers during a day/night cycle.
Supplementary Fig. S2. Sequence alignment of AcNES1 with TPS enzymes from fruit and flowers.
Supplementary Fig. S3. (E,E)-farnesol and (E,E)-farnesal accumulation in A. chinensis flowers.
Supplementary Table S1. Terpene synthase accession numbers.
Supplementary Table S2. Primers.
Acknowledgments
We would like to thank Natalia Dudareva, Dinesh Nagegowda and Jennifer Sturgis for their help in acquiring confocal microscopy data; Inhwan Hwang (POSTEC, Korea) for providing the p326-SGFP vector. We would also like to thank Jinquan Feng for his help with the statistical analysis and Mirco Montefiori, Cyril Brendolise and William Laing for critically reviewing the manuscript. Work at Plant and Food Research was funded by the New Zealand Foundation for Research Science and Technology (CO6X0403).
References
- Adam K-P, Zapp J. Biosynthesis of the isoprene units of chamomile sesquiterpenes. Phytochemistry. 1998;48:953–959. [Google Scholar]
- Aharoni A, Giri AP, Deuerlein S, Griepink F, de Kogel W-J, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. The Plant Cell. 2003;15:2866–2884. doi: 10.1105/tpc.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Giri AP, Verstappen FWA, Bertea CM, Sevenier R, Sun Z, Jongsma MA, Schwab W, Bouwmeester HJ. Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. The Plant Cell. 2004;16:3110–3131. doi: 10.1105/tpc.104.023895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Jongsma M, Kim T-Y, Ri M-B, Giri A, Verstappen F, Schwab W, Bouwmeester H. Metabolic engineering of terpenoid biosynthesis in plants. Phytochemistry Reviews. 2006;5:49–58. [Google Scholar]
- Anet EFLJ. Superficial scald, a functional disorder of stored apples VIII. Volatile products from the autoxidation of alpha-farnesen. Journal of the Science of Food and Agriculture. 1972;23:605–608. [Google Scholar]
- Arigoni D, Eisenreich W, Latzel C, Sagner S, Radykewicz T, Zenk MH, Bacher A. Dimethylallyl pyrophosphate is not the committed precursor of isopentenyl pyrophosphate during terpenoid biosynthesis from 1-deoxyxylulose in higher plants. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1309–1314. doi: 10.1073/pnas.96.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlmann J, Meyer-Gauen G, Croteau R. Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4126–4133. doi: 10.1073/pnas.95.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland W, Gäbler A, Gilbert M, Feng Z. Biosynthesis of C11 and C16 homoterpenes in higher plants; stereochemistry of the C–C-bond cleavage reaction. Tetrahedron. 1998;54:14725–14736. [Google Scholar]
- Bouwmeester HJ, Verstappen FWA, Posthumus MA, Dicke M. Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and Lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiology. 1999;121:173–180. doi: 10.1104/pp.121.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane DE. Enzymic formation of sesquiterpenes. Chemical Reviews. 1990;90:1089–1103. [Google Scholar]
- Chen X, Yauk Y, Nieuwenhuizen NJ, Matich AJ, Wang MY, Perez RL, Atkinson RG, Beuning LL. Characterisation of an (S)-linalool synthase from kiwifruit (Actinidia arguta) that catalyses the first committed step in the production of floral lilac compounds. Functional Plant Biology. 2010;37:232–243. [Google Scholar]
- Comeskey DJ, Bunn BJ, Fielder S. Stereospecific synthesis of all four isomeric 6,8-heneicosadien−11-ones: sex pheromone components of the painted apple moth Teia anartoides. Tetrahedron Letters. 2004;45:7651–7654. [Google Scholar]
- Croteau R. Biosynthesis and catabolism of monoterpenoids. Chemical Reviews. 1987;87:929–954. [Google Scholar]
- Croteau R, Alonso WR, Koepp AE, Johnson MA. Biosynthesis of monoterpenes: partial purification, characterization, and mechanism of action of 1,8-cineole synthase. Archives of Biochemistry and Biophysics. 1994;309:184–192. doi: 10.1006/abbi.1994.1101. [DOI] [PubMed] [Google Scholar]
- Crowhurst R, Gleave A, MacRae E, et al. Analysis of expressed sequence tags from Actinidia: applications of a cross species EST database for gene discovery in the areas of flavor, health, color and ripening. BMC Genomics. 2008;9:351. doi: 10.1186/1471-2164-9-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner H, Boeckler GA, Irmisch S, Yuan JS, Chen F, Gershenzon J, Unsicker SB, Köllner TG. Four terpene synthases produce major compounds of the gypsy moth feeding-induced volatile blend of Populus trichocarpa. Phytochemistry. 2011;72:897–908. doi: 10.1016/j.phytochem.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Degenhardt J, Gershenzon J. Demonstration and characterization of (E)-nerolidol synthase from maize: a herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl−1,3,7-nonatriene biosynthesis. Planta. 2000;210:815–822. doi: 10.1007/s004250050684. [DOI] [PubMed] [Google Scholar]
