Abstract
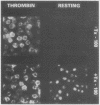
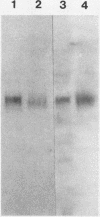
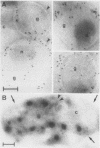
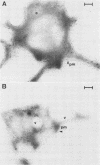
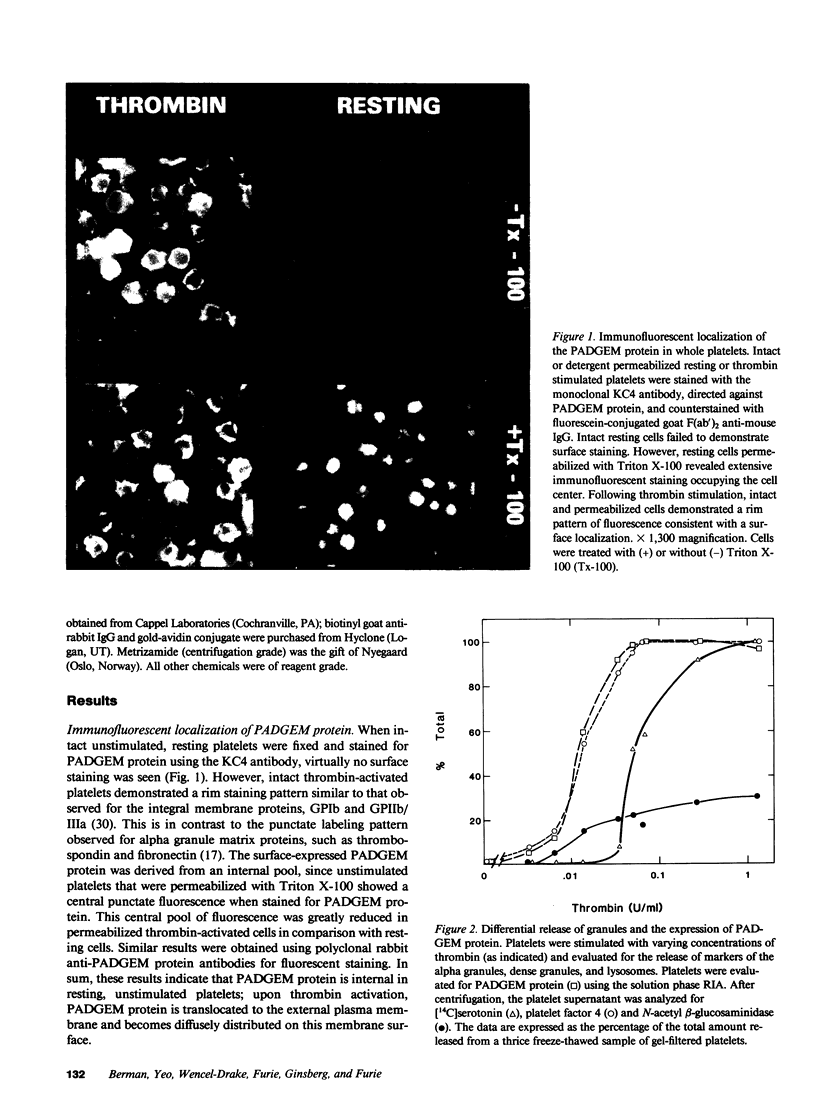
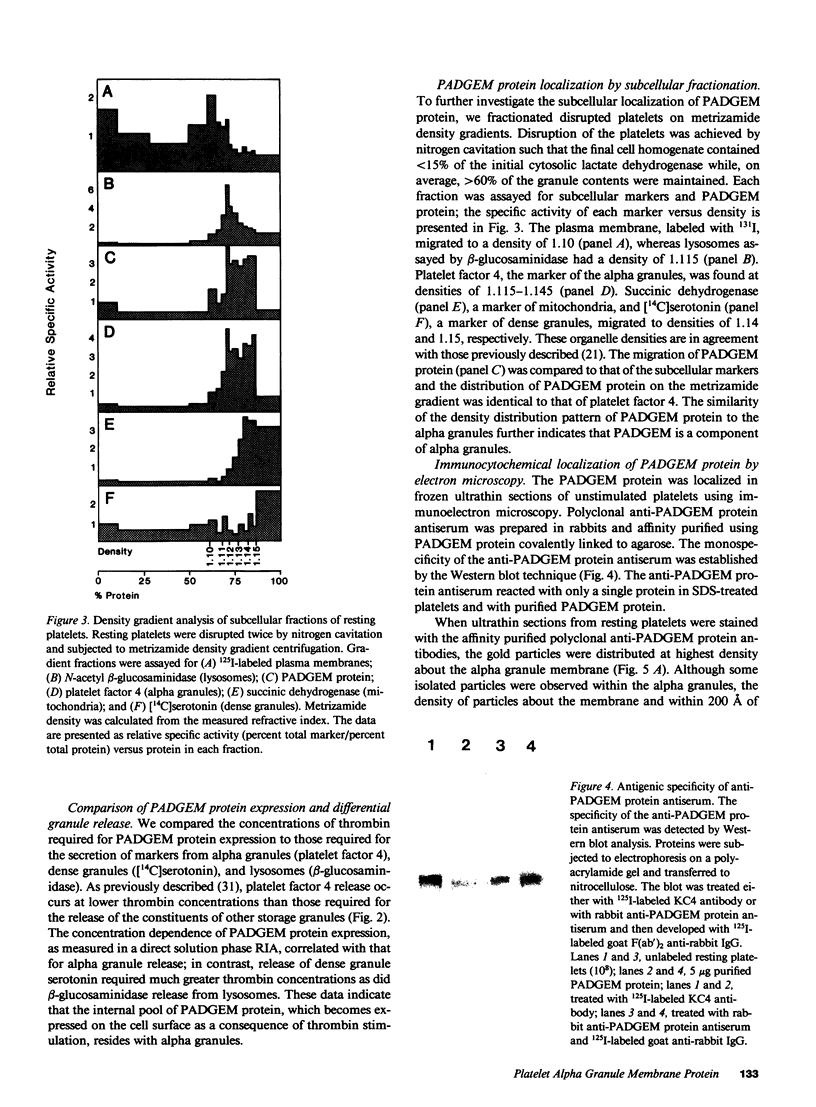
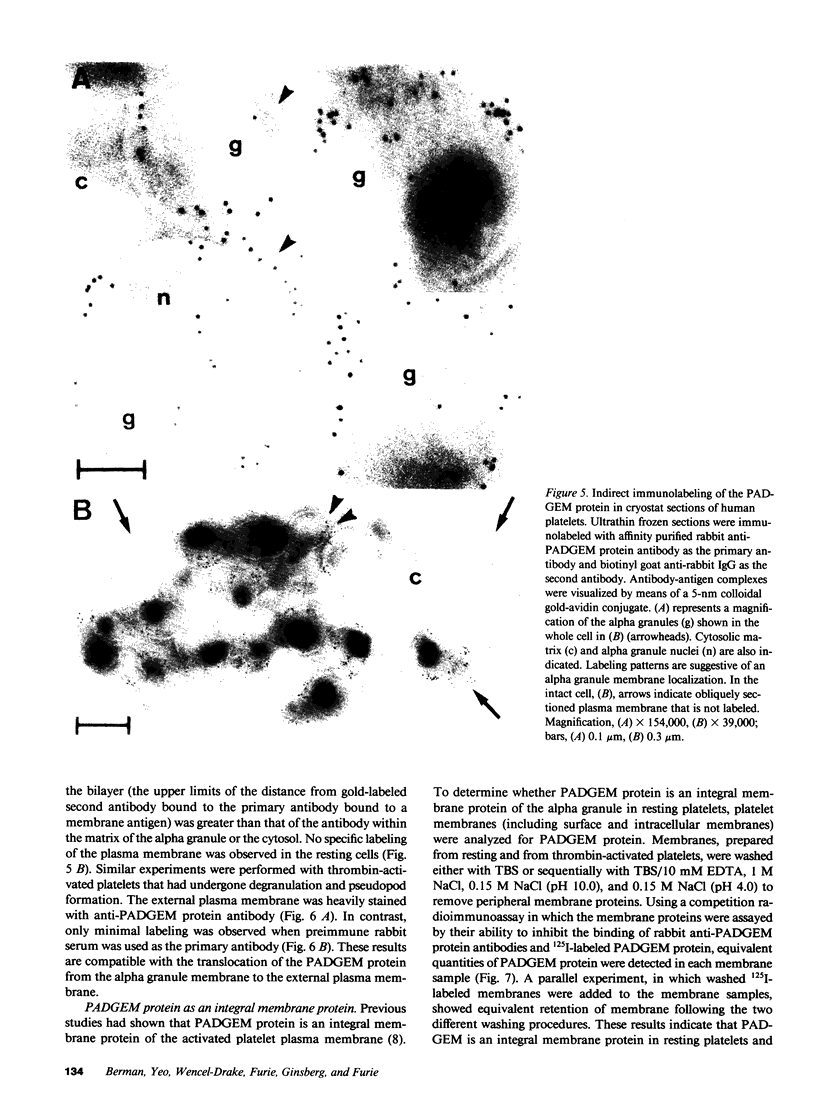
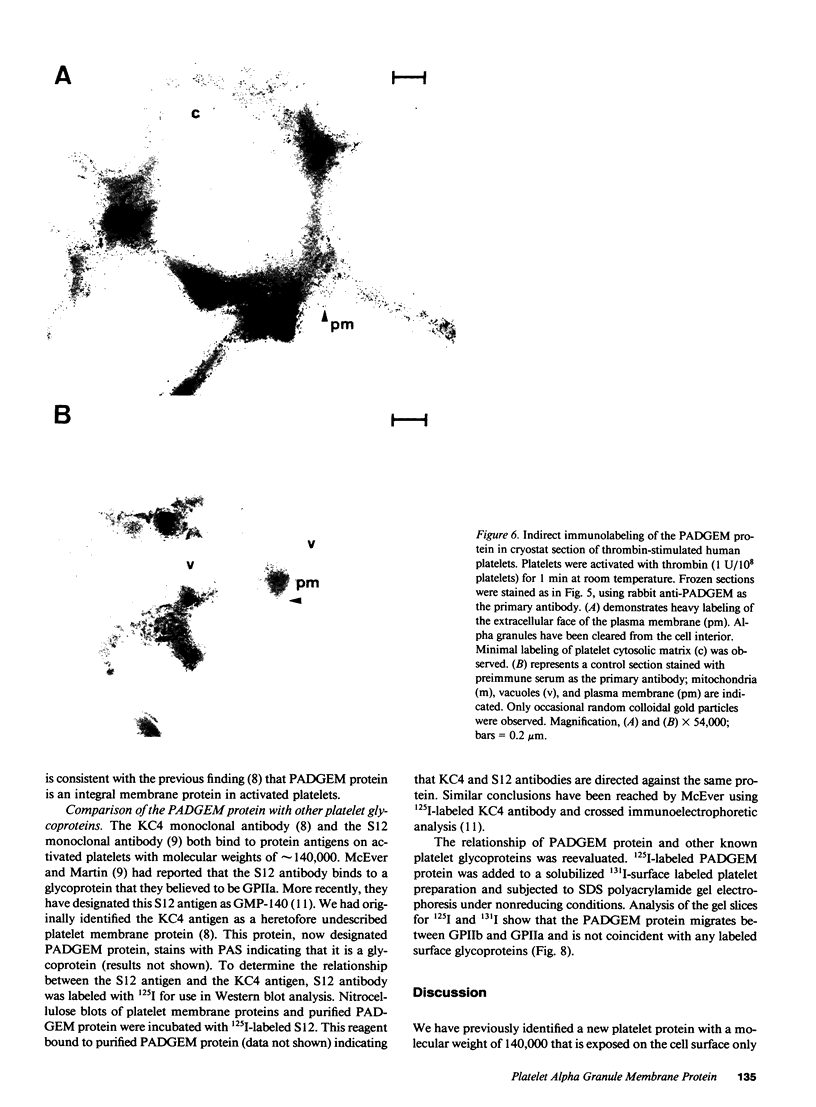
We have identified and purified a platelet integral membrane protein (140,000 mol wt), using the KC4 monoclonal antibody specific for activated platelets, that is internal in resting platelets but exposed on activated platelets (Hsu-Lin S.-C., C.L. Berman, B.C. Furie, D. August, and B. Furie, 1984, J. Biol. Chem. 259: 9121-9126.). The expression of the protein on the platelet surface is secretion-dependent. This protein has been named platelet activation-dependent granule-external membrane (PADGEM) protein. PADGEM protein is distinct from the surface glycoproteins of resting platelets, but identical to the S12 antigen, GMP-140. Using immunofluorescent staining, resting platelets failed to stain for PADGEM protein with the KC4 antibody, but after permeabilization showed a punctate staining of the cell interior. Thrombin-stimulated intact platelets stained with a peripheral rim pattern thus demonstrating the translocation of PADGEM protein from an internal location to the cell surface. PADGEM protein expression on the platelet surface at varying thrombin concentrations correlated with alpha granule release, as measured by the secretion of platelet factor 4. Further evidence for an alpha granule localization of PADGEM protein was provided by nitrogen cavitation of