Abstract
As part of a continuing effort to elucidate mechanisms that regulate the magnitude of ethylene signalling, an Arabidopsis mutant with an enhanced ethylene response was identified. Subsequent characterization of this loss-of-function mutant revealed severe hypocotyl shortening in the presence of saturating ethylene along with increased expression in leaves of a subset of ethylene-responsive genes. It was subsequently determined by map-based cloning that the mutant (sar1-7) represents a loss-of-function mutation in the previously described nucleoporin AtNUP160 (At1g33410, SAR1). In support of previously reported results, the sar1-7 mutant partially restored auxin responsiveness to roots of an rce1 loss-of-function mutant, indicating that AtNUP160/SAR1 is required for proper expression of factors responsible for the repression of auxin signalling. Analysis of arf7-1/sar1-7 and arf19-1/sar1-7 double mutants revealed that mutations affecting either ARF7 or ARF19 function almost fully blocked manifestation of the sar1-7-dependent ethylene hypersensitivity phenotype, suggesting that ARF7- and ARF19-mediated auxin signalling is responsible for regulating the magnitude of and/or competence for the ethylene response in Arabidopsis etiolated hypocotyls. Consistent with this, addition of auxin to ethylene-treated seedlings resulted in severe hypocotyl shortening, reminiscent of that seen for other eer (enhanced ethylene response) mutants, suggesting that auxin functions in part synergistically with ethylene to control hypocotyl elongation and other ethylene-dependent phenomena.
Keywords: Arabidopsis, AtNUP160, auxin, ethylene, hypocotyls, nucleoporin, SAR1
Introduction
The mechanisms underlying regulation of ethylene signalling have received intensive scrutiny, largely due to the importance of ethylene-dependent phenomena to agriculture (Stepanova and Alonso, 2009). Ethylene is the simplest unsaturated hydrocarbon, having prominent roles in a wide range of processes that occur throughout the life cycle of the plant. These include several phenomena of agricultural relevance that are positively regulated by ethylene including tissue senescence, fruit ripening, and pathogen response. Additionally, ethylene is critical for manifestation of other growth programmes including the triple response in etiolated seedlings, which results in hypocotyl shortening and thickening, formation of a pronounced apical hook, and inhibition of root growth in conjunction with promotion of root hair formation.
The seedling triple response has provided an important means by which to isolate mutants that have defects affecting the ethylene-signalling pathway, thus allowing identification of core factors required for propagation of an ethylene signal. Mutants include those that are insensitive to ethylene, those that have a constitutive ethylene response independent of ethylene perception, and those that have an enhanced ethylene response due to genetic lesions impacting on negative regulators of the ethylene response or pathways that antagonize ethylene signalling. Through the use of this approach, a skeletal understanding of how an ethylene-initiated signal is propagated and subsequently terminated in Arabidopsis has developed (Stepanova and Alonso, 2009). Ethylene perception is initiated through hormone binding to a family of five transmembrane receptors (ETR1, ETR2, EIN4, ERS1 and ERS2) (Schaller and Bleecker, 1995; Hua and Meyerowitz, 1998; Hua et al., 1998) responsible for maintaining CTR1, a negative regulator of the pathway, in an active state (Kieber et al., 1993). Ethylene binding causes inactivation of this mitogen-activated protein kinase kinase kinase (MAPKKK), which relieves repression of the ethylene response and causes manifestation of ethylene phenomena in a manner dependent on EIN2, a transmembrane protein of unknown function (Alonso et al., 1999). Ultimately, the primary ethylene signalling pathway terminates in a transcriptional cascade mediated by at least two transcription factors, EIN3 and EIL1, which are normally degraded rapidly in the absence of ethylene, yet are stabilized and accumulate in the nucleus following ethylene signalling (Chao et al., 1997; Solano et al., 1998; Guo and Ecker, 2003; An et al., 2010).
Through the identification of Arabidopsis mutants that have increased ethylene responsiveness, significant advances have also been made recently regarding how the magnitude of ethylene signalling is regulated. Reported mutants can be sorted into various groups including: those that lead to greater activity or stability of known components of the ethylene signalling pathway; those of a more cryptic nature that, while exhibiting an extreme ethylene response at a morphological level, also show ethylene insensitivity with regard to expression of a subset of ethylene-regulated genes; and those that affect ethylene signalling by negatively affecting opposing signalling pathways. The first group of mutants with an increase in ethylene response is represented by several F-box factors that are required for proper degradation of various components of the pathway, including EBF1 and EBF2 (Guo and Ecker, 2003; Binder et al., 2007), which target EIN3 and EIL1 for degradation, along with ETP1 and ETP2 (Qiao et al., 2009), which function to target EIN2 for degradation. Loss of any of these factors leads to inappropriate accumulation of their specific target(s) and perpetuation of ethylene signalling.
Enhanced ethylene response (eer) mutants such as eer3, eer4, and eer5 have increased ethylene responsiveness at the morphological level that is correlated with loss of expression of a subset of ethylene-regulated genes (Christians and Larsen, 2007; Robles et al., 2007; Christians et al., 2008). These mutants probably represent defects in an ethylene-dependent pathway that promotes increased expression of an undefined group of factors required for resetting the ethylene-signalling pathway.
Finally, it is possible to increase ethylene responsiveness by decreasing the regulatory effect of an opposing pathway, which was found for a feronia loss-of-function mutant (Deslauriers and Larsen, 2010). These fer mutants have a reduced response to brassinosteroids in etiolated hypocotyls, which subsequently increases the magnitude of the ethylene response. Consequently, a perturbation in the brassinosteroid pathway, which actively promotes hypocotyl elongation, results in a greater contribution of ethylene to the promotion of hypocotyl shortening, as brassinosteroid-dependent opposition of this inhibition is diminished.
Mutations that increase the activity of a pathway synergistically with the ethylene response may also magnify the impact that ethylene has on hypocotyl shortening, with auxin signalling being a candidate for this. As with elucidation of the ethylene-signalling pathway, a similar mutagenesis-based approach has been used to define the auxin-signalling pathway, which is dependent on proteolytic degradation of transcriptional repressors following auxin perception (Chapman and Estelle, 2009). Initiation of an auxin response depends on activation of a RUB1 conjugation system that activates a Cullin-based ubiquitin ligase, which, depending on the F-box associated with it, targets various components of the auxin-signalling pathway for degradation (Gray et al., 2001). In this scheme, repressors of auxin (or indole-3-acetic acid (IAA))-dependent transcription, are proteolytically degraded, thus allowing transcriptional activators, collectively known as ARFs (auxin response factors), to promote auxin-dependent gene expression. Mutations that block degradation of IAA repressor proteins result in dominant mutants that are largely insensitive to auxin due to failure to alleviate the repression of ARF-dependent transcription (e.g. Tian et al., 2003).
