Abstract
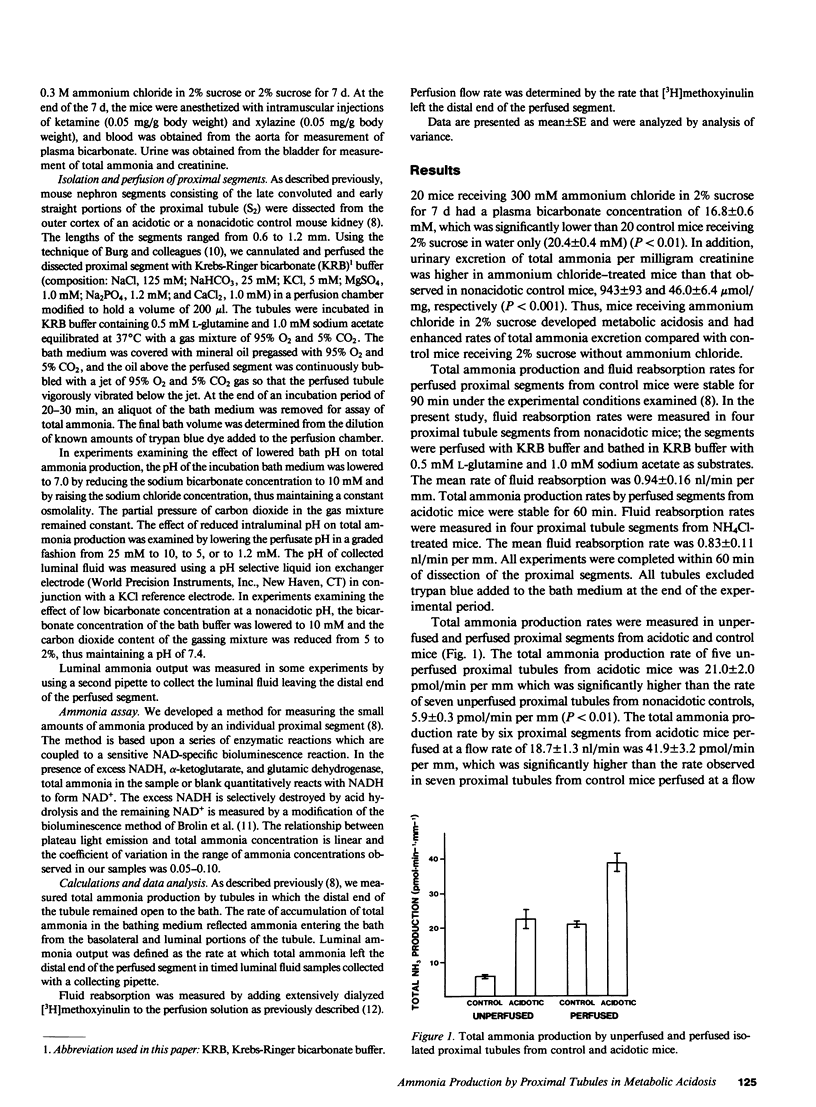
We examined the effects of metabolic acidosis in vivo and reduced bath and luminal pH in vitro on total NH3 (NH3 + NH+4) production rates by isolated mouse proximal tubule segments. Midproximal tubule segments were obtained from mice with NH4Cl-induced metabolic acidosis and from nonacidotic controls. The segments were perfused with modified Krebs-Ringer bicarbonate (KRB) buffer, incubated in KRB buffer containing 0.5 mM L-glutamine and 1.0 mM sodium acetate, and gassed with 95% O2 and 5% CO2. Isolated unperfused and perfused proximal tubules from acidotic mice produced total NH3 at higher rates than corresponding tubules from nonacidotic mice. Perfusion of the tubular lumen stimulated total NH3 production by tubules from both acidotic and nonacidotic mice. In contrast, lowering the bath pH to 7.0 by lowering the HCO3- concentration increased total NH3 production rates by tubules from nonacidotic mice but not by tubules from acidotic mice. Reducing the HCO3- concentration of the bath buffer to 10 mM while maintaining a pH of 7.4 had no significant effect on total NH3 production by tubules from nonacidotic mice. Lowering the luminal fluid pH by reducing the perfusate HCO-3 from 25 mM to 10, 5, or 1.2 mM while maintaining a bath pH of 7.4 lowered collected luminal fluid pH but had no effect on total NH3 production by proximal tubules from nonacidotic mice. These observations demonstrated that metabolic acidosis in vivo stimulated total NH3 production in isolated mouse proximal tubule segments and that low peritubular pH and HCO-3 stimulated total NH3 production by proximal tubule segments from nonacidotic mice in vitro.
