Abstract
The title compound, C19H19NO10, was obtained from the reaction of α-d-1-bromo-2,3,4-tri-O-acetylxylose with N-hydroxyphthalimide in the presence of potassium carbonate. The asymmetric unit contains two independent molecules, in which the O—CH—O—N torsion angles are 73.0 (4) and 65.0 (4)°. The hexapyranosyl rings adopt chair conformations and the substituent groups are in equatorial positions. In the crystal, molecules are linked by nonclassical C—H⋯O hydrogen bonds.
Related literature
For related structures, see: Yang et al. (2004 ▶); Wang et al. (2008 ▶); Bai et al. (2008 ▶).
Experimental
Crystal data
C19H19NO10
M r = 421.35
Monoclinic,
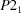
a = 11.722 (2) Å
b = 9.2270 (18) Å
c = 19.615 (4) Å
β = 104.52 (3)°
V = 2053.8 (7) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 295 K
0.40 × 0.30 × 0.20 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.957, T max = 0.978
4180 measured reflections
3977 independent reflections
2784 reflections with I > 2σ(I)
R int = 0.058
3 standard reflections every 200 reflections intensity decay: 1%
Refinement
R[F 2 > 2σ(F 2)] = 0.047
wR(F 2) = 0.131
S = 1.00
3977 reflections
541 parameters
6 restraints
H-atom parameters constrained
Δρmax = 0.24 e Å−3
Δρmin = −0.22 e Å−3
Data collection: CAD-4 EXPRESS (Enraf–Nonius, 1994 ▶); cell refinement: CAD-4 EXPRESS; data reduction: XCAD4 (Harms & Wocadlo, 1995 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812004369/rk2311sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812004369/rk2311Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11A⋯O15i | 0.98 | 2.42 | 3.339 (6) | 157 |
| C22—H22A⋯O1ii | 0.96 | 2.39 | 3.329 (7) | 165 |
| C26—H26A⋯O3iii | 0.98 | 2.54 | 3.385 (6) | 144 |
| C30—H30B⋯O5 | 0.97 | 2.56 | 3.429 (6) | 149 |
| C35—H35⋯O5iv | 0.93 | 2.54 | 3.294 (8) | 138 |
Symmetry codes: (i) 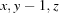 ; (ii)
; (ii) 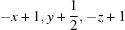 ; (iii)
; (iii) 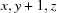 ; (iv)
; (iv) 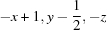 .
.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (grant No. 30711041) and the Fundamental Research Funds for the Central Universities (grant No. 1106020824)
supplementary crystallographic information
Comment
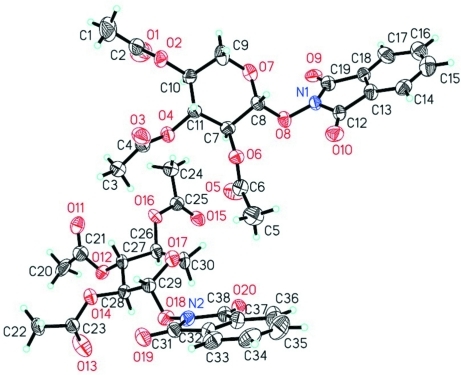
In the present work, the structure of 2,3,4-tri-O-acetyl-β-D-xyloside-N-hydroxyphthalimide, I, has been determined (Fig. 1). The asymmetric unit of I contains two independent molecules. The molecules are twisted at the CH–O bonds with the O7–C8–O8–N1 and O17–C29–O18–N2 torsion angles of 73.0 (4)° (molecule 1) and 65.0 (4)° (molecule 2), respectively. The bond lengths and angles in the title molecules show normal values. The hexapyranosyl ring adopts chair conformation (Fig. 1) and the substituented groups are individually planar and occupy equatorial positions (Yang et al., 2004; Wang et al., 2008; Bai et al., 2008).
Experimental
The solution of α-D-1-bromo-2,3,4-tri-O-acetyl-xylose (0.1 mol) and N-hydroxyphthalimide (0.1 mol) in chloroform (100 ml) and water (100 ml) was treated with sodium carbonate (0.1 mol) with triethyl benzyl ammonium chloride in present at room temperature overnight. The chloroform layer was separated, washed with water and allowed to evaporate slowly. The residual 2,3,4-tri-O-acetyl-β-D-xyloside-N- hydroxyphthalimide was then recrystallized to constant melting point (m.p. 455.6-456 K) from ethyl acetate. The purity of the compound was checked and characterized by NMR spectra. Fine block colourless crystals for X-ray diffraction were obtained by slow evaporation of an ethyl acetate at room temperature. 1H NMR, 500 MHz, CDCl3, δ: 8.15 (d, J = 9.6 Hz, 1H, Ar–H), 7.85 (d, J = 9.3 Hz, 1H, Ar–H), 5.82(d, J = 8.2 Hz, 1H, G–H), 5.25 (t, J = 9.9 Hz, 1H, G–H), 4.87(t, J = 9.6 Hz, 1H, G–H), 4.62 (t, J = 9.0 Hz, 1H, G–H), 3.80 (m, 2H, G–H), 2.14, 2.12, 2.09 (3s, COCH3).
Refinement
Hydrogen atoms were placed in calculated positions with appropriate riding models: C–H = 0.96Å for methyl H; C–H = 0.93Å for aryl H; C–H = 0.98Å for methine H and Uiso(H) = 1.2(1.5)Ueq(C). The atom C25 restrictive refinement by AFIX2 command.
Figures
Fig. 1.
The asymmetric unit of the title compound, showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms are presented as a small spheres of arbitrary radius.