Several Arabidopsis mutants that are auxin insensitive also have an altered ethylene response. A notable example is tir1, which represents a defect in an auxin-specific F-box protein and auxin receptor that, when mutated, not only impacts on the auxin response but also results in a measurable decrease in the ethylene response (Alonso et al., 2003). This has also been reported for an arf7/arf19 double mutant, which represents mutations in two auxin-responsive transcription factors (Li et al., 2006). It is not currently clear why alterations in the auxin response are linked to changes in the ethylene response. In this study, the results from analysis of a loss-of-function mutation in the nucleoporin AtNUP160, which in part results in improper expression of IAA repressor proteins (Parry et al., 2006), argue that auxin works synergistically with ethylene to control the magnitude of the ethylene response, with enhancement of auxin signalling concomitantly increasing the impact of ethylene signalling.
Materials and methods
Phenotypic analysis
All growth experiments were conducted as described previously (Larsen and Chang, 2001). For all growth experiments, mean ±SE values were determined from 30 seedlings. Measurement of ethylene production was conducted as described previously (Larsen and Cancel, 2003). Northern blot analysis was performed as described previously (Larsen and Cancel, 2003). For all northern blot analyses, the 18S rRNA gene was used to assess loading accuracy.
Map-based cloning
To generate a mapping population, sar1-7 (male; ecotype Ws-2) was crossed with La-0 wild type (wt) (female) and 4-d-old etiolated F2 seedlings with the sar1-7 phenotype when treated with 100 μl l−1 ethylene were isolated for collection of leaf tissue and genomic DNA isolation (Larsen and Cancel, 2003), after which the DNA was used as template for PCR.
To narrowly define the sar1-7 map position, novel cleaved amplified polymorphic sequence (CAPS) markers were developed for the region to which sar1-7 localized. For At1g34850, the primers were 5′-GTTGTTGATCACGTTGTAGTTTG-3′ and 5′-CAAAACGAGATATTGGTTCAGTG-3′. SspI digestion resulted in one DNA fragment for Ws-2 and two for La-0. For At1g34260, the primers were 5′-TGGCAGCTGCAAGGTACATAC-3′ and 5′-CTGCTGGAGAAAGATGAATGTC-3′, with BstNI digestion giving two bands for Ws-2 and one for La-0. For At1g34065, the primers were 5′-CTGTAATACGTGTCTCAAGTTTG-3′ and 5′-CTGCTGGAGAAAGATGAATGTTC-3′. XmnI digestion gave two DNA fragments for Ws-2 and one for La-0.
Candidate genes identified within the genetic window were amplified from both sar1-7 and Ws-2 wt by PCR using PfuTurbo (Stratagene) as described previously (Larsen and Cancel, 2003).
Generation of transgenic plants
A genomic construct representing 1.5 kb of upstream sequence, the complete coding sequence for At2g19560 including all exons and introns, and its predicted 3′ untranslated region (UTR) was generated by PCR for functional complementation. This construct was sequenced and subcloned into pBI101, after which it was introduced into sar1-7 by Agrobacterium-mediated transformation. Primary transformants were identified and analysed as described previously (Larsen and Cancel, 2003).
Genetic analysis
To generate double mutants, sar1-7 (male) was crossed with arf7-1, arf19-1, ein2-5 or ein3-1 (female). Isolated individuals homozygous for the various mutations were subsequently genotyped by sequencing to identify those homozygous for the sar1-7 mutation. A sequenced fragment of At1g33410 was amplified by PCR using primers 5′-CATGGTTCCCAATGTAGAGCTTC-3′ and 5′-GATAAGAGAGGAAAAGGATTCTTG-3′.
Protein–protein interaction analysis
For yeast two-hybrid analyses, the coding sequence of ARF7 was cloned into pACTII and used to test for interactions with the coding sequence of IAA3 cloned into pLEX-NLS. A chlorophenol red-β-D-galactopyranoside-based β-galactosidase assay was performed as described previously (Cancel and Larsen, 2002). An in vitro binding assay was subsequently used, with 35S-labelled in vitro-translated ARF7 being tested for interaction with bacterially produced maltose-binding protein (MBP) fusion proteins, including MBP–IAA3, as described previously (Cancel and Larsen, 2002).
RESULTS
Characterization of an enhanced ethylene response Arabidopsis mutant
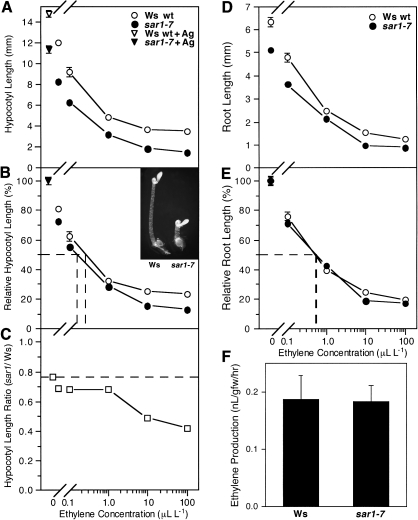
A screen was performed in which ethyl methanesulfonate-mutagenized Arabidopsis seedlings of the Ws-2 ecotype were grown for 4 d in the dark in the presence of 100 μl l−1 ethylene after which the population was checked for mutant seedlings that displayed exaggerated hypocotyl shortening. A mutant that presented severe hypocotyl inhibition coupled with increased radial expansion compared with Ws-2 wt was chosen as a candidate for having an enhanced ethylene response phenotype. As shown in Fig. 1A–C, growth analysis of this mutant compared with Ws-2 wt in the presence of 5 μM AgNO3, a potent inhibitor of ethylene action, revealed that, in the absence of ethylene, the mutant was capable of near wt growth with regard to hypocotyl elongation. The addition of increasing amounts of ethylene in the presence of the ethylene biosynthesis inhibitor AVG showed that the mutant had a severely exaggerated response to saturating ethylene. In contrast, analysis of root growth of etiolated seedlings of the mutant and Ws-2 wt in the presence of either air or increasing concentrations of ethylene revealed that mutant roots responded similarly to ethylene compared with Ws-2 wt (Fig. 1E, F), suggesting that the mutated factor was not related to modulation of the ethylene response in this tissue.
Fig. 1.