- Dicke M, Baldwin IT. The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends in Plant Science. 2010;15:167–175. doi: 10.1016/j.tplants.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Donath J, Boland W. Biosynthesis of acyclic homoterpenes: enzyme selectivity and absolute configuration of the nerolidol precursor. Phytochemistry. 1995;39:785–790. [Google Scholar]
- Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gershenzon J. The nonmevalonate pathway supports both monoterpene and sesquiterpene formation in snapdragon flowers. Proceedings of the National Academy of Sciences of the United States of America. 2005:933–938. doi: 10.1073/pnas.0407360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Cseke L, Blanc VM, Pichersky E. Evolution of floral scent in Clarkia: novel patterns of S-linalool synthase gene expression in the C. breweri flower. The Plant Cell. 1996;8:1137–1148. doi: 10.1105/tpc.8.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish CM, Kolosova N, Gorenstein N, Faldt J, Miller B, Bohlmann J. (E)-β-ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. The Plant Cell. 2003;15:1227–1241. doi: 10.1105/tpc.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, Pichersky E. Floral scent metabolic pathways: their regulation and evolution. Biology of Floral Scent. 2006:55–78. [Google Scholar]
- Eisenreich W, Schwarz M, Cartayrade A, Arigoni D, Zenk MH, Bacher A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chemistry and Biology. 1998;5:R221. doi: 10.1016/s1074-5521(98)90002-3. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, von Heijne G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Science. 1999;8:978–984. doi: 10.1110/ps.8.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AH, Bhandari J, Crowell DN. Farnesol kinase is involved in farnesol metabolism, ABA signaling and flower development in Arabidopsis. The Plant Journal. 2011;66:1078–1088. doi: 10.1111/j.1365-313X.2011.04572.x. [DOI] [PubMed] [Google Scholar]
- Gondet L, Weber T, Maillot-Vernier P, Benveniste P, Bach TJ. Regulatory role of microsomal 3-hydroxy-3-methylglutaryl-coenzyme a reductase in a tobacco mutant that overproduces sterols. Biochemical and Biophysical Research Communications. 1992;186:888–893. doi: 10.1016/0006-291x(92)90829-a. [DOI] [PubMed] [Google Scholar]
- Goodwin TW. The prenyl lipids of the membranes of higher plants. In: M Tevini, HK Lichtenthaler, editors. Lipids and Lipid Polymers in Higher Plants. Berlin: Springer Verlag; 1977. pp. 29–44. [Google Scholar]
- Green S, Friel EN, Matich A, Beuning LL, Cooney JM, Rowan DD, MacRae E. Unusual features of a recombinant apple alpha-farnesene synthase. Phytochemistry. 2007;68:176–188. doi: 10.1016/j.phytochem.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Greenhagen B, Chappell J. Molecular scaffolds for chemical wizardry: learning nature’s rules for terpene cyclases. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13479–13481. doi: 10.1073/pnas.261562898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB. Recent advances in the ecological chemistry of plant terpenoids. In: JB Harborne, FA Tomas-Barberan, editors. Ecological Chemistry and Biochemistry of Plant Terpenoids. Oxford: Clarendon Press; 1991. pp. 396–426. [Google Scholar]
- Hartmann M-A, Bach TJ. Incorporation of all-trans-farnesol into sterols and ubiquinone in Nicotiana tabacum L. cv Bright Yellow-2 cell cultures. Tetrahedron Letters. 2001;42:655–657. [Google Scholar]
- Hellens R, Allan A, Friel E, Bolitho K, Grafton K, Templeton M, Karunairetnam S, Gleave A, Laing W. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerlin A, Bach TJ. Farnesol-induced cell death and stimulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in tobacco cv Bright Yellow-2 cells. Plant Physiology. 2000;123:1257–1268. doi: 10.1104/pp.123.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerlin A, Hoeffler J-F, Meyer O, Tritsch D, Kagan IA, Grosdemange-Billiard C, Rohmer M, Bach TJ. Cross-talk between the cytosolic mevalonate and the plastidial methylerythritol phosphate pathways in tobacco bright yellow-2 cells. Journal of Biological Chemistry. 2003;278:26666–26676. doi: 10.1074/jbc.M302526200. [DOI] [PubMed] [Google Scholar]