Full text
PDF



Selected References
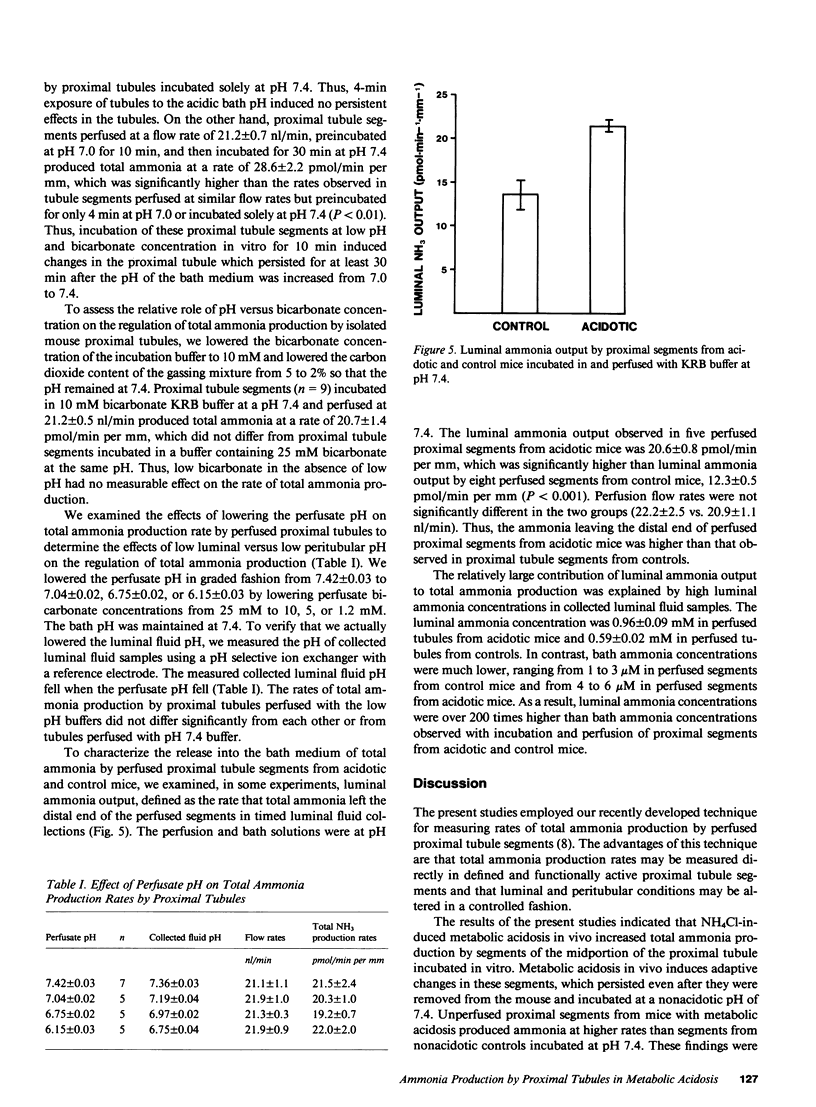
These references are in PubMed. This may not be the complete list of references from this article.