Crystal data
| C19H19NO10 | F(000) = 880 |
| Mr = 421.35 | Dx = 1.363 Mg m−3 |
| Monoclinic, P21 | Melting point = 455.6–456 K |
| Hall symbol: P 2yb | Mo Kα radiation, λ = 0.71073 Å |
| a = 11.722 (2) Å | Cell parameters from 25 reflections |
| b = 9.2270 (18) Å | θ = 9–12° |
| c = 19.615 (4) Å | µ = 0.11 mm−1 |
| β = 104.52 (3)° | T = 295 K |
| V = 2053.8 (7) Å3 | Block, colourless |
| Z = 4 | 0.40 × 0.30 × 0.20 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | 2784 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.058 |
| Graphite monochromator | θmax = 25.3°, θmin = 1.1° |
| ω/2θ scans | h = −14→13 |
| Absorption correction: ψ scan (North et al., 1968) | k = 0→11 |
| Tmin = 0.957, Tmax = 0.978 | l = 0→23 |
| 4180 measured reflections | 3 standard reflections every 200 reflections |
| 3977 independent reflections | intensity decay: 1% |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.047 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.131 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0835P)2] where P = (Fo2 + 2Fc2)/3 |
| 3977 reflections | (Δ/σ)max < 0.001 |
| 541 parameters | Δρmax = 0.24 e Å−3 |
| 6 restraints | Δρmin = −0.22 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.7869 (7) | −0.0615 (11) | 0.5590 (4) | 0.122 (3) | |
| H1A | 0.7057 | −0.0752 | 0.5347 | 0.183* | |
| H1B | 0.7919 | −0.0293 | 0.6062 | 0.183* | |
| H1C | 0.8286 | −0.1514 | 0.5604 | 0.183* | |
| O1 | 0.8981 (5) | 0.1670 (8) | 0.5445 (3) | 0.134 (2) | |
| O2 | 0.8245 (3) | 0.0151 (4) | 0.45501 (15) | 0.0650 (9) | |
| O3 | 0.5499 (3) | 0.0723 (5) | 0.3498 (2) | 0.0833 (11) | |
| O4 | 0.6896 (3) | 0.2426 (3) | 0.36472 (17) | 0.0570 (8) | |
| O5 | 0.7255 (4) | 0.4574 (4) | 0.2203 (2) | 0.0873 (12) | |
| O6 | 0.7291 (3) | 0.2142 (3) | 0.22473 (15) | 0.0532 (7) | |
| O7 | 1.0122 (3) | 0.1240 (4) | 0.34194 (17) | 0.0668 (9) | |
| O8 | 0.9724 (3) | 0.2386 (3) | 0.23545 (16) | 0.0564 (8) | |
| O9 | 1.2187 (3) | 0.2686 (4) | 0.28619 (19) | 0.0749 (10) | |
| O10 | 0.9277 (3) | 0.0843 (5) | 0.10753 (18) | 0.0738 (10) | |
| N1 | 1.0553 (3) | 0.1711 (4) | 0.20799 (18) | 0.0505 (9) | |
| C2 | 0.8407 (6) | 0.0501 (10) | 0.5213 (3) | 0.098 (2) | |
| C3 | 0.5069 (4) | 0.3108 (7) | 0.3831 (3) | 0.0703 (15) | |
| H3A | 0.4296 | 0.2725 | 0.3798 | 0.105* | |
| H3B | 0.5018 | 0.3899 | 0.3507 | 0.105* | |
| H3C | 0.5402 | 0.3447 | 0.4302 | 0.105* | |
| C4 | 0.5824 (4) | 0.1965 (6) | 0.3658 (3) | 0.0603 (12) | |
| C5 | 0.6116 (6) | 0.3217 (9) | 0.1216 (3) | 0.097 (2) | |
| H5A | 0.5418 | 0.2725 | 0.1262 | 0.146* | |
| H5B | 0.6499 | 0.2650 | 0.0928 | 0.146* | |
| H5C | 0.5906 | 0.4146 | 0.0999 | 0.146* | |
| C6 | 0.6941 (4) | 0.3424 (6) | 0.1934 (3) | 0.0607 (12) | |
| C7 | 0.8168 (4) | 0.2182 (5) | 0.2902 (2) | 0.0500 (10) | |
| H7A | 0.8351 | 0.3192 | 0.3041 | 0.060* | |
| C8 | 0.9257 (4) | 0.1452 (5) | 0.2785 (2) | 0.0501 (10) | |
| H8A | 0.9045 | 0.0520 | 0.2546 | 0.060* | |
| C9 | 0.9693 (4) | 0.0343 (7) | 0.3882 (3) | 0.0712 (15) | |
| H9A | 1.0326 | 0.0122 | 0.4292 | 0.085* | |
| H9B | 0.9409 | −0.0561 | 0.3648 | 0.085* | |
| C10 | 0.8700 (4) | 0.1095 (6) | 0.4110 (2) | 0.0560 (12) | |
| H10A | 0.8986 | 0.1993 | 0.4363 | 0.067* | |
| C11 | 0.7720 (4) | 0.1427 (5) | 0.3462 (2) | 0.0491 (10) | |
| H11A | 0.7314 | 0.0530 | 0.3273 | 0.059* | |
| C12 | 1.0261 (4) | 0.1045 (6) | 0.1424 (2) | 0.0562 (12) | |