resting platelets followed by metrizamide density gradient centrifugation; PADGEM protein codistributed with platelet factor 4. Using immunoelectron microscopy, the protein was localized to the alpha granule in frozen ultrathin sections of resting platelets labeled using rabbit anti-PADGEM protein antibodies, whereas in thrombin-activated platelets, the plasma membrane was labeled. These studies indicate that PADGEM protein is a component of the alpha granule membrane of resting platelets and is incorporated into the plasma membrane upon activation and secretion.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Coller B. S. Interaction of normal, thrombasthenic, and Bernard-Soulier platelets with immobilized fibrinogen: defective platelet-fibrinogen interaction in thrombasthenia. Blood. 1980 Feb;55(2):169–178. [PubMed] [Google Scholar]
- Davis G. A., Bloom F. E. Subcellular particles separated through a histochemical reaction. Anal Biochem. 1973 Feb;51(2):429–435. doi: 10.1016/0003-2697(73)90496-x. [DOI] [PubMed] [Google Scholar]
- Dean G. E., Fishkes H., Nelson P. J., Rudnick G. The hydrogen ion-pumping adenosine triphosphatase of platelet dense granule membrane. Differences from F1F0- and phosphoenzyme-type ATPases. J Biol Chem. 1984 Aug 10;259(15):9569–9574. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gluck S., Cannon C., Al-Awqati Q. Exocytosis regulates urinary acidification in turtle bladder by rapid insertion of H+ pumps into the luminal membrane. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4327–4331. doi: 10.1073/pnas.79.14.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogstad G. O. A method for the isolation of alpha-granules from human platelets. Thromb Res. 1980 Dec 1;20(5-6):669–681. doi: 10.1016/0049-3848(80)90155-3. [DOI] [PubMed] [Google Scholar]