Response of Ws wt and mutant etiolated seedlings to ethylene. (A) For hypocotyl responsiveness, seedlings were grown for 4 d in the dark with 5 μM AgNO3 (triangles) or 5 μM 2-aminoethoxyvinyl glycine (AVG) (circles), with the latter supplemented with increasing concentrations of ethylene ranging from 0 to 100 μl l−1. (B) Relative hypocotyl length (length/length at 5 μM AgNO3), with the ethylene concentration giving 50% inhibition denoted by a dashed line. The inset shows 4-d-old dark-grown seedlings of Ws wt and mutant exposed to 100 μl l−1 ethylene. (C) The ratio of mutant to Ws wt hypocotyl length for each ethylene concentration tested, with the dashed line representing the predicted ratio if the mutant was not ethylene hyper-responsive. (D, E) For root responsiveness, Ws wt and mutant seedlings were grown in the dark for 4 d in the presence of air or increasing concentrations of ethylene. Actual root length is shown in (D), whereas (E) shows relative root length (length/length in air), with the ethylene concentration giving 50% inhibition denoted by a dashed line. (F) Ethylene biosynthetic rates for 4-d-old dark-grown seedlings of Ws wt and mutant. Ethylene was measured using a gas chromatography system with production rates calculated based on tissue fresh weight. Mean ±SE values were determined from five samples of 100 seedlings each.
As an exaggerated hypocotyl shortening phenotype could be interpreted as arising from alterations in ethylene production rates, it was necessary to measure the amount of ethylene generated by etiolated mutant seedlings compared with Ws-2 wt. As shown in Fig. 1F, measurement of ethylene levels using gas chromatography demonstrated that there was no discernible difference in ethylene production rates when comparing Ws-2 wt with the mutant, indicating that the exaggerated ethylene-dependent phenotype did not arise from overproduction of ethylene but rather from an increased ethylene response.
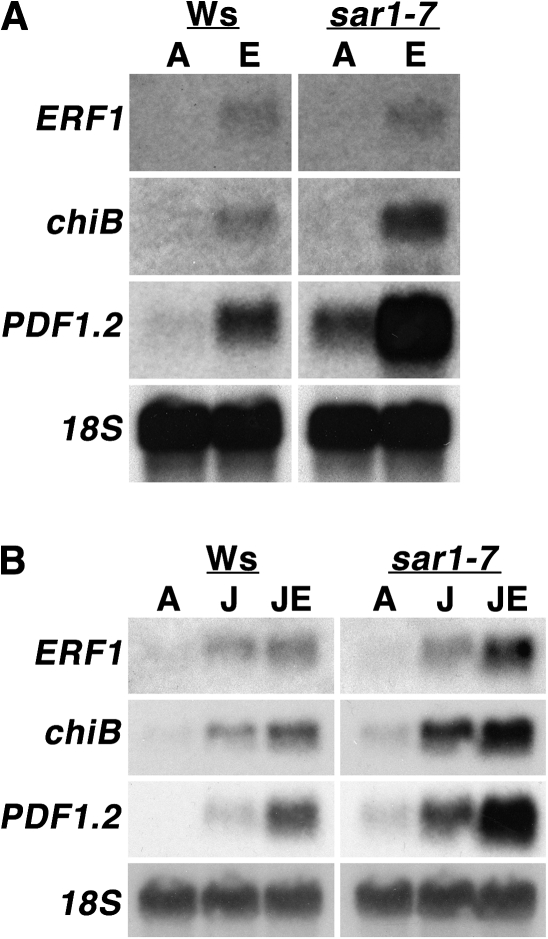
Ethylene-dependent gene expression is enhanced in mutant leaves
Patterns of ethylene-dependent gene expression were determined for leaves of the mutant compared with Ws-2 wt. While expression levels of the ethylene-inducible transcription factor ERF1 were comparable between the mutant and Ws-2 wt, ethylene treatment resulted in a substantive increase in expression levels of both basic chitinase (chiB) and PDF1.2 in the mutant compared with the wt (Fig. 2A). This subset of genes is regulated by both ethylene and jasmonic acid (JA) (Penninckx et al., 1998) making it of interest to determine whether the mutant also had increased gene expression in response to JA. Mutant leaves had a pronounced increase in expression of both chiB and PDF1.2 following treatment with 100 μM (+/–)-JA, with profound increases in expression of ERF1, chiB, and PDF1.2 following treatment with a combination of saturating ethylene and JA (Fig. 2B). This was consistent with the observation that ethylene and JA signalling are inextricably linked at the level of regulation of EIN3, EIL1, and ERF1 (Lorenzo et al., 2003; Zhu et al., 2011), which represents a point at which both signalling pathways intersect to regulate gene expression.
Fig. 2.
Ethylene-responsive gene expression in mutant leaves. (A) Four-week-old adult plants of Ws wt and the mutant were exposed to air (A) or 100 μl l−1 ethylene (E) for 24 h, after which Northern blot analysis was performed to test for expression of ethylene-inducible genes. (B) As several genes are coordinately regulated by both ethylene and jasmonic acid, gene expression was also determined following exposure of leaves to air (A), 100 μM (+/–)-jasmonic acid (J) or 100 μM jasmonic acid with 100 μl l−1 ethylene (JE).
Loss of the nucleoporin AtNUP160 increases the ethylene response
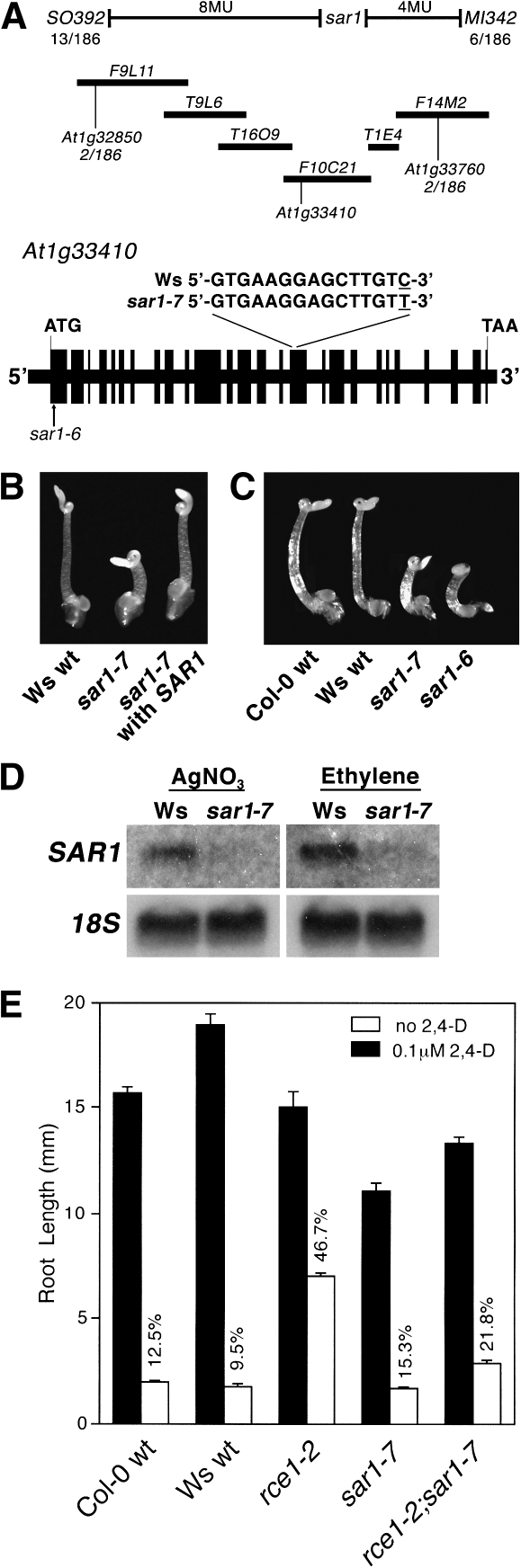
A mapping population was created by crossing the mutant (Ws-2 background) with La-0, after which F2 seedlings displaying exaggerated hypocotyl shortening in the presence of saturating ethylene were identified for isolation of genomic DNA. PCR-based polymorphic markers were used to determine that the mutation was located in the top half of chromosome 1 (Fig. 3A). Fine mapping and subsequent sequencing of candidate genes revealed that the mutation represented a substitution of T for C in the 16th exon of a previously characterized gene, At1g33410, that encodes the nucleoporin AtNUP160, which is required for a normal auxin response in Arabidopsis roots (Dong et al., 2006; Parry et al., 2006). Analysis of our loss-of-function allele, sar1-7, indicated that the nucleotide change resulted in the introduction of an inappropriate stop codon that caused premature termination of the protein.