| C13 | 1.1421 (4) | 0.0666 (5) | 0.1293 (2) | 0.0569 (12) | |
| C14 | 1.1708 (5) | −0.0142 (6) | 0.0768 (3) | 0.0657 (14) | |
| H14 | 1.1125 | −0.0563 | 0.0412 | 0.079* | |
| C15 | 1.2878 (6) | −0.0308 (7) | 0.0784 (4) | 0.0845 (17) | |
| H15 | 1.3087 | −0.0842 | 0.0432 | 0.101* | |
| C16 | 1.3747 (5) | 0.0298 (7) | 0.1309 (3) | 0.0835 (17) | |
| H16 | 1.4533 | 0.0196 | 0.1302 | 0.100* | |
| C17 | 1.3452 (5) | 0.1074 (6) | 0.1859 (3) | 0.0793 (16) | |
| H17 | 1.4032 | 0.1463 | 0.2226 | 0.095* | |
| C18 | 1.2312 (4) | 0.1231 (5) | 0.1834 (2) | 0.0529 (11) | |
| C19 | 1.1734 (4) | 0.1982 (6) | 0.2336 (2) | 0.0564 (11) | |
| O11 | 0.4128 (4) | 0.7342 (6) | 0.4406 (2) | 0.0991 (14) | |
| O12 | 0.3423 (3) | 0.8357 (4) | 0.33808 (15) | 0.0564 (8) | |
| O13 | 0.0676 (3) | 0.8442 (5) | 0.2317 (3) | 0.0949 (14) | |
| O14 | 0.1691 (3) | 0.6402 (4) | 0.26372 (16) | 0.0538 (8) | |
| O15 | 0.7126 (3) | 0.7988 (4) | 0.29705 (18) | 0.0669 (9) | |
| O16 | 0.5774 (2) | 0.6731 (4) | 0.33357 (16) | 0.0566 (8) | |
| O17 | 0.4092 (3) | 0.5377 (3) | 0.19975 (17) | 0.0581 (8) | |
| O18 | 0.2382 (3) | 0.6188 (3) | 0.12131 (16) | 0.0591 (8) | |
| O19 | 0.0646 (3) | 0.4089 (4) | 0.07830 (19) | 0.0710 (10) | |
| O20 | 0.3982 (3) | 0.5904 (5) | 0.03530 (19) | 0.0799 (11) | |
| N2 | 0.2382 (4) | 0.5133 (5) | 0.0720 (2) | 0.0599 (10) | |
| C20 | 0.3261 (6) | 0.9705 (9) | 0.4350 (3) | 0.104 (2) | |
| H20A | 0.3465 | 0.9642 | 0.4854 | 0.156* | |
| H20B | 0.2427 | 0.9845 | 0.4180 | 0.156* | |
| H20C | 0.3669 | 1.0507 | 0.4207 | 0.156* | |
| C21 | 0.3611 (5) | 0.8328 (8) | 0.4048 (3) | 0.0790 (16) | |
| C22 | −0.0176 (4) | 0.6513 (7) | 0.2828 (3) | 0.0678 (14) | |
| H22A | 0.0112 | 0.6384 | 0.3327 | 0.102* | |
| H22B | −0.0351 | 0.5585 | 0.2606 | 0.102* | |
| H22C | −0.0877 | 0.7094 | 0.2732 | 0.102* | |
| C23 | 0.0733 (4) | 0.7248 (6) | 0.2550 (2) | 0.0569 (12) | |
| C24 | 0.7675 (5) | 0.6422 (8) | 0.3972 (3) | 0.0842 (17) | |
| H24A | 0.7657 | 0.5396 | 0.3891 | 0.126* | |
| H24B | 0.7434 | 0.6620 | 0.4396 | 0.126* | |
| H24C | 0.8462 | 0.6777 | 0.4021 | 0.126* | |
| C25 | 0.6864 (4) | 0.7153 (6) | 0.3371 (2) | 0.059 | |
| C26 | 0.4855 (4) | 0.7379 (5) | 0.2796 (2) | 0.0525 (11) | |
| H26A | 0.4995 | 0.8422 | 0.2770 | 0.063* | |
| C27 | 0.3697 (3) | 0.7111 (5) | 0.3015 (2) | 0.0496 (10) | |
| H27A | 0.3784 | 0.6260 | 0.3323 | 0.060* | |
| C28 | 0.2668 (4) | 0.6885 (5) | 0.2378 (2) | 0.0473 (10) | |
| H28A | 0.2473 | 0.7789 | 0.2112 | 0.057* | |
| C29 | 0.2899 (4) | 0.5686 (5) | 0.1910 (2) | 0.0517 (11) | |
| H29A | 0.2490 | 0.4806 | 0.1998 | 0.062* | |
| C30 | 0.4795 (4) | 0.6667 (6) | 0.2084 (2) | 0.0614 (13) | |
| H30A | 0.4458 | 0.7344 | 0.1709 | 0.074* | |
| H30B | 0.5585 | 0.6428 | 0.2051 | 0.074* | |
| C31 | 0.1439 (4) | 0.4156 (5) | 0.0513 (2) | 0.0523 (11) | |
| C32 | 0.1726 (4) | 0.3348 (5) | −0.0062 (2) | 0.0517 (11) | |
| C33 | 0.1103 (5) | 0.2213 (6) | −0.0463 (2) | 0.0698 (14) | |
| H33 | 0.0399 | 0.1875 | −0.0387 | 0.084* | |
| C34 | 0.1565 (6) | 0.1625 (7) | −0.0968 (3) | 0.0941 (19) | |
| H34 | 0.1193 | 0.0848 | −0.1235 | 0.113* | |
| C35 | 0.2655 (7) | 0.2222 (10) | −0.1092 (4) | 0.125 (3) | |
| H35 | 0.2941 | 0.1859 | −0.1459 | 0.149* | |
| C36 | 0.3239 (6) | 0.3267 (8) | −0.0691 (4) | 0.097 (2) | |
| H36 | 0.3956 | 0.3599 | −0.0752 | 0.117* | |
