Abstract
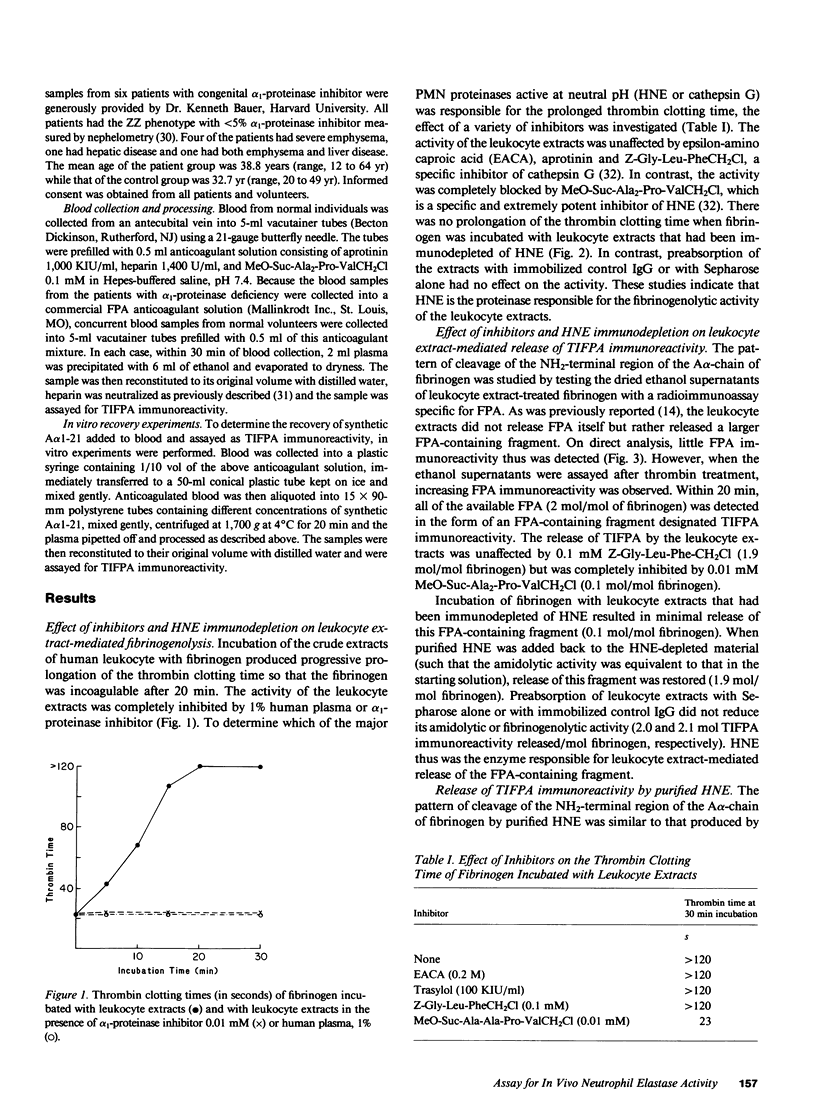
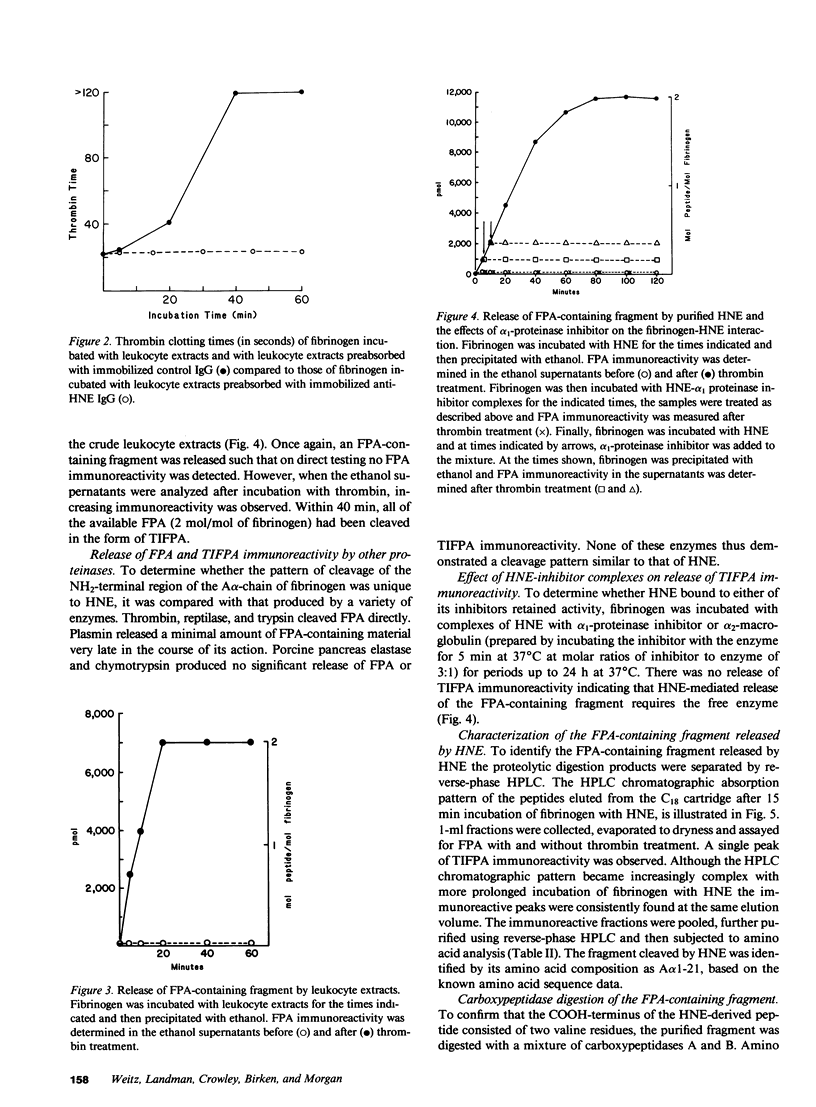
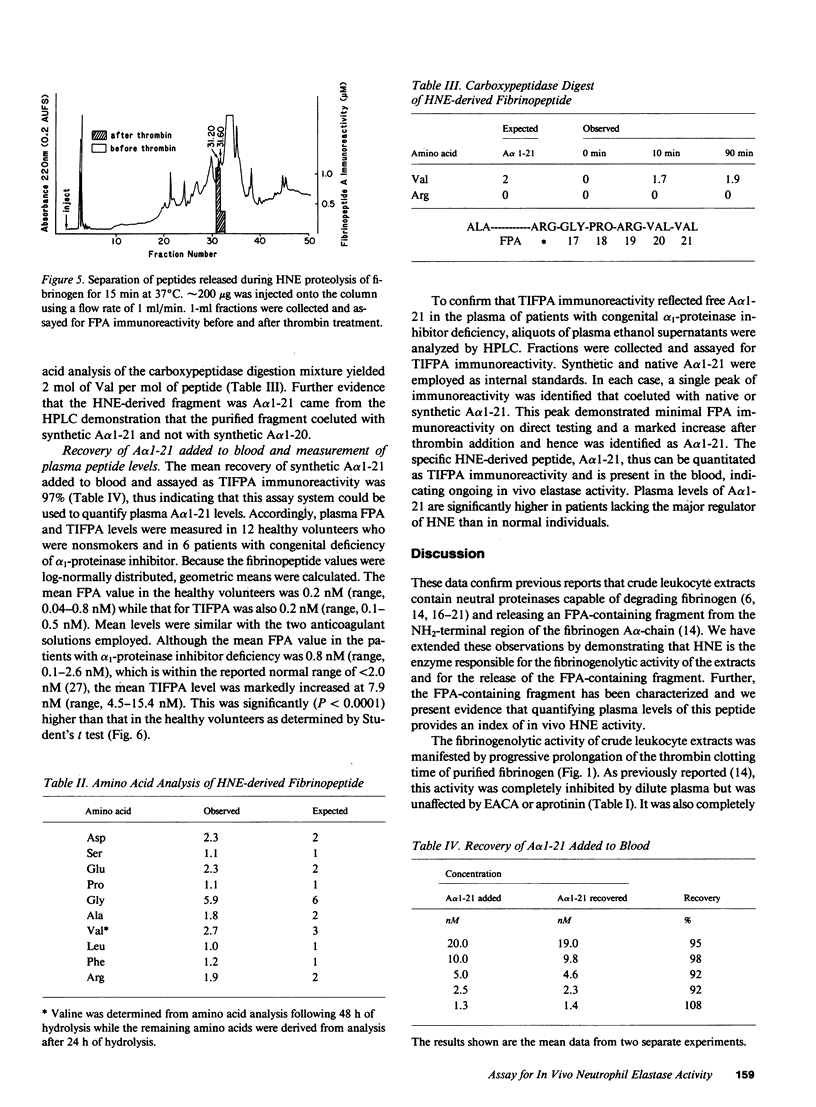
Leukocyte extracts contain enzymes that digest fibrinogen and release a fibrinopeptide A-containing fragment. This study was undertaken to identify the responsible proteinase and to characterize the fibrinopeptide A-containing fragment so that it could be used as an index of enzyme activity. Both the fibrinogenolytic activity and the release of the fibrinopeptide A-containing fragment mediated by the leukocyte extracts were shown to be due to human neutrophil elastase (HNE) by the following criteria: activity was completely blocked by a specific HNE inhibitor or by adsorbing HNE from the extracts with a monospecific antibody and reconstitution with purified HNE restored the ability to release the fibrinopeptide A-containing fragment. This fragment was not released by a variety of other proteinases or by HNE-inhibitor complexes indicating that, at least with respect to the enzymes tested, it is a specific product of HNE and its release requires the free enzyme. By separating the products of HNE digestion of fibrinogen using high performance liquid chromatography, identifying the immunoreactive fractions and subjecting them to amino acid analysis, the fragment was identified as A alpha 1-21, indicating an HNE cleavage site at the Val(A alpha 21)-Glu(A alpha 22) bond. The mean plasma A alpha 1-21 level was markedly higher in patients with alpha 1-proteinase inhibitor deficiency as compared to healthy controls (0.2 nM vs. 7.9 nM; P less than 0.0001), consistent with increased in vivo HNE activity in these individuals.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart M. I. Importance of neutrophilic leukocytes in the resolution of fibrin. Fed Proc. 1965 Jul-Aug;24(4):846–853. [PubMed] [Google Scholar]
- Bauer K. A., Goodman T. L., Kass B. L., Rosenberg R. D. Elevated factor Xa activity in the blood of asymptomatic patients with congenital antithrombin deficiency. J Clin Invest. 1985 Aug;76(2):826–836. doi: 10.1172/JCI112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian S. B., Nossel H. L., Butler V. P., Jr, Canfield R. E. Radioimmunoassay of human fibrinopeptide B and kinetics of fibrinopeptide cleavage by different enzymes. J Clin Invest. 