Fig. 3.
A loss-of-function mutation in the nucleoporin-encoding gene AtNUP160/SAR1 causes an enhanced ethylene response. (A) Map-based cloning of the sar1-7 mutation. Solid bars represent the order of bacterial artificial chromosomes in the 11.8–12.2 Mb region of chromosome 1. The bacterial artificial chromosome containing AtNUP160/SAR1 and recombination frequencies at each CAPS marker surrounding sar1-7 are shown. The genomic structure of AtNUP160/SAR1 is illustrated either as filled boxes, which represent exons, or as intervening lines, which represent non-coding regions. The position of the sar1-7 mutation, which represents an inappropriate stop codon in exon 16, is shown. (B) Ws wt, sar1-7, and T2 progeny from sar1-7 transformed with the wt AtNUP160/SAR1 genomic construct were tested for an ethylene response after 4 d growth in the dark in the presence of 100 μl l−1 ethylene. (C) The mutant sar1-6 (Col-0 background), with a T-DNA insertion in the first exon of AtNUP160/SAR1, was tested for an ethylene response following growth in the dark for 4 d in the presence of 100 μl l−1 ethylene. (D) For Northern blot analysis of AtNUP160/SAR1 expression in etiolated seedlings, total RNA was isolated from dark-grown Ws wt and sar1-7 seedlings treated with 5 μM AgNO3 or 100 μl l−1 ethylene for 4 d. (E) Seedlings were grown in the light in the absence or presence of 0.1 μM 2,4-D for 7 d after which root length was measured.
In order to verify that the mutant phenotype arose from the identified mutation, a functional complementation approach was used in which a genomic construct consisting of 1.5 kb of the promoter, the 5' UTR, exons, introns, and the 3' UTR was cloned into a binary vector and introduced by Agrobacterium-mediated transformation into the sar1-7 mutant. Transgenic sar1-7 seedlings homozygous for the transgene were grown in the dark in the presence of 100 μl l−1 ethylene after which it was determined that introduction of a wt version of At1g33410 fully restored normal ethylene responsiveness to the sar1-7 mutant (Fig. 3B). In conjunction, a previously identified T-DNA allele, sar1-6, that represents an insertion in the first exon of At1g33410 was obtained (Dong et al., 2006). This allele, which is in a Col-0 background, was grown in the presence of 100 μl l−1 ethylene and its growth phenotype was compared with wt and sar1-7. As shown in Fig. 3C, ethylene treatment of sar1-6 resulted in the same exaggeration of hypocotyl shortening that was seen for sar1-7, thus confirming that functional AtNUP160 is required for a normal ethylene response in Arabidopsis hypocotyls. Northern blot analysis using At1g33410 as the probe revealed that the sar1-7 mutation severely reduced transcript levels, which was consistent with nonsense-mediated decay arising from the inappropriate stop codon (Fig. 3D). There was no apparent increase in expression of At1g33410 following ethylene treatment.
It was reported previously that a sar1 loss-of-function mutation partially restored auxin responsiveness to roots of rce1-1 (Parry et al., 2006), which is a loss-of-function mutation that destroys the function of RUB conjugating enzyme 1 (RCE1) and causes a reduced auxin response. Because of this, it was of interest to determine whether our sar1-7 mutation could reverse the phenotypic changes seen for rce1. Root growth of sar1-7, rce1-2, and rce1-2/sar1-7 roots in the absence or presence of the auxin analogue 2,4-dichlorophenoxyacetic acid (2,4-D) was determined for each mutant in comparison with the respective wt lines (Fig. 3E). As with previous reports, rce1-2 roots had a reduced auxin response that was consistent with loss of degradation of repressors of auxin signalling, whereas the sar1-7/rce1-2 double mutant had auxin-responsive roots that were more comparable to the respective wt controls than to rce1-2 (Fig. 3E) (Larsen and Cancel, 2004; Parry et al., 2006). These results suggested that loss-of-function mutations affecting AtNUP160 lead to increased auxin responsiveness, presumably through reduced or inappropriate expression of factors required for repressing auxin signalling, although it cannot be ruled out that these mutations modified auxin synthesis and/or transport, as altered auxin transport can affect hypocotyl morphology (Garbers et al., 1996).
Genetic analysis of the relationship between sar1-7 and ethylene signalling factors
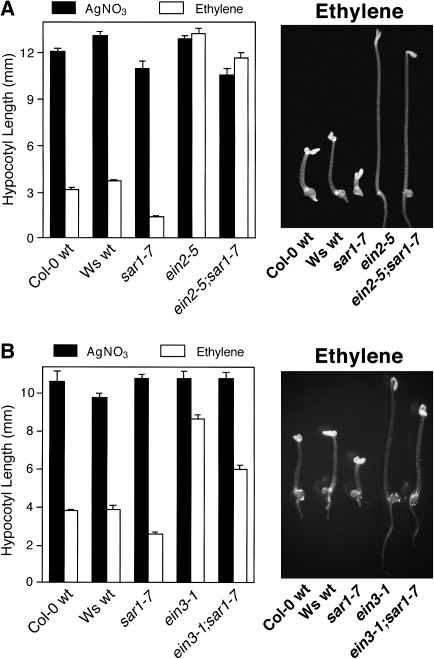
An ein2-5/sar1-7 double mutant was generated in order to demonstrate further the dependence of the sar1-7 extreme hypocotyl shortening phenotype on ethylene signalling. Seedlings of the double mutant and the respective parents were grown in the dark for 4 d in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene, after which hypocotyl length was determined (Fig. 4A). There was no discernible difference in hypocotyl length for the ein2-5/sar1-7 double mutant in the presence or absence of ethylene, indicating that the enhanced shortening seen for the sar1-7 mutant was indeed ethylene dependent.