| C37 | 0.2751 (4) | 0.3856 (6) | −0.0175 (2) | 0.0610 (13) | |
| C38 | 0.3176 (4) | 0.5078 (6) | 0.0295 (2) | 0.0577 (12) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.140 (6) | 0.152 (8) | 0.090 (5) | −0.014 (6) | 0.062 (4) | 0.032 (5) |
| O1 | 0.155 (5) | 0.141 (5) | 0.104 (3) | −0.035 (4) | 0.029 (3) | −0.008 (4) |
| O2 | 0.082 (2) | 0.069 (2) | 0.0509 (19) | −0.0061 (19) | 0.0297 (16) | 0.0099 (17) |
| O3 | 0.073 (2) | 0.068 (3) | 0.115 (3) | −0.013 (2) | 0.034 (2) | −0.020 (2) |
| O4 | 0.0583 (18) | 0.0449 (18) | 0.0749 (19) | −0.0017 (15) | 0.0299 (15) | −0.0017 (16) |
| O5 | 0.095 (3) | 0.043 (2) | 0.111 (3) | 0.002 (2) | 0.001 (2) | 0.015 (2) |
| O6 | 0.0639 (18) | 0.0388 (17) | 0.0554 (17) | −0.0029 (15) | 0.0120 (14) | 0.0043 (15) |
| O7 | 0.0572 (18) | 0.070 (2) | 0.076 (2) | 0.0014 (18) | 0.0216 (16) | 0.017 (2) |
| O8 | 0.0640 (18) | 0.0413 (17) | 0.0710 (19) | 0.0000 (15) | 0.0302 (16) | 0.0071 (16) |
| O9 | 0.073 (2) | 0.073 (3) | 0.078 (2) | −0.014 (2) | 0.0159 (18) | −0.028 (2) |
| O10 | 0.062 (2) | 0.083 (3) | 0.070 (2) | −0.003 (2) | 0.0042 (18) | −0.005 (2) |
| N1 | 0.052 (2) | 0.052 (2) | 0.053 (2) | −0.0043 (18) | 0.0235 (16) | 0.0007 (18) |
| C2 | 0.095 (4) | 0.144 (7) | 0.057 (3) | −0.037 (5) | 0.022 (3) | −0.008 (4) |
| C3 | 0.063 (3) | 0.076 (4) | 0.073 (3) | −0.002 (3) | 0.018 (3) | −0.007 (3) |
| C4 | 0.056 (3) | 0.055 (3) | 0.071 (3) | −0.006 (2) | 0.019 (2) | −0.007 (3) |
| C5 | 0.093 (4) | 0.105 (5) | 0.084 (4) | 0.001 (4) | 0.002 (3) | 0.024 (4) |
| C6 | 0.063 (3) | 0.056 (3) | 0.062 (3) | 0.008 (3) | 0.013 (2) | 0.006 (3) |
| C7 | 0.065 (3) | 0.031 (2) | 0.055 (2) | −0.006 (2) | 0.016 (2) | 0.003 (2) |
| C8 | 0.053 (2) | 0.042 (2) | 0.057 (2) | 0.000 (2) | 0.016 (2) | 0.006 (2) |
| C9 | 0.066 (3) | 0.082 (4) | 0.070 (3) | 0.020 (3) | 0.026 (3) | 0.030 (3) |
| C10 | 0.061 (3) | 0.059 (3) | 0.049 (2) | −0.006 (2) | 0.016 (2) | 0.000 (2) |
| C11 | 0.052 (2) | 0.041 (2) | 0.055 (2) | 0.000 (2) | 0.015 (2) | −0.004 (2) |
| C12 | 0.065 (3) | 0.059 (3) | 0.048 (2) | −0.012 (2) | 0.020 (2) | 0.006 (2) |
| C13 | 0.070 (3) | 0.042 (3) | 0.062 (3) | 0.000 (2) | 0.024 (2) | 0.007 (2) |
| C14 | 0.094 (4) | 0.047 (3) | 0.057 (3) | 0.001 (3) | 0.021 (3) | −0.002 (2) |
| C15 | 0.114 (5) | 0.055 (3) | 0.098 (4) | 0.016 (3) | 0.053 (4) | −0.006 (3) |
| C16 | 0.077 (3) | 0.078 (4) | 0.105 (4) | 0.008 (3) | 0.039 (3) | −0.002 (4) |
| C17 | 0.076 (4) | 0.062 (4) | 0.104 (4) | −0.007 (3) | 0.029 (3) | −0.001 (3) |
| C18 | 0.062 (3) | 0.049 (3) | 0.049 (2) | −0.002 (2) | 0.018 (2) | 0.011 (2) |
| C19 | 0.063 (3) | 0.047 (3) | 0.060 (3) | 0.002 (2) | 0.018 (2) | 0.003 (2) |
| O11 | 0.124 (3) | 0.108 (4) | 0.066 (2) | 0.033 (3) | 0.025 (2) | 0.002 (3) |
| O12 | 0.0646 (18) | 0.0526 (19) | 0.0551 (19) | 0.0081 (16) | 0.0207 (15) | −0.0096 (16) |
| O13 | 0.072 (2) | 0.061 (3) | 0.159 (4) | 0.024 (2) | 0.042 (3) | 0.039 (3) |
| O14 | 0.0517 (17) | 0.0447 (17) | 0.0716 (19) | 0.0041 (15) | 0.0278 (15) | 0.0066 (16) |
| O15 | 0.066 (2) | 0.052 (2) | 0.089 (2) | −0.0079 (17) | 0.0298 (18) | 0.0012 (19) |
| O16 | 0.0430 (16) | 0.056 (2) | 0.074 (2) | −0.0074 (15) | 0.0196 (15) | 0.0050 (17) |
| O17 | 0.0638 (19) | 0.0404 (18) | 0.076 (2) | 0.0079 (15) | 0.0285 (16) | −0.0060 (16) |
| O18 | 0.078 (2) | 0.0381 (17) | 0.0626 (19) | −0.0034 (16) | 0.0193 (16) | −0.0046 (16) |