- Greenberg C. S., Shuman M. A. Specific binding of blood coagulation factor XIIIa to thrombin-stimulated platelets. J Biol Chem. 1984 Dec 10;259(23):14721–14727. [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J Cell Biol. 1971 Jul;50(1):135–158. doi: 10.1083/jcb.50.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lingg G., Fischer-Colbrie R., Schmidt W., Winkler H. Exposure of an antigen of chromaffin granules on cell surface during exocytosis. Nature. 1983 Feb 17;301(5901):610–611. doi: 10.1038/301610a0. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Miletich J. P. Relationships between platelets and coagulation factors in hemostasis. Annu Rev Med. 1978;29:41–49. doi: 10.1146/annurev.me.29.020178.000353. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Martin M. N. A monoclonal antibody to a membrane glycoprotein binds only to activated platelets. J Biol Chem. 1984 Aug 10;259(15):9799–9804. [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Properties of the factor Xa binding site on human platelets. J Biol Chem. 1978 Oct 10;253(19):6908–6916. [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg P. E., McEver R. P., Shuman M. A., Jacques Y. V., Bainton D. F. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985 Sep;101(3):880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg P. E., Shuman M. A., Levine S. P., Bainton D. F. Redistribution of alpha-granules and their contents in thrombin-stimulated platelets. J Cell Biol. 1984 Feb;98(2):748–760. doi: 10.1083/jcb.98.