Fig. 4.
Analysis of double mutants between sar1-7 and known ethylene-insensitive mutants. (A) Seedlings of Col-0 wt, Ws wt, sar1-7, ein2-5, and ein2-5/sar1-7 were grown in the dark in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene for 4 d, after which hypocotyl length was determined. (B) Seedlings of Col-0 wt, Ws wt, sar1-7, ein3-1, and ein3-1/sar1-7 were grown in the dark in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene for 4 d, after which hypocotyl length was assessed.
An ein3-1/sar1-7 double mutant was also generated. Seedlings of the double mutant and the respective parents were grown in the dark for 4 d in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene, after which hypocotyl length was determined (Fig. 4B). It was found that sar1-7 significantly restored ethylene-dependent hypocotyl shortening to an ein3-1 background, with the sar1-7 mutation probably resulting in amplification of the limited amount of ethylene signalling that still occurs in ein3-1 (Chao et al., 1997), similar to that seen for other eer mutants (Christians et al., 2008; Robles et al., 2007). In conjunction with the increased ethylene responsiveness of ein3-1/sar1-7 hypocotyls, ethylene treatment resulted in presentation of a full apical hook in ein3-1/sar1-7 in contrast to ein3-1, which was incapable of ethylene-dependent exaggerated apical hook formation. It should be noted that, while all double mutants represented hybrid crosses between the sar1-7 mutant (Ws background) and mutants in the Col-0 background, there was no measurable increase in variability in F2 progeny that might be expected from such hybrid crosses.
The enhanced ethylene response in sar1-7 is auxin dependent
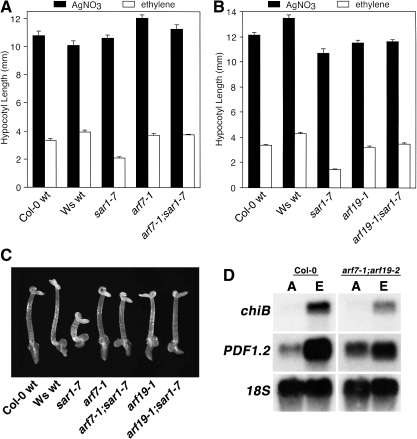
As SAR1 is required for proper expression of factors associated with auxin signalling, it was determined whether the increased ethylene responsiveness in sar1-7 was auxin dependent. A genetic approach was used in which double mutants were generated between sar1-7 and either arf7-1 or arf19-1, both of which are loss-of-function mutations affecting auxin-responsive transcription factors that have been implicated in cross-talk between the auxin and ethylene response (Li et al., 2006). Analysis of the arf7-1/sar1-7 and arf19-1/sar1-7 double mutants revealed that loss-of-function mutations affecting either ARF7 or ARF19 almost completely restored the ethylene responsiveness of the sar1-7 mutant to that of wt (Fig. 5A–C). These results indicated that the increase in the ethylene response seen for the sar1-7 mutant was dependent on a normally functioning auxin-signalling pathway.
Fig. 5.
The ethylene hyper-response phenotype of sar1-7 requires functional ARF7 and ARF19. (A) An arf7-1/sar1-7 double mutant was generated and analysed for its response to ethylene by growth for 4 d in the dark in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene, after which hypocotyl length was measured. (B) An arf19-1/sar1-7 double mutant was generated and analysed for ethylene responsiveness following growth for 4 d in the dark in the presence or absence of 5 μM AgNO3 or 100 μl l−1 ethylene, after which hypocotyl length was measured. (C) Photograph showing the ethylene responsiveness of the analysed mutants. (D) Leaves of arf7-1/arf19-2 have reduced ethylene-dependent expression of chiB and PDF1.2. For this analysis, 4-week-old adult plants were exposed to air (A) or 100 μl l−1 ethylene (E) for 24 h, after which Northern blot analysis was performed for chiB and PDF1.2.
As sar1-7 had increased expression of chiB and PDF1.2 in response to ethylene, it was of interest to determine whether leaves of an arf7-1/arf19-2 double mutant had altered ethylene-dependent expression of these defence genes (Fig. 6D). The arf7-1/arf19-2 double mutant had reduced ethylene-responsive expression of both chiB and PDF1.2, which supports the argument that auxin is required to condition the ethylene response and is consistent with our observation that loss of SAR1 function and the consequent ARF7- and ARF19-dependent enhanced auxin responsiveness leads to increased expression of these genes.
Fig. 6.
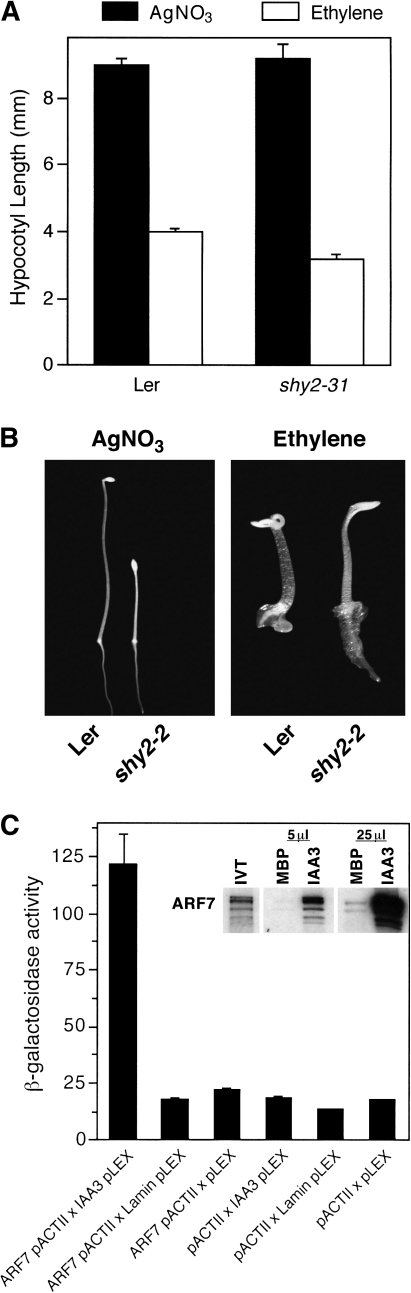
IAA3 is required for a normal response to ethylene. (A) Seedlings of Ler and shy2-31, which is a loss-of-function mutation affecting IAA3, were grown in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene, after which hypocotyl length was measured. (B) Manifestation of the ethylene response in dark-grown seedlings of shy2-2, which represents an amino acid change that prevents auxin-dependent degradation of the repressor of auxin response IAA3, was assessed following growth for 4 d in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene. (C) For the yeast two-hybrid assay, the full coding sequence of IAA3 fused to the DNA-binding domain of pLEX-NLS was tested for an interaction with the full coding sequence of ARF7 fused to the GAL4 activation domain in pACTII. The inset shows an in vitro pull-down assay in which in vitro-translated ARF7 radiolabelled with [35S]methionine was tested for its ability to interact with bacterially produced MBP fused to IAA3.
