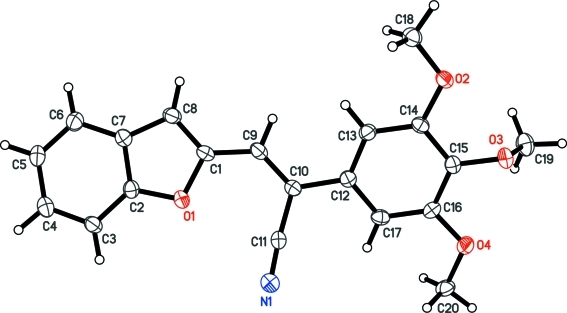
Abstract
In the title compound, C20H17NO4, the double bond of the acrylonitrile group separating the 1-benzofuran moiety from the 3,4,5-trimethoxyphenyl ring has Z geometry. The 1-benzofuran groups are π–π stacked with inversion-related counterparts such that the furan ring centroid–centroid distance is 3.804 (5) Å. The dihedral angle between the planes of the trimethoxyphenyl ring and the acrylonitrile group is 24.2 (2)°.
Related literature
For the biological activity, see: Naruto et al. (1983 ▶); Parmar et al. (1988 ▶); Shiba (1996 ▶); Sanna et al. (1999 ▶, 2000 ▶); Ohsumi et al. (1998 ▶); Saczewski et al. (2004 ▶). For similar structures, see: Choi et al. (2007 ▶); Seo et al. (2009 ▶); Sonar et al. (2007 ▶).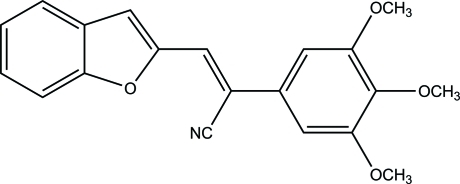
Experimental
Crystal data
C20H17NO4
M r = 335.35
Monoclinic,

a = 28.0892 (5) Å
b = 6.9555 (1) Å
c = 20.0908 (4) Å
β = 122.678 (1)°
V = 3303.93 (10) Å3
Z = 8
Mo Kα radiation
μ = 0.09 mm−1
T = 90 K
0.24 × 0.20 × 0.14 mm
Data collection
Nonius KappaCCD diffractometer
26416 measured reflections
3790 independent reflections
2183 reflections with I > 2σ(I)
R int = 0.085
Refinement
R[F 2 > 2σ(F 2)] = 0.064
wR(F 2) = 0.186
S = 1.02
3790 reflections
229 parameters
H-atom parameters constrained
Δρmax = 0.51 e Å−3
Δρmin = −0.35 e Å−3
Data collection: COLLECT (Nonius, 1998 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO-SMN (Otwinowski & Minor, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXL97 and local procedures.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812005831/hg5136sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812005831/hg5136Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812005831/hg5136Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This investigation was supported by NIH/National Cancer Institute grant No. RO1 CA140409.
supplementary crystallographic information
Comment
Acrylonitrile analogs that incorporate 1,2,4-triazole, benzimidazole, or 1,3,5-triazine heterocyclic groups have been found to possess interesting biological properties such as spasmolytic (Naruto et al., 1983), antioxidative (Parmar et al., 1988), insecticidal (Shiba, 1996), antitubercular (Sanna et al., 1999, 2000) and cytotoxic (Ohsumi et al., 1998; Saczewski et al., 2004) activities. From our previous studies, we reported the X-ray crystallographic data of two benzothiophene acrylonitrile analogs (Sonar et al., 2007). Based on this, and to compare the structure–activity relationships of different substituted acrylonitrile analogs, we have now prepared the title compound, (I), by the reaction of benzofuran-2-carbaldehyde with 2-(3,4,5-trimethoxyphenyl)acetonitrile in methanolic and sodium methoxide under reflux. The title compound was crystallized from the methanol. The molecular structure is shown in Fig.1. The 1-benzofuran ring is planar, with bond distances and angles comparable with those previously reported for other 1-benzofuran derivatives (Choi et al., 2007; Seo et al., 2009). The X-ray crystallographic studies revealed that the title compound is the Z isomer, since the 1-benzofuran ring is trans relative to the bulky 3,4,5-trimethoxy phenyl group. The 1-benzofuran groups are π—π stacked with inversion-related (1 - x, 1 - y, 1 - z) counterparts with a furan ring centroid—centroid distance of 3.804 (5) Å. Since the stacked benzofurans are inversion related, they are exactly parallel with perpendicular spacing of 3.409 (3) Å. The dihedral angle between the planes of the trimethoxy phenyl ring and the acrylonitrile group is 24.2 (2) Å.