| O19 | 0.065 (2) | 0.067 (2) | 0.088 (2) | −0.0089 (18) | 0.0313 (19) | −0.001 (2) |
| O20 | 0.084 (2) | 0.080 (3) | 0.087 (2) | −0.024 (2) | 0.043 (2) | −0.014 (2) |
| N2 | 0.071 (3) | 0.052 (2) | 0.059 (2) | −0.008 (2) | 0.022 (2) | −0.015 (2) |
| C20 | 0.110 (5) | 0.128 (6) | 0.077 (4) | 0.025 (5) | 0.028 (4) | −0.047 (4) |
| C21 | 0.088 (4) | 0.086 (4) | 0.064 (4) | 0.010 (4) | 0.023 (3) | −0.007 (4) |
| C22 | 0.048 (2) | 0.092 (4) | 0.071 (3) | 0.002 (3) | 0.029 (2) | 0.008 (3) |
| C23 | 0.054 (3) | 0.061 (3) | 0.056 (3) | 0.003 (3) | 0.014 (2) | 0.002 (3) |
| C24 | 0.072 (3) | 0.099 (5) | 0.080 (3) | −0.015 (4) | 0.017 (3) | −0.012 (4) |
| C25 | 0.059 | 0.059 | 0.059 | 0.000 | 0.015 | 0.000 |
| C26 | 0.057 (2) | 0.039 (2) | 0.071 (3) | 0.004 (2) | 0.032 (2) | 0.002 (2) |
| C27 | 0.053 (2) | 0.040 (2) | 0.064 (3) | −0.001 (2) | 0.028 (2) | −0.002 (2) |
| C28 | 0.058 (2) | 0.035 (2) | 0.054 (2) | −0.003 (2) | 0.024 (2) | 0.0039 (19) |
| C29 | 0.061 (3) | 0.033 (2) | 0.065 (3) | −0.007 (2) | 0.024 (2) | −0.005 (2) |
| C30 | 0.062 (3) | 0.064 (3) | 0.071 (3) | 0.007 (3) | 0.040 (2) | 0.003 (3) |
| C31 | 0.052 (2) | 0.049 (3) | 0.058 (3) | −0.003 (2) | 0.018 (2) | 0.001 (2) |
| C32 | 0.064 (3) | 0.049 (3) | 0.045 (2) | 0.005 (2) | 0.018 (2) | 0.002 (2) |
| C33 | 0.098 (4) | 0.048 (3) | 0.059 (3) | −0.003 (3) | 0.010 (3) | −0.002 (3) |
| C34 | 0.140 (5) | 0.063 (4) | 0.074 (4) | 0.005 (4) | 0.015 (4) | −0.023 (3) |
| C35 | 0.166 (7) | 0.119 (7) | 0.107 (5) | 0.018 (6) | 0.068 (5) | −0.046 (5) |
| C36 | 0.121 (5) | 0.083 (5) | 0.110 (5) | −0.007 (4) | 0.072 (4) | −0.017 (4) |
| C37 | 0.064 (3) | 0.066 (3) | 0.057 (3) | 0.007 (3) | 0.022 (2) | 0.005 (3) |
| C38 | 0.057 (3) | 0.061 (3) | 0.059 (3) | 0.001 (3) | 0.022 (2) | 0.000 (3) |
Geometric parameters (Å, º)
| C1—C2 | 1.497 (10) | O11—C21 | 1.214 (7) |
| C1—H1A | 0.9600 | O12—C21 | 1.272 (6) |
| C1—H1B | 0.9600 | O12—C27 | 1.433 (5) |
| C1—H1C | 0.9600 | O13—C23 | 1.188 (6) |
| O1—C2 | 1.292 (10) | O14—C23 | 1.343 (6) |
| O2—C2 | 1.307 (7) | O14—C28 | 1.435 (5) |
| O2—C10 | 1.421 (5) | O15—C25 | 1.194 (6) |
| O3—C4 | 1.224 (6) | O16—C25 | 1.321 (6) |
| O4—C4 | 1.332 (6) | O16—C26 | 1.437 (5) |
| O4—C11 | 1.446 (5) | O17—C29 | 1.395 (5) |
| O5—C6 | 1.201 (6) | O17—C30 | 1.433 (6) |
| O6—C6 | 1.349 (6) | O18—N2 | 1.373 (5) |
| O6—C7 | 1.430 (5) | O18—C29 | 1.426 (5) |
| O7—C8 | 1.408 (5) | O19—C31 | 1.180 (5) |
| O7—C9 | 1.410 (6) | O20—C38 | 1.198 (6) |
| O8—N1 | 1.373 (4) | N2—C38 | 1.397 (6) |
| O8—C8 | 1.410 (5) | N2—C31 | 1.405 (6) |
| O9—C19 | 1.222 (6) | C20—C21 | 1.501 (9) |
| O10—C12 | 1.199 (5) | C20—H20A | 0.9600 |
| N1—C19 | 1.372 (6) | C20—H20B | 0.9600 |
| N1—C12 | 1.388 (6) | C20—H20C | 0.9600 |
| C3—C4 | 1.471 (7) | C22—C23 | 1.477 (7) |
| C3—H3A | 0.9600 | C22—H22A | 0.9600 |
| C3—H3B | 0.9600 | C22—H22B | 0.9600 |
| C3—H3C | 0.9600 | C22—H22C | 0.9600 |
| C5—C6 | 1.508 (8) | C24—C25 | 1.479 (8) |
| C5—H5A | 0.9600 | C24—H24A | 0.9600 |
| C5—H5B | 0.9600 | C24—H24B | 0.9600 |
| C5—H5C | 0.9600 | C24—H24C | 0.9600 |
| C7—C11 | 1.502 (6) | C26—C30 | 1.530 (7) |
| C7—C8 | 1.511 (6) | C26—C27 | 1.542 (5) |
| C7—H7A | 0.9800 | C26—H26A | 0.9800 |
| C8—H8A | 0.9800 | C27—C28 | 1.517 (6) |
| C9—C10 | 1.516 (6) | C27—H27A | 0.9800 |
| C9—H9A | 0.9700 | C28—C29 | 1.506 (6) |
| C9—H9B | 0.9700 | C28—H28A | 0.9800 |