Mutational loss of IAA3 results in an enhanced ethylene response
A previous report on SAR1 showed that it is required for proper expression of IAA proteins, which are repressors of ARF function (Parry et al., 2006). Interestingly, downregulation of Sl-IAA3 resulted in an altered ethylene response in tomato, including exaggeration of apical hook formation in etiolated seedlings (Chaabouni et al., 2009). As the transcriptional activators ARF7 and ARF19 are required for manifestation of the enhanced ethylene response in sar1-7, it was of interest to determine what effect the loss of IAA3 would have on ethylene sensitivity. For this analysis, wt and shy2-31 seedlings, the latter representing a loss-of function mutant affecting IAA3, were grown for 4 d in the dark in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene, after which hypocotyl length was determined (Fig. 6A). From this, it was found that, while indistinguishable from the Ler ecotype in the presence of AgNO3, shy2-31 hypocotyls were significantly shorter than those of Ler when grown in the presence of saturating ethylene. Consistent with this, the dominant shy2-2 mutant, which represents a gain-of-function mutation that blocks IAA3 turnover (Tian et al., 2003), had a reduced capability to respond to ethylene, including failure to produce an apical hook along with increased root length in the presence of saturating ethylene (Fig. 6B).
Because loss-of-function mutations affecting ARF7 and ARF19 block manifestation of the sar1-7 phenotype and shy2-31 has an increased ethylene response, it was determined whether there was a biochemical relationship between these transcription factors and IAA3. Yeast two-hybrid and in vitro pull-down analyses were performed to test for a physical interaction between IAA3 and ARF7. As shown in Fig. 6C, there was a strong interaction observed between ARF7 and IAA3 in the yeast two-hybrid assay. This was also observed using an in vitro pull-down approach in which IAA3 was generated in bacteria as an MBP fusion that was tested for its capability to interact with in vitro-translated ARF7 radiolabelled with [35S]methionine.
Auxin controls the magnitude of the ethylene response in Arabidopsis
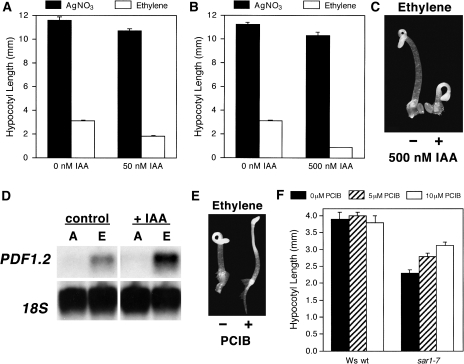
Based on the reported role of SAR1 in regulation of the auxin response coupled with our observations regarding sar1 loss-of-function mutants having ethylene hypersensitivity, we hypothesized that auxin plays a synergistic role with regard to modulating the level of the ethylene response in Arabidopsis. To test this, etiolated wt Arabidopsis seedlings were grown in the presence of 5 μM AgNO3 or 100 μl l−1 ethylene with or without 50 or 500 nM IAA for 4 d. As shown in Fig. 7A–C, whereas there was only a slight difference in hypocotyl length when comparing the AgNO3 control with AgNO3+IAA, there was a severe reduction in hypocotyl length for seedlings treated with both saturating ethylene and IAA compared with treatment with ethylene alone. The phenotype of seedlings that were treated with a combination of saturating ethylene and IAA was indistinguishable from the phenotypes of several published eer mutants including eer1/rcn1, eer3, and eer5 (Larsen and Cancel, 2003; Christians and Larsen, 2007; Robles et al., 2007; Christians et al., 2008).
Fig. 7.
Auxin signalling controls the magnitude of and competence for the ethylene response. (A–C) Wild-type seedlings were grown in the dark for 4 d with 5 μM AgNO3 or 100 μl l−1 ethylene supplemented with 0, 50 (A,), or 500 nM (B, C) IAA, after which hypocotyl length was determined for each treatment. (D) Col-0 wt seedlings were grown for 7 d hydroponically, after which all samples were treated with 10 μM (+/–)-JA for 24 h either in the absence (A) or presence (E) of 10 μM ACC. As part of this treatment, sample pairs (i.e. control and +ACC) were additionally supplemented with 1 μM IAA for the final 8 h of the ACC treatment and compared with a control pair that was treated with ethanol only. Following this, whole seedlings were collected and total RNA was isolated for Northern blot analysis for PDF1.2. (E) Wild-type seedlings were grown for 4 d in the dark with 100 μl l−1 ethylene supplemented with either 0 or 50 μM PCIB, an auxin-response inhibitor. (F) Wild-type and sar1-7 etiolated seedings were grown for 4 d in the presence of 100 μl l−1 ethylene supplemented with 0, 5, or 10 μM PCIB after which hypocotyl length was measured.
As our sar1-7 mutant was observed to have an increase in ethylene-responsive gene expression in ethylene-treated leaves, it was determined whether the addition of exogenous auxin would result in a similar increase in PDF1.2 expression. For this analysis, wt seedlings were grown in a sterile hydroponic environment for 7 d, after which all samples were supplemented with 10 μM (+/–)-JA along with either 0 or 10 μM 1-aminocyclopropane-1-carboxylic acid (ACC). After 16 h of JA±ACC treatment, one pair of samples (i.e. no ACC and 10 μM ACC) was further supplemented with 1 μM IAA, whereas the control pair (i.e. no ACC and 10 μM ACC) was treated with ethanol. Seedling tissue was subsequently collected after 8 h of IAA treatment and total RNA was isolated for northern blot analysis for PDF1.2. As shown in Fig. 7D, treatment with 10 μM JA+10 μM ACC resulted in increased PDF1.2 expression compared with control seedlings that were treated with 10 μM JA only. Consistent with our results for the auxin hypersensitive sar1-7 mutant, supplementation of the JA+ACC-treated seedlings with 1 μM IAA resulted in a substantive increase in PDF1.2 expression compared with control seedlings that were treated with JA+ACC only. This indicated that auxin functions in a synergistic manner with ethylene and JA to regulate PDF1.2 expression, which supports our model that the magnitude of and/or competence for the ethylene response is in part regulated by auxin signalling.
p-Chlorophenoxyisobutyric acid (PCIB) is an effective anti-auxin that also blocks manifestation of several auxin-dependent aspects of the ethylene-dependent triple response (Fig. 7E) (Oono et al., 2003). As we predicted that the enhanced ethylene response of sar1-7 was auxin dependent, it was of interest to determine whether chemical blockage of auxin signalling using PCIB would block manifestation of the sar1-7 ethylene-response phenotype. For this analysis, etiolated wt and sar1-7 seedlings were grown in the presence of 100 μl l−1 ethylene with or without 5 or 10 μM PCIB. As shown in Fig. 7F, treatment with increasing concentrations of PCIB resulted in a progressive reversal of the ethylene hypersensitivity seen for sar1-7, which further argues that the ethylene hypersensitivity seen for sar1-7 is auxin dependent.
