Abstract
A squamous cell carcinoma of 33-yr-old patient who developed marked leukocytosis and hypercalcemia was transplanted into nude mice in which more marked leukocytosis and hypercalcemia also developed. This tumor (LJC-1-JCK) produced a colony-stimulating factor (CSF) and formed a cyst in the tumor from which a CSF-producing cell line (T3M-1) was established. The CSF causes predominantly formation of granulocytic colonies in addition to macrophage colonies. Bone-resorbing activity (BRA) was detected in the cystic fluid and was eluted as two separate peaks with proteins of an apparent molecular weight of 30,000-50,000 and 10,000-20,000. Colony-stimulating activity (CSA) was eluted at an apparent 30,000 mol wt. The conditioned medium of the T3M-1 cells also contained a BRA with an apparent 14,000 mol wt, whereas CSA eluted at an apparent 30,000 mol wt. PTH, epidermal growth factor, transforming growth factor-alpha, prostaglandin Es, and vitamin D could not account for the powerful BRA. In contrast to CSA, BRA was not inactivated by trypsin and more stable at 70 degrees C. When T3M-1 cells were transplanted into nude mice, marked hypercalcemia developed in addition to granulocytosis. Our findings suggest that the tumor produces and secretes a powerful BRA in vivo and in vitro, which is different from CSA in terms of molecular weight, heat stability, and trypsin treatment. We speculate that the synergistic action of CSF that stimulates macrophage colony formation and recruits osteoclast precursors, and BRA, which stimulates mononuclear phagocytes and/or osteoclasts were responsible for a marked increase in osteoclastic bone resorption and humoral hypercalcemia in the patient.
Full text
PDF



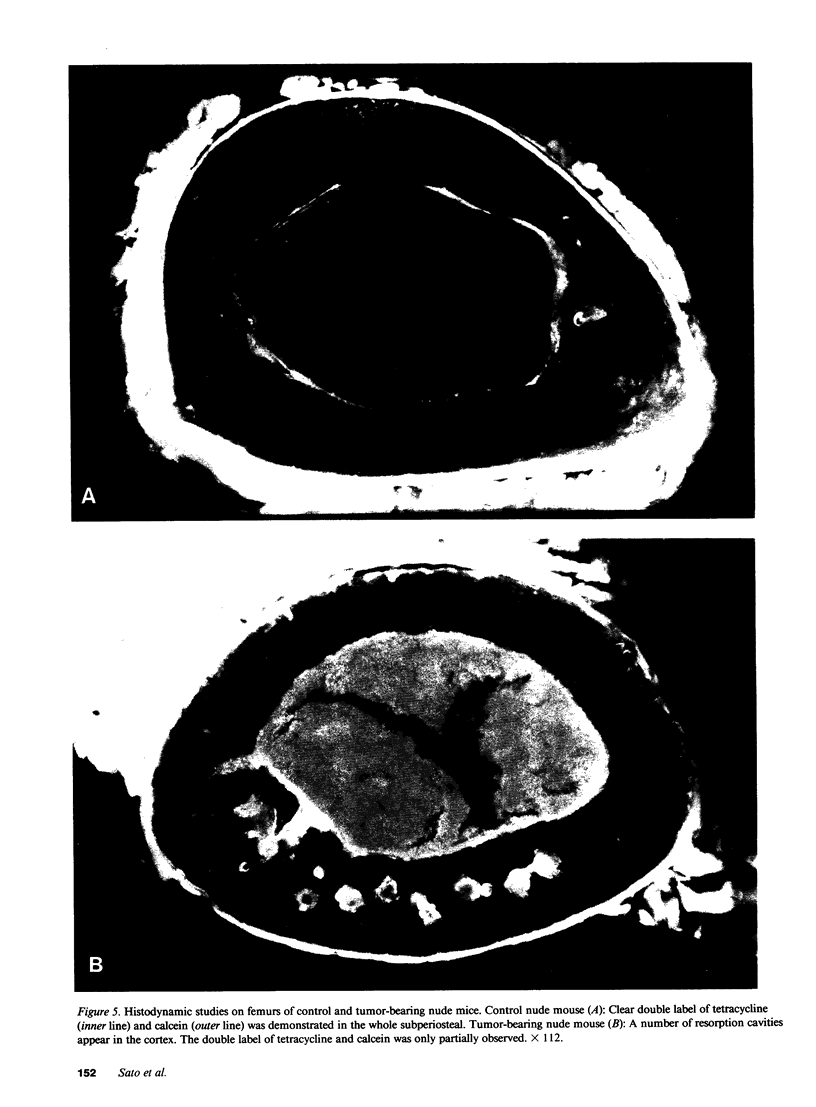


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Stathakos D., Scher C. D. Isolation of a cationic polypeptide from human serum that stimulates proliferation of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2635–2639. doi: 10.1073/pnas.72.7.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash P., Loutit J. F., Townsend K. M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980 Feb 14;283(5748):669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- Block N. L., Whitmore W. F., Jr Leukemoid reaction, thrombocytosis and hypercalcemia associated with bladder cancer. J Urol. 1973 Dec;110(6):660–663. doi: 10.1016/s0022-5347(17)60308-2. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., McGuire M. K., Russell R. G. An interleukin 1 like factor stimulates bone resorption in vitro. Nature. 1983 Nov 24;306(5941):378–380. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- Hocking W., Goodman J., Golde D. Granulocytosis associated with tumor cell production of colony-stimulating activity. Blood. 1983 Mar;61(3):600–603. [PubMed] [Google Scholar]
- Ibbotson K. J., D'Souza S. M., Ng K. W., Osborne C. K., Niall M., Martin T. J., Mundy G. R. Tumor-derived growth factor increases bone resorption in a tumor associated with humoral hypercalcemia of malignancy. Science. 1983 Sep 23;221(4617):1292–1294. doi: 10.1126/science.6577602. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Twardzik D. R., D'Souza S. M., Hargreaves W. R., Todaro G. J., Mundy G. R. Stimulation of bone resorption in vitro by synthetic transforming growth factor-alpha. Science. 1985 May 24;228(4702):1007–1009. doi: 10.1126/science.3859011. [DOI] [PubMed] [Google Scholar]
- Kakiuchi T., Chesnut R. W., Grey H. M. B cells as antigen-presenting cells: the requirement for B cell activation. J Immunol. 1983 Jul;131(1):109–114. [PubMed] [Google Scholar]
- Kimura N., Shibuya T., Niho Y., Nakamura H., Matsuo S., Imamura T., Miyamoto N., Yanase T. Human lung cancer cell line (KSNY) producing colony-stimulating activity which affects both human and mouse marrow cells. Gan. 1979 Dec;70(6):807–810. [PubMed] [Google Scholar]