| C10—C11 | 1.516 (6) | C29—H29A | 0.9800 |
| C10—H10A | 0.9800 | C30—H30A | 0.9700 |
| C11—H11A | 0.9800 | C30—H30B | 0.9700 |
| C12—C13 | 1.488 (7) | C31—C32 | 1.461 (6) |
| C13—C14 | 1.381 (7) | C32—C37 | 1.359 (6) |
| C13—C18 | 1.390 (6) | C32—C33 | 1.401 (6) |
| C14—C15 | 1.372 (8) | C33—C34 | 1.355 (8) |
| C14—H14 | 0.9300 | C33—H33 | 0.9300 |
| C15—C16 | 1.373 (9) | C34—C35 | 1.466 (5) |
| C15—H15 | 0.9300 | C34—H34 | 0.9300 |
| C16—C17 | 1.407 (8) | C35—C36 | 1.321 (9) |
| C16—H16 | 0.9300 | C35—H35 | 0.9300 |
| C17—C18 | 1.334 (7) | C36—C37 | 1.392 (7) |
| C17—H17 | 0.9300 | C36—H36 | 0.9300 |
| C18—C19 | 1.497 (7) | C37—C38 | 1.463 (7) |
| C2—C1—H1A | 109.5 | C21—O12—C27 | 119.7 (4) |
| C2—C1—H1B | 109.5 | C23—O14—C28 | 119.4 (4) |
| H1A—C1—H1B | 109.5 | C25—O16—C26 | 116.9 (4) |
| C2—C1—H1C | 109.5 | C29—O17—C30 | 111.9 (3) |
| H1A—C1—H1C | 109.5 | N2—O18—C29 | 111.7 (3) |
| H1B—C1—H1C | 109.5 | O18—N2—C38 | 124.3 (4) |
| C2—O2—C10 | 118.0 (5) | O18—N2—C31 | 121.3 (4) |
| C4—O4—C11 | 119.5 (4) | C38—N2—C31 | 113.5 (4) |
| C6—O6—C7 | 117.1 (4) | C21—C20—H20A | 109.5 |
| C8—O7—C9 | 110.9 (3) | C21—C20—H20B | 109.5 |
| N1—O8—C8 | 112.1 (3) | H20A—C20—H20B | 109.5 |
| C19—N1—O8 | 121.5 (4) | C21—C20—H20C | 109.5 |
| C19—N1—C12 | 114.3 (4) | H20A—C20—H20C | 109.5 |
| O8—N1—C12 | 122.0 (4) | H20B—C20—H20C | 109.5 |
| O1—C2—O2 | 119.3 (6) | O11—C21—O12 | 122.9 (6) |
| O1—C2—C1 | 130.3 (6) | O11—C21—C20 | 123.5 (6) |
| O2—C2—C1 | 110.3 (7) | O12—C21—C20 | 113.2 (6) |
| C4—C3—H3A | 109.5 | C23—C22—H22A | 109.5 |
| C4—C3—H3B | 109.5 | C23—C22—H22B | 109.5 |
| H3A—C3—H3B | 109.5 | H22A—C22—H22B | 109.5 |
| C4—C3—H3C | 109.5 | C23—C22—H22C | 109.5 |
| H3A—C3—H3C | 109.5 | H22A—C22—H22C | 109.5 |
| H3B—C3—H3C | 109.5 | H22B—C22—H22C | 109.5 |
| O3—C4—O4 | 122.0 (5) | O13—C23—O14 | 123.5 (5) |
| O3—C4—C3 | 124.3 (5) | O13—C23—C22 | 126.3 (5) |
| O4—C4—C3 | 113.6 (5) | O14—C23—C22 | 110.1 (5) |
| C6—C5—H5A | 109.5 | C25—C24—H24A | 109.5 |
| C6—C5—H5B | 109.5 | C25—C24—H24B | 109.5 |
| H5A—C5—H5B | 109.5 | H24A—C24—H24B | 109.5 |
| C6—C5—H5C | 109.5 | C25—C24—H24C | 109.5 |
| H5A—C5—H5C | 109.5 | H24A—C24—H24C | 109.5 |
| H5B—C5—H5C | 109.5 | H24B—C24—H24C | 109.5 |
| O5—C6—O6 | 123.3 (4) | O15—C25—O16 | 124.1 (4) |
| O5—C6—C5 | 125.2 (5) | O15—C25—C24 | 126.8 (5) |
| O6—C6—C5 | 111.4 (5) | O16—C25—C24 | 109.1 (4) |
| O6—C7—C11 | 109.9 (3) | O16—C26—C30 | 110.4 (4) |
| O6—C7—C8 | 107.2 (3) | O16—C26—C27 | 106.2 (3) |
| C11—C7—C8 | 111.6 (4) | C30—C26—C27 | 110.1 (4) |
| O6—C7—H7A | 109.4 | O16—C26—H26A | 110.1 |
| C11—C7—H7A | 109.4 | C30—C26—H26A | 110.1 |
| C8—C7—H7A | 109.4 | C27—C26—H26A | 110.1 |
| O7—C8—O8 | 108.2 (3) | O12—C27—C28 | 106.9 (3) |
| O7—C8—C7 | 112.1 (3) | O12—C27—C26 | 109.5 (4) |
| O8—C8—C7 | 106.7 (3) | C28—C27—C26 | 111.5 (3) |
| O7—C8—H8A | 109.9 | O12—C27—H27A | 109.6 |
| O8—C8—H8A | 109.9 | C28—C27—H27A | 109.6 |
| C7—C8—H8A | 109.9 | C26—C27—H27A | 109.6 |
| O7—C9—C10 | 110.2 (4) | O14—C28—C29 | 105.6 (3) |
| O7—C9—H9A | 109.6 | O14—C28—C27 | 107.0 (3) |
| C10—C9—H9A | 109.6 | C29—C28—C27 | 112.1 (4) |
| O7—C9—H9B | 109.6 | O14—C28—H28A | 110.7 |
| C10—C9—H9B | 109.6 | C29—C28—H28A | 110.7 |
| H9A—C9—H9B | 108.1 | C27—C28—H28A | 110.7 |
| O2—C10—C11 | 108.2 (4) | O17—C29—O18 | 110.9 (3) |
| O2—C10—C9 | 109.4 (4) | O17—C29—C28 | 113.8 (4) |