1975 Aug;56(2):438–445. doi: 10.1172/JCI108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikian S. B., Nossel H. L. Unique pattern of fibrinogen cleavage by human leukocyte proteases. Blood. 1977 Jul;50(1):21–28. [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Blow A. M. Action of human lysosomal elastase on the oxidized B chain of insulin. Biochem J. 1977 Jan 1;161(1):13–16. doi: 10.1042/bj1610013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdillon M. C., Brechemier D., Blaes N., Derouette J. C., Hornebeck W., Robert L. Elastase-like enzymes in skin fibroblasts and rat aorta smooth muscle cells. Cell Biol Int Rep. 1980 Mar;4(3):313–320. doi: 10.1016/0309-1651(80)90064-8. [DOI] [PubMed] [Google Scholar]
- Brower M. S., Harpel P. C. Alpha-1-antitrypsin-human leukocyte elastase complexes in blood: quantification by an enzyme-linked differential antibody immunosorbent assay and comparison with alpha-2-plasmin inhibitor-plasmin complexes. Blood. 1983 May;61(5):842–849. [PubMed] [Google Scholar]
- Brower M. S., Harpel P. C. Proteolytic cleavage and inactivation of alpha 2-plasmin inhibitor and C1 inactivator by human polymorphonuclear leukocyte elastase. J Biol Chem. 1982 Aug 25;257(16):9849–9854. [PubMed] [Google Scholar]
- Campbell E. J. Human leukocyte elastase, cathepsin G, and lactoferrin: family of neutrophil granule glycoproteins that bind to an alveolar macrophage receptor. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6941–6945. doi: 10.1073/pnas.79.22.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. Proteolysis by neutrophils. Relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J Clin Invest. 1982 Oct;70(4):845–852. doi: 10.1172/JCI110681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield R. E., Dean J., Nossel H. L., Butler V. P., Jr, Wilner G. D. Reactivity of fibrinogen and fibrinopeptide A containing fibrinogen fragments with antisera to fibrinopeptide A. Biochemistry. 1976 Mar 23;15(6):1203–1209. doi: 10.1021/bi00651a004. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp H., Janoff A. Potential mediator of inflammation. Phagocyte-derived oxidants suppress the elastase-inhibitory capacity of alpha 1-proteinase inhibitor in vitro. J Clin Invest. 