- Baverel G., Lund P. A role for bicarbonate in the regulation of mammalian glutamine metabolism. Biochem J. 1979 Dec 15;184(3):599–606. doi: 10.1042/bj1840599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Ammonium handling by superficial and juxtamedullary nephrons in the rat. Evidence for an ammonia shunt between the loop of Henle and the collecting duct. J Clin Invest. 1982 Jul;70(1):1–12. doi: 10.1172/JCI110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- GLABMAN S., KOSE R. M., GIEBISCH G. Micropuncture study of ammonia excretion in the rat. Am J Physiol. 1963 Jul;205:127–132. doi: 10.1152/ajplegacy.1963.205.1.127. [DOI] [PubMed] [Google Scholar]
- Good D. W., Burg M. B. Ammonia production by individual segments of the rat nephron. J Clin Invest. 1984 Mar;73(3):602–610. doi: 10.1172/JCI111250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYES C. P., Jr, MAYSON J. S., OWEN E. E., ROBINSON R. R. A MICROPUNCTURE EVALUATION OF RENAL AMMONIA EXCRETION IN THE RAT. Am J Physiol. 1964 Jul;207:77–83. doi: 10.1152/ajplegacy.1964.207.1.77. [DOI] [PubMed] [Google Scholar]
- Jones E. R., Beck T. R., Kapoor S., Shay R., Narins R. G. Prostaglandins inhibit renal ammoniagenesis in the rat. J Clin Invest. 1984 Sep;74(3):992–1002. doi: 10.1172/JCI111520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman J. G., Ware R. A., Schwartz J. H. Anion transport regulates intracellular pH in renal cortical tissue. Biochim Biophys Acta. 1981 Oct 20;648(1):87–92. doi: 10.1016/0005-2736(81)90127-9. [DOI] [PubMed] [Google Scholar]
- Nagami G. T., Kurokawa K. Regulation of ammonia production by mouse proximal tubules perfused in vitro. Effect of luminal perfusion. J Clin Invest. 1985 Mar;75(3):844–849. doi: 10.1172/JCI111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajo I. M., Goldstein M. B., Sonnenberg H., Stinebaugh B. J., Wilson D. R., Halperin M. L. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1981 Sep;20(3):353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- Scaduto R. C., Jr, Schoolwerth A. C. Effect of bicarbonate on glutamine and glutamate metabolism by rat kidney cortex mitochondria. Am J Physiol. 1985 Oct;249(4 Pt 2):F573–F581. doi: 10.1152/ajprenal.1985.249.4.F573. [DOI] [PubMed] [Google Scholar]
- Silbernagl S. Ammoniagenesis in the lumen of the proximal tubule or not? Contrib Nephrol. 1985;47:157–164. doi: 10.1159/000411224. [DOI] [PubMed] [Google Scholar]
- Simpson D. P., Hager S. R. Bicarbonate-carbon dioxide buffer system: a determinant of the mitochondrial pH gradient. Am J Physiol. 1984 Sep;247(3 Pt 2):F440–F446. doi: 10.1152/ajprenal.1984.247.3.F440. [DOI] [PubMed] [Google Scholar]
- Tannen R. L. Ammonia metabolism. Am J Physiol. 1978 Oct;235(4):F265–F277. doi: 10.1152/ajprenal.1978.235.4.F265. [DOI] [PubMed] [Google Scholar]
- Vinay P., Lemieux G., Gougoux A., Lemieux C. Response of the rat and dog kidney to H+ concentration in vitro--a comparative study with slices and tubules. Int J Biochem. 1980;12(1-2):89–98. doi: 10.1016/0020-711x(80)90048-8. [DOI] [PubMed] [Google Scholar]
- Yanagawa N., Nagami G. T., Jo O., Uemasu J., Kurokawa K. Dissociation of gluconeogenesis from fluid and phosphate reabsorption in isolated rabbit proximal tubules. Kidney Int. 1984 Jun;25(6):869–873. doi: 10.1038/ki.1984.103. [DOI] [PubMed] [Google Scholar]