| C11—C10—C9 | 108.7 (4) | O18—C29—C28 | 104.3 (4) |
| O2—C10—H10A | 110.2 | O17—C29—H29A | 109.2 |
| C11—C10—H10A | 110.2 | O18—C29—H29A | 109.2 |
| C9—C10—H10A | 110.2 | C28—C29—H29A | 109.2 |
| O4—C11—C7 | 105.4 (4) | O17—C30—C26 | 111.0 (3) |
| O4—C11—C10 | 109.3 (3) | O17—C30—H30A | 109.4 |
| C7—C11—C10 | 112.2 (4) | C26—C30—H30A | 109.4 |
| O4—C11—H11A | 109.9 | O17—C30—H30B | 109.4 |
| C7—C11—H11A | 109.9 | C26—C30—H30B | 109.4 |
| C10—C11—H11A | 109.9 | H30A—C30—H30B | 108.0 |
| O10—C12—N1 | 125.2 (4) | O19—C31—N2 | 123.8 (5) |
| O10—C12—C13 | 130.9 (4) | O19—C31—C32 | 132.8 (5) |
| N1—C12—C13 | 103.9 (4) | N2—C31—C32 | 103.4 (4) |
| C14—C13—C18 | 119.7 (5) | C37—C32—C33 | 121.6 (4) |
| C14—C13—C12 | 131.3 (5) | C37—C32—C31 | 109.6 (4) |
| C18—C13—C12 | 108.9 (4) | C33—C32—C31 | 128.9 (4) |
| C15—C14—C13 | 118.1 (5) | C34—C33—C32 | 117.6 (5) |
| C15—C14—H14 | 120.9 | C34—C33—H33 | 121.2 |
| C13—C14—H14 | 120.9 | C32—C33—H33 | 121.2 |
| C14—C15—C16 | 121.4 (5) | C33—C34—C35 | 119.6 (5) |
| C14—C15—H15 | 119.3 | C33—C34—H34 | 120.2 |
| C16—C15—H15 | 119.3 | C35—C34—H34 | 120.2 |
| C15—C16—C17 | 120.2 (5) | C36—C35—C34 | 121.3 (6) |
| C15—C16—H16 | 119.9 | C36—C35—H35 | 119.4 |
| C17—C16—H16 | 119.9 | C34—C35—H35 | 119.4 |
| C18—C17—C16 | 117.7 (6) | C35—C36—C37 | 118.1 (6) |
| C18—C17—H17 | 121.2 | C35—C36—H36 | 120.9 |
| C16—C17—H17 | 121.2 | C37—C36—H36 | 120.9 |
| C17—C18—C13 | 122.7 (5) | C32—C37—C36 | 121.7 (5) |
| C17—C18—C19 | 129.9 (5) | C32—C37—C38 | 109.7 (4) |
| C13—C18—C19 | 107.4 (4) | C36—C37—C38 | 128.5 (5) |
| O9—C19—N1 | 126.2 (4) | O20—C38—N2 | 123.3 (5) |
| O9—C19—C18 | 129.0 (4) | O20—C38—C37 | 133.3 (4) |
| N1—C19—C18 | 104.8 (4) | N2—C38—C37 | 103.4 (4) |
| C8—O8—N1—C19 | −104.9 (4) | C29—O18—N2—C38 | −102.4 (5) |
| C8—O8—N1—C12 | 93.0 (4) | C29—O18—N2—C31 | 89.3 (5) |
| C10—O2—C2—O1 | −0.8 (9) | C27—O12—C21—O11 | 7.8 (9) |
| C10—O2—C2—C1 | 179.9 (5) | C27—O12—C21—C20 | −179.1 (5) |
| C11—O4—C4—O3 | −1.9 (7) | C28—O14—C23—O13 | −6.3 (7) |
| C11—O4—C4—C3 | −178.0 (4) | C28—O14—C23—C22 | 177.7 (4) |
| C7—O6—C6—O5 | −6.3 (7) | C26—O16—C25—O15 | −2.8 (7) |
| C7—O6—C6—C5 | 173.9 (4) | C26—O16—C25—C24 | 177.5 (4) |
| C6—O6—C7—C11 | 123.6 (4) | C25—O16—C26—C30 | 78.9 (5) |
| C6—O6—C7—C8 | −114.9 (4) | C25—O16—C26—C27 | −161.8 (4) |
| C9—O7—C8—O8 | 178.3 (4) | C21—O12—C27—C28 | 135.9 (5) |
| C9—O7—C8—C7 | 60.9 (5) | C21—O12—C27—C26 | −103.2 (5) |
| N1—O8—C8—O7 | 73.0 (4) | O16—C26—C27—O12 | 95.9 (4) |
| N1—O8—C8—C7 | −166.2 (3) | C30—C26—C27—O12 | −144.7 (4) |
| O6—C7—C8—O7 | −171.4 (3) | O16—C26—C27—C28 | −146.0 (4) |
| C11—C7—C8—O7 | −51.0 (5) | C30—C26—C27—C28 | −26.6 (5) |
| O6—C7—C8—O8 | 70.3 (4) | C23—O14—C28—C29 | −125.7 (4) |
| C11—C7—C8—O8 | −169.3 (3) | C23—O14—C28—C27 | 114.7 (4) |
| C8—O7—C9—C10 | −65.7 (5) | O12—C27—C28—O14 | −70.1 (4) |
| C2—O2—C10—C11 | −129.1 (5) | C26—C27—C28—O14 | 170.2 (4) |
| C2—O2—C10—C9 | 112.6 (6) | O12—C27—C28—C29 | 174.6 (3) |
| O7—C9—C10—O2 | 177.4 (4) | C26—C27—C28—C29 | 54.9 (5) |
| O7—C9—C10—C11 | 59.4 (6) | C30—O17—C29—O18 | 76.9 (4) |
| C4—O4—C11—C7 | 129.4 (4) | C30—O17—C29—C28 | −40.3 (5) |
| C4—O4—C11—C10 | −109.8 (5) | N2—O18—C29—O17 | 65.0 (4) |
| O6—C7—C11—O4 | −75.7 (4) | N2—O18—C29—C28 | −172.1 (3) |
| C8—C7—C11—O4 | 165.5 (3) | O14—C28—C29—O17 | −137.4 (4) |