- Hemmerlin A, Reents R, Mutterer J, Feldtrauer J-F, Waldmann H, Bach TJ. Monitoring farnesol-induced toxicity in tobacco BY-2 cells with a fluorescent analog. Archives of Biochemistry and Biophysics. 2006;448:93–103. doi: 10.1016/j.abb.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Hey SJ, Powers SJ, Beale MH, Hawkins ND, Ward JL, Halford NG. Enhanced seed phytosterol accumulation through expression of a modified HMG-CoA reductase. Plant Biotechnology Journal. 2006;4:219–229. doi: 10.1111/j.1467-7652.2005.00174.x. [DOI] [PubMed] [Google Scholar]
- Keller RK, Thompson R. Rapid synthesis of isoprenoid diphosphates and their isolation in one step using either thin layer or flash chromatography. Journal of Chromatography A. 1993;645:161–167. doi: 10.1016/0021-9673(93)80630-q. [DOI] [PubMed] [Google Scholar]
- Kreck M, Püschel S, Wüst M, Mosandl A. Biogenetic studies in Syringa vulgaris L.: synthesis and bioconversion of deuterium-labeled precursors into lilac aldehydes and lilac alcohols. Journal of Agricultural and Food Chemistry. 2002;51:463–469. doi: 10.1021/jf020845p. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim DH, Kim Y-W, Hwang I. Identification of a signal that distinguishes between the chloroplast outer envelope membrane and the endomembrane system in vivo. The Plant Cell. 2001;13:2175–2190. doi: 10.1105/tpc.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesburg CA, Caruthers JM, Paschall CM, Christianson DW. Managing and manipulating carbocations in biology: terpenoid cyclase structure and mechanism. Current Opinion in Structural Biology. 1998;8:695–703. doi: 10.1016/s0959-440x(98)80088-2. [DOI] [PubMed] [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, et al. Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiology. 2001;127:1256–1265. [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:47–65. doi: 10.1146/annurev.arplant.50.1.47. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Rohmer M, Schwender J. Two independent biochemical pathways for isopentenyl diphosphate and isoprenoid biosynthesis in higher plants. Physiologia Plantarum. 1997;101:643. [Google Scholar]
- Martin D, Aubourg S, Schouwey M, Daviet L, Schalk M, Toub O, Lund S, Bohlmann J. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biology. 2010;10:226. doi: 10.1186/1471-2229-10-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matich AJ, Bunn BJ, Hunt MB. The enantiomeric composition of linalool and linalool oxide in the flowers of kiwifruit (Actinidia) species. Chirality. 2010;22:110–119. doi: 10.1002/chir.20713. [DOI] [PubMed] [Google Scholar]
- Matich AJ, Young H, Allen JM, Wang MY, Fielder S, McNeilage MA, MacRae EA. Actinidia arguta: volatile compounds in fruit and flowers. Phytochemistry. 2003;63:285–301. doi: 10.1016/s0031-9422(03)00142-0. [DOI] [PubMed] [Google Scholar]
- Maurey K, Wolf F, Golbeck J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase activity in Ochromonas malhamensis: a system to study the relationship between enzyme activity and rate of steroid biosynthesis. Plant Physiology. 1986;82:523–527. doi: 10.1104/pp.82.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello L, Dugo P, Dugo G. The chiral compounds of citrus essential oils. In: G Dugo, A Di Giacomo, editors. Citrus The Genus Citrus. London: Taylor and Francis; 2002. pp. 461–495. [Google Scholar]
- Montefiori M, Espley RV, Stevenson D, Cooney J, Datson PM, Saiz A, Atkinson RG, Hellens RP, Allan AC. Identification and characterisation of F3GT1 and F3GGT1, two glycosyltransferases responsible for anthocyanin biosynthesis in red-fleshed kiwifruit (Actinidia chinensis) The Plant Journal. 2011;65:106–118. doi: 10.1111/j.1365-313X.2010.04409.x. [DOI] [PubMed] [Google Scholar]
- Muñoz-Bertomeu J, Sales E, Ros R, Arrillaga I, Segura J. Up-regulation of an N-terminal truncated 3-hydroxy-3-methylglutaryl CoA reductase enhances production of essential oils and sterols in transgenic Lavandula latifolia. Plant Biotechnology Journal. 2007;5:746–758. doi: 10.1111/j.1467-7652.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- Nagegowda D, Gutensohn M, Wilkerson C, Dudareva N. Two nearly identical terpene synthases catalyze the formation of nerolidol and linalool in snapdragon flowers. The Plant Journal. 2008;55:224–239. doi: 10.1111/j.1365-313X.2008.03496.x. [DOI] [PubMed] [Google Scholar]