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons S., Hawiger J. Separation of human platelets from plasma proteins including factor VIII VWF by a combined albumin gradient-gel filtration method using HEPES buffer. Thromb Res. 1978 Feb;12(2):297–306. doi: 10.1016/0049-3848(78)90300-6. [DOI] [PubMed] [Google Scholar]
- Valdorf-Hansen J. F., Zucker M. B. Effect of temperature and inhibitors on serotonin-14C release from human platelets. Am J Physiol. 1971 Jan;220(1):105–111. doi: 10.1152/ajplegacy.1971.220.1.105. [DOI] [PubMed] [Google Scholar]
- Walsh P. N., Gagnatelli G. Platelet antiheparin activity: storage site and release mechanism. Blood. 1974 Aug;44(2):157–168. [PubMed] [Google Scholar]
- Wencel-Drake J. D., Painter R. G., Zimmerman T. S., Ginsberg M. H. Ultrastructural localization of human platelet thrombospondin, fibrinogen, fibronectin, and von Willebrand factor in frozen thin section. Blood. 1985 Apr;65(4):929–938. [PubMed] [Google Scholar]
- Wencel-Drake J. D., Plow E. F., Zimmerman T. S., Painter R. G., Ginsberg M. H. Immunofluorescent localization of adhesive glycoproteins in resting and thrombin-stimulated platelets. Am J Pathol. 1984 May;115(2):156–164. [PMC free article] [PubMed] [Google Scholar]
- Witte L. D., Kaplan K. L., Nossel H. L., Lages B. A., Weiss H. J., Goodman D. S. Studies of the release from human platelets of the growth factor for cultured human arterial smooth muscle cells. Circ Res. 1978 Mar;42(3):402–409. doi: 10.1161/01.res.42.3.402. [DOI] [PubMed] [Google Scholar]