Discussion
The biochemical mechanisms underlying ethylene and auxin signalling are clearly intertwined, with many of the identified mutants for each pathway displaying phenotypes related to both hormones. For example, TIR1, the first demonstrated auxin receptor (Dharmasiri et al., 2005), has an as yet undefined role in mediation of ethylene response, as loss-of-function tir1 mutants also display weak ethylene insensitivity in conjunction with an aberrant auxin response (Alonso et al., 2003). This is also true of other auxin-insensitive mutants such as arf7, arf19, aux1, and shy2-2, all of which show clear differences in auxin responsiveness while at the same time demonstrating aspects of ethylene insensitivity (Pickett et al., 1990; Larsen and Cancel, 2004; Li et al., 2006). With regard to ethylene signalling, work on HOOKLESS, which encodes an ethylene responsive N-acetyltransferase, has revealed that loss of ethylene-responsive apical hook formation is attributable to reduced auxin responsiveness in the region of apical hook formation, with overexpression of HOOKLESS leading to constitutive presentation of an apical hook (Lehman et al., 1996). A similar phenomenon was also reported for seedlings treated with the polar auxin transport inhibitor, N-1-naphthylphthalamic acid, which blocks exaggerated apical hook formation in the presence of ethylene (Garbers et al., 1996). In conjunction with our work, these results indicate that there is probably a synergistic relationship between auxin and ethylene signalling. This is supported by work with wei8, which has reduced auxin levels in roots in conjunction with decreased ethylene responsiveness (Stepanova et al., 2008). Work with sar1-7, which represents a reduced capability to produce repressors of auxin signalling in conjunction with an increased auxin response, provides a probable causal relationship between auxin and ethylene signalling in which functional auxin signalling is required for modulation of competence for and/or the magnitude of the ethylene response.
SAR1 encodes a putative nucleoporin that is required for proper expression of factors that are necessary for repression of auxin signalling, as evidenced by prior work showing inappropriate accumulation of AXR3/IAA17 in the cytoplasm of sar1 loss-of-function mutant root cells (Parry et al., 2006). Loss of SAR1 function is also associated with regulation of the cold stress response (Dong et al., 2006), although it is unlikely based on our results that this aspect of the sar1 loss-of-function phenotype is related to enhanced ethylene signalling. Instead, the increased ethylene response seen for sar1 loss-of-function mutants probably results from increased auxin signalling, as evidenced by the capability of sar1 loss-of-function mutants to restore auxin responsiveness to rce1 loss-of-function mutants along with suppression of the sar1-7 ethylene hyper-response phenotype by combination of this with mutations that block auxin signalling, such as arf7-1 and arf19-2.
Most striking was that addition of exogenous auxin to moderate levels had an additive effect on the consequences of ethylene signalling in both etiolated seedlings and leaves. This was especially true for the seedling triple response, which showed extreme ethylene responsiveness when ethylene and auxin were applied simultaneously. While our results are not consistent with what has been reported previously in which it was argued that auxin and ethylene contribute independently to hypocotyl shortening (Stepanova et al., 2007), it should be noted that our experimental approach was significantly different. In the previous report, ethylene signalling mutants were used to show that auxin is still capable of promoting severe hypocotyl shortening, even in severely ethylene-insensitive mutants such as ein2-5. In contrast, our approach relied on treatment of seedlings with AgNO3, which is a potent inhibitor of ethylene action that effectively blocks initiation of an ethylene-signalling event at the point of ethylene binding. As ein2-5 and other ethylene-signalling mutants are arguably still capable of a limited ethylene response (Deslauriers and Larsen, 2010), especially under extreme conditions such as high auxin levels, it is likely that the discrepancy arises from the promotion of ethylene signalling by auxin in these mutants. This is supported by our finding that treatment of wt seedlings with levels of auxin at or well above 1 μM in the presence of AgNO3 (data not shown) had only a mild inhibitory effect on hypocotyl elongation compared with that found previously for ein2-5. Consequently, our results argue that auxin and ethylene largely function synergistically, rather than independently, to control hypocotyl elongation.
In support of the model that auxin conditions the magnitude of the ethylene response, ethylene and auxin appeared to be synergistic with regard to expression of a subset of ethylene-regulated genes. This was demonstrated by PDF1.2 expression, which was significantly upregulated both in the sar1-7 mutant and following addition of auxin in conjunction with ethylene and JA treatment. Conversely, mutations that blocked auxin signalling or treatment with PCIB, which blocks the auxin response, led to a reduced or lost capability to manifest ethylene-dependent phenomena such as apical hook formation, root growth inhibition, and ethylene-dependent gene expression. This has also been observed previously with N-1-naphthylphthalamic acid, which inhibits auxin transport and blocks apical hook formation (Garbers et al., 1996; Lehman et al., 1996). Taken together, these results indicate that auxin controls manifestation of the ethylene response in terms of both competence to respond and the magnitude of the response.
Currently, it is unclear how auxin functions with ethylene in this synergistic manner. Work with arf7-1 and arf19-2 argues that the respective transcription factors act in part to either directly or indirectly modulate the level of ethylene-responsive phenomena. Based on current knowledge of auxin signalling, these factors are regulated by repressors of the auxin response such as IAA3 (Tian et al., 2003; Li et al., 2006). Consistent with this model, mutational loss of IAA3 results in increased ethylene responsiveness, probably due to a reduced capability to regulate ARF7 and ARF19 function in the shy2-31 mutant. As sar1 loss-of-function mutants have increased auxin responsiveness due to improper expression of IAA factors such as IAA17, the increased ethylene response in sar1-7 arguably arises from loss of expression of a subset of IAA repressors including IAA3 that regulate ARF7- and ARF19-dependent modulation of ethylene signalling.
From our results, we conclude that the auxin and ethylene responses are intricately tied together, with auxin in part serving as a positive regulator of ethylene signalling. While the mechanism underlying this synergistic relationship requires further investigation, our work indicates that modification of auxin signalling may be an alternative approach for regulating the ethylene response in agricultural crops, with inhibitors of auxin action possibly delaying or preventing the manifestation of ethylene-regulated phenomena such as fruit ripening or tissue senescence.