- Nah J, Song S-J, Back K. Partial characterization of farnesyl and geranylgeranyl diphosphatases induced in rice seedlings by UV-C irradiation. Plant and Cell Physiology. 2001;42:864–867. doi: 10.1093/pcp/pce102. [DOI] [PubMed] [Google Scholar]
- Nes WR, McKean ML. Occurence, physiology and ecology of sterols. In: WR Nes, ML McKean, editors. Biochemistry of Steroids and Other Isopentenoids. Baltimore: University Park Press; 1977. pp. 411–533. [Google Scholar]
- Nieuwenhuizen NJ, Beuning LL, Sutherland PW, Sharma NN, Cooney JM, Bieleski LRF, Shroeder R, MacRae EA, Atkinson RG. Identification and characterisation of acidic and novel basic forms of actinidin, the highly abundant cysteine protease from kiwifruit. Functional Plant Biology. 2007;34:946–961. doi: 10.1071/FP07121. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen NJ, Wang MY, Matich AJ, Green SA, Chen X, Yauk Y-K, Beuning LL, Nagegowda DA, Dudareva N, Atkinson RG. Two terpene synthases are responsible for the major sesquiterpenes emitted from the flowers of kiwifruit (Actinidia deliciosa) Journal of Experimental Botany. 2009;60:3203–3219. doi: 10.1093/jxb/erp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Current Opinion in Plant Biology. 2002;5:237–243. doi: 10.1016/s1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends in Genetics. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Concepcion M, Boronat A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiology. 2002;130:1079–1089. doi: 10.1104/pp.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohmer M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Natural Product Reports. 1999;16:565. doi: 10.1039/a709175c. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Savage T, Hatch M, Croteau R. Monoterpene synthases of Pinus contorta and related conifers. A new class of terpenoid cyclase. Journal of Biological Chemistry. 1994;269:4012–4020. [PubMed] [Google Scholar]
- Schnee C, Kollner TG, Gershenzon J, Degenhardt J. The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiology. 2002;130:2049–2060. doi: 10.1104/pp.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer AG, Hampton RY. Lipid-mediated, reversible misfolding of a sterol-sensing domain protein. The EMBO Journal. 2005;24:149–159. doi: 10.1038/sj.emboj.7600498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon SL, Porter JW. Introductions. In: JW Porter, S Spurgeon, editors. Biosynthesis of Isoprenoid Compounds. 1981. limited edn. New York: John Wiley and Sons, 1–46. [Google Scholar]
- Studier FW. Protein production by auto-induction in high density shaking cultures. Protein Expression and Purification. 2005;41:207–234. doi: 10.1016/j.pep.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thai L, Rush JS, Maul JE, Devarenne T, Rodgers DL, Chappell J, Waechter CJ. Farnesol is utilized for isoprenoid biosynthesis in plant cells via farnesyl pyrophosphate formed by successive monophosphorylation reactions. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13080–13085. doi: 10.1073/pnas.96.23.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholl D, Chen F, Petri J, Gershenzon J, Pichersky E. Two sesquiterpene synthases are responsible for the complex mixture of sesquiterpenes emitted from Arabidopsis flowers. The Plant Journal. 2005;42:757–771. doi: 10.1111/j.1365-313X.2005.02417.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsicker SB, Kunert G, Gershenzon J. Protective perfumes: the role of vegetative volatiles in plant defense against herbivores. Current Opinion in Plant Biology. 2009;12:479–485. doi: 10.1016/j.pbi.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Wu S, Schalk M, Clark A, Miles RB, Coates R, Chappell J. Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nature Biotechnology. 2006;24:1441–1447. doi: 10.1038/nbt1251. [DOI] [PubMed] [Google Scholar]
- Young H, Paterson VJ. Characterisation of bound flavour components in kiwifruit. Journal of the Science of Food and Agriculture. 1995;68:257–260. [Google Scholar]
- Zuckerkandl E, Pauling L. Evolutionary divergence and convergence in proteins. In: V Bryson, HJ Vogel, editors. Evolving Genes and Proteins. New York: Academic Press; 1965. pp. 97–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.