1980 Nov;66(5):987–995. doi: 10.1172/JCI109968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibber B. A., Deutsch D. G., Mertz E. T. Affinity chromatography of plasminogen. Methods Enzymol. 1974;34:424–432. doi: 10.1016/s0076-6879(74)34051-7. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Stone P. J., El Hag A., Calore J. D., Franzblau C. Myeloperoxidase-catalyzed inactivation of alpha 1-protease inhibitor by human neutrophils. J Biol Chem. 1981 Apr 10;256(7):3348–3353. [PubMed] [Google Scholar]
- Egbring R., Schmidt W., Fuchs G., Havemann K. Demonstration of granulocytic proteases in plasma of patients with acute leukemia and septicemia with coagulation defects. Blood. 1977 Feb;49(2):219–231. [PubMed] [Google Scholar]
- Galloway M. J., Mackie M. J., McVerry B. A. Elastase-alpha 1 antitrypsin complexes in acute leukaemia. Thromb Res. 1985 May 15;38(4):311–320. doi: 10.1016/0049-3848(85)90131-8. [DOI] [PubMed] [Google Scholar]
- Gramse M., Bingenheimer C., Schmidt W., Egbring R., Havemann K. Degradation products of fibrinogen by elastase-like neutral protease from human granulocytes. Characterization and effects on blood coagulation in vitro. J Clin Invest. 1978 Apr;61(4):1027–1033. doi: 10.1172/JCI109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENRY R. L. LEUKOCYTES AND THROMBOSIS. Thromb Diath Haemorrh. 1965 Mar 15;13:35–46. [PubMed] [Google Scholar]
- Harpel P. C. Human alpha2-macroglobulin. Methods Enzymol. 1976;45:639–652. doi: 10.1016/s0076-6879(76)45055-3. [DOI] [PubMed] [Google Scholar]
- Harpel P. C., Mosesson M. W. Degradation of human fibrinogen by plasms alpha2-macroglobulin-enzyme complexes. J Clin Invest. 1973 Sep;52(9):2175–2184. doi: 10.1172/JCI107402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. Elastase in tissue injury. Annu Rev Med. 1985;36:207–216. doi: 10.1146/annurev.me.36.020185.001231. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Varani J. Substrate hydrolysis by immune complex-activated neutrophils: effect of physical presentation of complexes and protease inhibitors. J Immunol. 1981 Nov;127(5):1875–1879. [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. II. Internalization of alpha-macroglobulin . trypsin complexes by rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7329–7335. [PubMed] [Google Scholar]