- Kondo Y., Sato K., Ohkawa H., Ueyama Y., Okabe T., Sato N., Asano S., Mori M., Ohsawa N., Kosaka K. Association of hypercalcemia with tumors producing colony-stimulating factor(s). Cancer Res. 1983 May;43(5):2368–2374. [PubMed] [Google Scholar]
- Lee M. Y., Baylink D. J. Hypercalcemia, excessive bone resorption, and neutrophilia in mice bearing a mammary carcinoma. Proc Soc Exp Biol Med. 1983 Apr;172(4):424–429. doi: 10.3181/00379727-172-41582. [DOI] [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- Loutit J. F., Nisbet N. W. The origin of osteoclasts. Immunobiology. 1982 Apr;161(3-4):193–203. doi: 10.1016/S0171-2985(82)80074-0. [DOI] [PubMed] [Google Scholar]
- Minkin C., Fredericks R. S., Pokress S., Rude R. K., Sharp C. F., Jr, Tong M., Singer F. R. Bone resorption and humoral hypercalcemia of malignancy: stimulation of bone resorption in vitro by tumor extracts is inhibited by prostaglandin synthesis inhibitors. J Clin Endocrinol Metab. 1981 Nov;53(5):941–947. doi: 10.1210/jcem-53-5-941. [DOI] [PubMed] [Google Scholar]
- Moore R. N., Oppenheim J. J., Farrar J. J., Carter C. S., Jr, Waheed A., Shadduck R. K. Production of lymphocyte-activating factor (Interleukin 1) by macrophages activated with colony-stimulating factors. J Immunol. 1980 Sep;125(3):1302–1305. [PubMed] [Google Scholar]
- Mundy G. R., Ibbotson K. J., D'Souza S. M., Simpson E. L., Jacobs J. W., Martin T. J. The hypercalcemia of cancer. Clinical implications and pathogenic mechanisms. N Engl J Med. 1984 Jun 28;310(26):1718–1727. doi: 10.1056/NEJM198406283102607. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Obara T., Ito Y., Kodama T., Fujimoto Y., Mizoguchi H., Oshimi K., Takahashi M., Hirayama A. A case of gastric carcinoma associated with excessive granulocytosis. Production of a colony-stimulating factor by the tumor. Cancer. 1985 Aug 15;56(4):782–788. doi: 10.1002/1097-0142(19850815)56:4<782::aid-cncr2820560414>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Okabe T., Nomura H., Sato N., Ohsawa N. Large-scale preparation and characterization of human colony-stimulating factor. J Cell Physiol. 1982 Jan;110(1):43–49. doi: 10.1002/jcp.1041100108. [DOI] [PubMed] [Google Scholar]
- Okabe T., Sato N., Kondo Y., Asano S., Ohsawa N., Kosaka K., Ueyama Y. Establishment and characterization of a human cancer cell line that produces human colony-stimulating factor. Cancer Res. 1978 Nov;38(11 Pt 1):3910–3917. [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Luben R. A., Mundy G. R., Dietrich J. W., Horton J. E., Trummel C. L. Effect of osteoclast activating factor from human leukocytes on bone metabolism. J Clin Invest. 1975 Aug;56(2):408–413. doi: 10.1172/JCI108106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G., Simmons H. A., Sandberg A. L., Canalis E. Direct stimulation of bone resorption by epidermal growth factor. Endocrinology. 1980 Jul;107(1):270–273. doi: 10.1210/endo-107-1-270. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Lamb L. C., Newton D. L., Sporn M. B., De Larco J. E., Todaro G. J. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3494–3498. doi: 10.1073/pnas.77.6.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Insogna K. L., Vignery A. M., Stewart A. F., Broadus A. E., D'Souza S. M., Bertolini D. R., Mundy G. R., Rodan G. A. Factors associated with humoral hypercalcemia of malignancy stimulate adenylate cyclase in osteoblastic cells. J Clin Invest. 1983 Oct;72(4):1511–1515. doi: 10.1172/JCI111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Kuratomi Y., Yamamoto K., Saito T., Kuzuya T., Yoshida S., Moriyama S. I., Takahashi A. Primary squamous cell carcinoma of the thyroid associated with marked leukocytosis and hypercalcemia. Cancer. 