Acknowledgments
We thank Dr Jason Reed of the University of North Carolina, NC, USA, for seed stocks.
Glossary
Abbreviations
- 2
4-D
- 2
4-dichlorophenoxyacetic acid
- ACC
1-aminocyclopropane-1-carboxylic acid
- ARF
auxin response factor
- AVG
aminoethoxyvinyl glycine
- IAA
indole-3-acetic acid
- JA
jasmonic acid
- MBP
maltose-binding protein
- PCIB
p-Chlorophenoxyisobutyric acid
- UTR
untranslated region
- wt
wild type
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proceedings of the National Academy of Sciences USA. 2003;100:2992–2997. doi: 10.1073/pnas.0438070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F, Zhao W, Ji Y, et al. Ethylene-induced stabilization of ETHYLENE-INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell. 2010;22:2384–2401. doi: 10.1105/tpc.110.076588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, Walker JM, Gagne JM, Emborg TJ, Hemmann G, Bleecker AB, Vierstra RD. The Arabidopsis EIN3 binding F-Box proteins EBF1 and EBF2 have distinct but overlapping roles in ethylene-signaling. Plant Cell. 2007;19:509–523. doi: 10.1105/tpc.106.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancel JD, Larsen PB. Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiology. 2002;129:1557–1567. doi: 10.1104/pp.003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaabouni S, Jones B, Delalande C, Wang H, Li Z, Mila I, Frasse P, Latché A, Pech J-C, Bouzayen M. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. Journal of Experimental Botany. 2009;60:1349–1362. doi: 10.1093/jxb/erp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE INSENSITIVE3 and related proteins. Cell. 1997;89:1133–1144. doi: 10.1016/s0092-8674(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M. Mechanism of auxin-regulated gene expression in plants. Annual Review of Genetics. 2009;43:265–285. doi: 10.1146/annurev-genet-102108-134148. [DOI] [PubMed] [Google Scholar]
- Christians MJ, Larsen PB. Mutational loss of the prohibitin AtPHB3 results in an extreme constitutive ethylene response phenotype coupled with partial loss of ethylene-inducible gene expression in Arabidopsis seedlings. Journal of Experimental Botany. 2007;58:2237–2248. doi: 10.1093/jxb/erm086. [DOI] [PubMed] [Google Scholar]
- Christians MJ, Robles LM, Zeller SM, Larsen PB. The eer5 mutation, which affects a novel proteasome-related subunit, indicates a prominent role for the COP9 signalosome in resetting the ethylene-signaling pathway in Arabidopsis. Plant Journal. 2008;55:467–477. doi: 10.1111/j.1365-313X.2008.03521.x. [DOI] [PubMed] [Google Scholar]
- Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Molecular Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dong C-H, Hu X, Tang W, Zheng X, Kim YS, Lee B-H, Zhu J- K. A putative Arabidopsis nucleoporin AtNUP160 is critical for RNA export and required for plant tolerance to cold stress. Molecular and Cellular Biology. 2006;26:9533–9543. doi: 10.1128/MCB.01063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers C, DeLong A, Deruere J, Bernasconi P, Soll D. A mutation in protein phosphatase 2A regulatory subunit A affects auxin transport in Arabidopsis. EMBO Journal. 1996;15:2115–2124. [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR. Plant responses to ethylene gas are mediated by SCFEBF1/EBF2 dependent proteolysis of EIN3 transcription factor. Cell. 2003;115:667–677. doi: 10.1016/s0092-8674(03)00969-3. [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell. 1998;94:261–271. doi: 10.1016/s0092-8674(00)81425-7. [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QG, Bleecker AB, Ecker JR, Meyerowitz EM. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis. Plant Cell. 1998;10:1321–1332. doi: 10.1105/tpc.10.8.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Cancel JD. Enhanced ethylene responsiveness in the Arabidopsis eer1 mutant results from a loss-of-function mutation in the protein phosphatase 2A A regulatory subunit, RCN1. Plant Journal. 2003;34:709–718. doi: 10.1046/j.1365-313x.2003.01762.x. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Cancel JD. A recessive mutation in the RUB1-conjugating enzyme, RCE1, reveals a requirement for RUB modification for control of ethylene biosynthesis and proper induction of basic chitinase and PDF1.2 in Arabidopsis. Plant Journal. 2004;38:626–638. doi: 10.1111/j.1365-313X.2004.02068.x. [DOI] [PubMed] [Google Scholar]
- Larsen PB, Chang C. The Arabidopsis eer1 mutant has enhanced ethylene responses in the hypocotyl and stem. Plant Physiology. 2001;125:1061–1073. doi: 10.1104/pp.125.2.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyls. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Li J, Dai X, Zhao Y. A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiology. 2006;140:899–908. doi: 10.1104/pp.105.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi K, Tanaka A, Uchimaya H. p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiology. 2003;133:1135–1147. doi: 10.1104/pp.103.027847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Ward S, Cernac A, Dharmasiri S, Estelle M. The Arabidopsis SUPRESSOR OF AUXIN RESISTANCE proteins are nucleoporins with an important role in hormone signaling and development. Plant Cell. 2006;18:15990–1603. doi: 10.1105/tpc.106.041566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiology. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR. Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes and Development. 2009;23:512–521. doi: 10.1101/gad.1765709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles LM, Wampole JS, Christians MJ, Larsen PB. Arabidopsis enhanced ethylene response 4 encodes an EIN3-interacting TFIID transcription factor required for proper ethylene response, including ERF1 induction. Journal of Experimental Botany. 2007;58:2627–2639. doi: 10.1093/jxb/erm080. [DOI] [PubMed] [Google Scholar]
- Schaller GE, Bleecker AB. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science. 1995;270:1809–1811. doi: 10.1126/science.270.5243.1809. [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. Nuclear events in ethylene-signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE RESPONSE-FACTOR1. Genes and Development. 1998;12:3703–3714. doi: 10.1101/gad.12.23.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likyacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 2008;133:177–191. doi: 10.1016/j.cell.2008.01.047. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Alonso JM. Ethylene signaling and response: where different regulatory modules meet. Current Opinion in Plant Biology. 2009;12:548–555. doi: 10.1016/j.pbi.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Tian Q, Nagpal P, Reed JW. Regulation of Arabidopsis SHY2/IAA3 protein turnover. Plant Journal. 2003;36:643–651. doi: 10.1046/j.1365-313x.2003.01909.x. [DOI] [PubMed] [Google Scholar]
- Zhu Z, An F, Feng Y, et al. Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proceedings of the National Academy of Sciences USA. 2011;108:12539–12544. doi: 10.1073/pnas.1103959108. [DOI] [PMC free article] [PubMed] [Google Scholar]