- Kettner C., Shaw E., White R., Janoff A. The specificity of macrophage elastase on the insulin B-chain. Biochem J. 1981 May 1;195(2):369–372. doi: 10.1042/bj1950369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake D. S., Hornebeck W., Bréchemier D., Robert L., Peters T. J. Properties and subcellular localization of elastase-like activities of arterial smooth muscle cells in culture. Biochim Biophys Acta. 1983 Nov 22;761(1):41–47. doi: 10.1016/0304-4165(83)90360-4. [DOI] [PubMed] [Google Scholar]
- Marglin A., Merrifield R. B. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1970;39:841–866. doi: 10.1146/annurev.bi.39.070170.004205. [DOI] [PubMed] [Google Scholar]
- Nossel H. L., Butler V. P., Jr, Wilner G. D., Canfield R. E., Harfenist E. J. Specificity of antisera to human fibrinopeptide A used in clinical fibrinopeptide A assays. Thromb Haemost. 1976 Feb 29;35(1):101–109. [PubMed] [Google Scholar]
- Nossel H. L. Relative proteolysis of the fibrinogen B beta chain by thrombin and plasmin as a determinant of thrombosis. Nature. 1981 May 14;291(5811):165–167. doi: 10.1038/291165a0. [DOI] [PubMed] [Google Scholar]
- Nossel H. L., Wasser J., Kaplan K. L., LaGamma K. S., Yudelman I., Canfield R. E. Sequence of fibrinogen proteolysis and platelet release after intrauterine infusion of hypertonic saline. J Clin Invest. 1979 Nov;64(5):1371–1378. doi: 10.1172/JCI109594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossel H. L., Yudelman I., Canfield R. E., Butler V. P., Jr, Spanondis K., Wilner G. D., Qureshi G. D. Measurement of fibrinopeptide A in human blood. J Clin Invest. 1974 Jul;54(1):43–53. doi: 10.1172/JCI107749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. Neutral proteases of human granulocytes. III. Interaction between human granulocyte elastase and plasma protease inhibitors. Scand J Clin Lab Invest. 1974 Dec;34(4):349–355. doi: 10.3109/00365517409049891. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. An alternative pathway for fibrinolysis. I. The cleavage of fibrinogen by leukocyte proteases at physiologic pH. J Clin Invest. 1975 Jul;56(1):30–38. doi: 10.1172/JCI108076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Gramse M., Havemann K. Immunochemical discrimination of leukocyte elastase from plasmic degradation products of fibrinogen. J Lab Clin Med. 1983 Dec;102(6):858–869. [PubMed] [Google Scholar]