1981 Nov 1;48(9):2080–2083. doi: 10.1002/1097-0142(19811101)48:9<2080::aid-cncr2820480927>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Sato N., Asano S., Ueyama Y., Mori M., Okabe T., Kondo Y., Ohsawa N., Kosaka K. Granulocytosis and colony-stimulating activity (CSA) produced by a human squamous cell carcinoma. Cancer. 1979 Feb;43(2):605–610. doi: 10.1002/1097-0142(197902)43:2<605::aid-cncr2820430230>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Shepard R. M., Horst R. L., Hamstra A. J., DeLuca H. F. Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55–69. doi: 10.1042/bj1820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. L., Mundy G. R., D'Souza S. M., Ibbotson K. J., Bockman R., Jacobs J. W. Absence of parathyroid hormone messenger RNA in nonparathyroid tumors associated with hypercalcemia. N Engl J Med. 1983 Aug 11;309(6):325–330. doi: 10.1056/NEJM198308113090601. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Stern P. H., Miller J. C., Chen S. F., Kahn D. J. A bone resorbing substance from bovine serum albumin (brA). Calcif Tissue Res. 1978 Aug 18;25(3):233–240. doi: 10.1007/BF02010775. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Insogna K. L., Goltzman D., Broadus A. E. Identification of adenylate cyclase-stimulating activity and cytochemical glucose-6-phosphate dehydrogenase-stimulating activity in extracts of tumors from patients with humoral hypercalcemia of malignancy. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1454–1458. doi: 10.1073/pnas.80.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. F., Vignery A., Silverglate A., Ravin N. D., LiVolsi V., Broadus A. E., Baron R. Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab. 1982 Aug;55(2):219–227. doi: 10.1210/jcem-55-2-219. [DOI] [PubMed] [Google Scholar]
- Strewler G. J., Williams R. D., Nissenson R. A. Human renal carcinoma cells produce hypercalcemia in the nude mouse and a novel protein recognized by parathyroid hormone receptors. J Clin Invest. 1983 Mar;71(3):769–774. doi: 10.1172/JCI110825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf M., Kowalski M. A., Mundy G. R. Effects of glucocorticoids on osteoclast-activating factor. J Lab Clin Med. 1978 Nov;92(5):772–778. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Hohmann E. L., Antoniades H. N., Levine L. Platelet-derived growth factor stimulates bone resorption via a prostaglandin-mediated mechanism. Endocrinology. 1982 Jul;111(1):118–124. doi: 10.1210/endo-111-1-118. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Levine L. Epidermal growth factor stimulates prostaglandin production and bone resorption in cultured mouse calvaria. Biochem Biophys Res Commun. 1978 Dec 14;85(3):966–975. doi: 10.1016/0006-291x(78)90638-1. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Singer F. R., Roberts A. B., Derynck R., Winkler M. E., Levine L. Alpha and beta human transforming growth factors stimulate prostaglandin production and bone resorption in cultured mouse calvaria. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4535–4538. doi: 10.1073/pnas.82.13.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toya M., Kuroda T., Obuchi M., Ikawa T., Habu K., Ishikawa T. [A case report of the maxillary cancer associated with marked leukocytosis and hypercalcemia (author's transl)]. Nihon Jibiinkoka Gakkai Kaiho. 1981 Dec 20;84(12):1554–1561. [PubMed] [Google Scholar]
- Uchida T., Ju S., Fay A., Liu Y., Dorf M. E. Functional analysis of macrophage hybridomas. I. Production and initial characterization. J Immunol. 1985 Feb;134(2):772–778. [PubMed] [Google Scholar]
- Yoneda T., Mundy G. R. Monocytes regulate osteoclast-activating factor production by releasing prostaglandins. J Exp Med. 1979 Aug 1;150(2):338–350. doi: 10.1084/jem.150.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]