| O6—C7—C11—C10 | 165.4 (4) | C27—C28—C29—O17 | −21.3 (5) |
| C8—C7—C11—C10 | 46.6 (5) | O14—C28—C29—O18 | 101.6 (4) |
| O2—C10—C11—O4 | 74.4 (5) | C27—C28—C29—O18 | −142.3 (3) |
| C9—C10—C11—O4 | −166.9 (4) | C29—O17—C30—C26 | 70.2 (5) |
| O2—C10—C11—C7 | −169.0 (4) | O16—C26—C30—O17 | 84.7 (4) |
| C9—C10—C11—C7 | −50.3 (5) | C27—C26—C30—O17 | −32.2 (5) |
| C19—N1—C12—O10 | −172.2 (5) | O18—N2—C31—O19 | −6.6 (7) |
| O8—N1—C12—O10 | −8.9 (7) | C38—N2—C31—O19 | −176.1 (5) |
| C19—N1—C12—C13 | 8.5 (5) | O18—N2—C31—C32 | 174.0 (4) |
| O8—N1—C12—C13 | 171.7 (4) | C38—N2—C31—C32 | 4.4 (5) |
| O10—C12—C13—C14 | −6.9 (9) | O19—C31—C32—C37 | 179.7 (5) |
| N1—C12—C13—C14 | 172.3 (5) | N2—C31—C32—C37 | −0.9 (5) |
| O10—C12—C13—C18 | 175.5 (5) | O19—C31—C32—C33 | −0.3 (9) |
| N1—C12—C13—C18 | −5.2 (5) | N2—C31—C32—C33 | 179.0 (5) |
| C18—C13—C14—C15 | −2.7 (7) | C37—C32—C33—C34 | 0.6 (7) |
| C12—C13—C14—C15 | 179.9 (5) | C31—C32—C33—C34 | −179.4 (5) |
| C13—C14—C15—C16 | 0.6 (9) | C32—C33—C34—C35 | −2.2 (9) |
| C14—C15—C16—C17 | 1.9 (10) | C33—C34—C35—C36 | 4.2 (12) |
| C15—C16—C17—C18 | −2.1 (9) | C34—C35—C36—C37 | −4.2 (12) |
| C16—C17—C18—C13 | −0.1 (8) | C33—C32—C37—C36 | −0.6 (8) |
| C16—C17—C18—C19 | 179.6 (5) | C31—C32—C37—C36 | 179.3 (5) |
| C14—C13—C18—C17 | 2.5 (8) | C33—C32—C37—C38 | 177.4 (4) |
| C12—C13—C18—C17 | −179.6 (5) | C31—C32—C37—C38 | −2.7 (6) |
| C14—C13—C18—C19 | −177.2 (4) | C35—C36—C37—C32 | 2.5 (10) |
| C12—C13—C18—C19 | 0.6 (5) | C35—C36—C37—C38 | −175.1 (6) |
| O8—N1—C19—O9 | 8.5 (7) | O18—N2—C38—O20 | 4.7 (8) |
| C12—N1—C19—O9 | 171.9 (5) | C31—N2—C38—O20 | 173.9 (5) |
| O8—N1—C19—C18 | −171.5 (4) | O18—N2—C38—C37 | −175.1 (4) |
| C12—N1—C19—C18 | −8.2 (5) | C31—N2—C38—C37 | −5.9 (5) |
| C17—C18—C19—O9 | 4.5 (9) | C32—C37—C38—O20 | −174.7 (6) |
| C13—C18—C19—O9 | −175.8 (5) | C36—C37—C38—O20 | 3.1 (10) |
| C17—C18—C19—N1 | −175.5 (5) | C32—C37—C38—N2 | 5.1 (5) |
| C13—C18—C19—N1 | 4.2 (5) | C36—C37—C38—N2 | −177.1 (6) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C7—H7A···O5 | 0.98 | 2.22 | 2.676 (6) | 107 |
| C10—H10A···O1 | 0.98 | 2.14 | 2.609 (7) | 107 |
| C11—H11A···O3 | 0.98 | 2.29 | 2.702 (6) | 104 |
| C11—H11A···O15i | 0.98 | 2.42 | 3.339 (6) | 157 |
| C22—H22A···O1ii | 0.96 | 2.39 | 3.329 (7) | 165 |
| C26—H26A···O3iii | 0.98 | 2.54 | 3.385 (6) | 144 |
| C27—H27A···O11 | 0.98 | 2.29 | 2.656 (6) | 101 |
| C28—H28A···O13 | 0.98 | 2.32 | 2.718 (6) | 103 |
| C30—H30B···O5 | 0.97 | 2.56 | 3.429 (6) | 149 |
| C35—H35···O5iv | 0.93 | 2.54 | 3.294 (8) | 138 |
Symmetry codes: (i) x, y−1, z; (ii) −x+1, y+1/2, −z+1; (iii) x, y+1, z; (iv) −x+1, y−1/2, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: RK2311).
References
- Bai, L., Wang, X. & Cai, B. (2008). Acta Cryst. E64, o1623. [DOI] [PMC free article] [PubMed]
- Enraf–Nonius (1994). CAD-4 EXPRESS Enraf–Nonius, Delft, The Netherlands.
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wang, X., Li, X., Yin, Y., Pang, Y. & Yang, Y. (2008). Acta Cryst. E64, o669. [DOI] [PMC free article] [PubMed]
- Yang, B., Zhang, S.-S., Wang, Y.-F., Li, X.-M., Jiao, K., Kassim, M. & Yamin, B. M. (2004). Acta Cryst. E60, o1902–o1904.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812004369/rk2311sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812004369/rk2311Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report