- Plow E. F. Leukocyte elastase release during blood coagulation. A potential mechanism for activation of the alternative fibrinolytic pathway. J Clin Invest. 1982 Mar;69(3):564–572. doi: 10.1172/JCI110482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F. The major fibrinolytic proteases of human leukocytes. Biochim Biophys Acta. 1980 Jun 5;630(1):47–56. doi: 10.1016/0304-4165(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Travis J. The degradation of human lung elastin by neutrophil proteinases. Biochim Biophys Acta. 1980 Jan 24;621(1):147–157. doi: 10.1016/0005-2795(80)90070-7. [DOI] [PubMed] [Google Scholar]
- Robert B., Szigeti M., Robert L., Legrand Y., Pignaud G., Caen J. Release of elastolytic activity from blood platelets. Nature. 1970 Sep 19;227(5264):1248–1249. doi: 10.1038/2271248a0. [DOI] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Isolation of elastase-like and chymotrypsin-like neutral proteases from human granulocytes. Hoppe Seylers Z Physiol Chem. 1974 Sep;355(9):1077–1082. doi: 10.1515/bchm2.1974.355.2.1077. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J., Landis J. A., Cox F. R., Kuhn C., Koren H. S. Elastase of U-937 monocytelike cells. Comparisons with elastases derived from human monocytes and neutrophils and murine macrophagelike cells. J Clin Invest. 1982 Feb;69(2):384–393. doi: 10.1172/JCI110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtfogel Y. T., Kucich U., James H. L., Scott C. F., Schapira M., Zimmerman M., Cohen A. B., Colman R. W. Human plasma kallikrein releases neutrophil elastase during blood coagulation. J Clin Invest. 1983 Nov;72(5):1672–1677. doi: 10.1172/JCI111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallén P. Plasmic degradation of fibrinogen. Scand J Haematol Suppl. 1971;13:3–14. doi: 10.1111/j.1600-0609.1971.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman M., Ashe B. M. Sbustrate specificity of the elastase and the chymotrypsin-like enzyme of the human granulocyte. Biochim Biophys Acta. 1977 Jan 11;480(1):241–245. doi: 10.1016/0005-2744(77)90337-0. [DOI] [PubMed] [Google Scholar]