Experimental
A mixture of benzofuran-2-carbaldehyde (0.3 g, 2.05 mmol), and 2-(3,4,5-trimethoxyphenyl)acetonitrile (0.45 g, 2.17 mmol) was refluxed in 5% methanolic sodium methoxide solution for 4 hrs. The reaction mixture was cooled to room temperature and added to ice cold water to afford a yellow crude solid, which was collected by filtration, washed with a 1:1 mixture of cold water and methanol, and suction–dried to afford the desired product. Crystallization from methanol gave a yellow crystalline product of (Z)-3-(benzofuran-2-yl)-2-(3,4,5-trimethoxyphenyl)acrylonitrile that was suitable for X-ray crystallographic analysis. 1H NMR (CDCl3): δ 3.90 (s, 3H), 3.91 (s, 6H), 6.89 (s, 2H), 7.26–7.31 (dd, 1H), 7.36–7.40 (m, 1H), 7.41 (s, 1H), 7.50 (s, 1H),7.53–7.56(dd, 1H), 7.63–7. 65 (m, 1H); 13C NMR (CDCl3): δ 56.53, 61.25, 103.30, 110.89, 111.10, 111.71, 117.60, 122.16, 123.81, 126.94, 127.66, 128.29, 129.17, 139.50, 151.21, 153.68, 155.20.
Refinement
H atoms were found in difference Fourier maps and subsequently placed in idealized positions with constrained distances of 0.98 Å (RCH3), 0.95 Å (Csp2H) and with Uiso(H) values set to either 1.2Ueq or 1.5Ueq (RCH3) of the attached atom.
Figures
Fig. 1.
A view of the molecular structure with displacement ellipsoids drawn at the 50% probability level and H atoms shown as small spheres of arbitrary radius.
Crystal data
| C20H17NO4 | F(000) = 1408 |
| Mr = 335.35 | Dx = 1.348 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 4101 reflections |
| a = 28.0892 (5) Å | θ = 1.0–27.5° |
| b = 6.9555 (1) Å | µ = 0.09 mm−1 |
| c = 20.0908 (4) Å | T = 90 K |
| β = 122.678 (1)° | Block, yellow |
| V = 3303.93 (10) Å3 | 0.24 × 0.20 × 0.14 mm |
| Z = 8 |
Data collection
| Nonius KappaCCD diffractometer | 2183 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.085 |
| Graphite monochromator | θmax = 27.5°, θmin = 1.7° |
| Detector resolution: 9.1 pixels mm-1 | h = −35→36 |
| ω scans at fixed χ = 55° | k = −8→9 |
| 26416 measured reflections | l = −26→25 |
| 3790 independent reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.064 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.186 | H-atom parameters constrained |
| S = 1.02 | w = 1/[σ2(Fo2) + (0.1061P)2] where P = (Fo2 + 2Fc2)/3 |
| 3790 reflections | (Δ/σ)max < 0.001 |
| 229 parameters | Δρmax = 0.51 e Å−3 |
| 0 restraints | Δρmin = −0.35 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against all reflections. The weighted R-value wR and goodness of fit S are based on F2. Conventional R-values R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-values based on F2 are statistically about twice as large as those based on F, and R-values based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.48054 (9) | 0.1350 (3) | 0.63530 (12) | 0.0314 (5) | |
| O1 | 0.54675 (6) | 0.4633 (2) | 0.60175 (9) | 0.0235 (4) | |
| O2 | 0.28706 (6) | 0.8492 (2) | 0.58677 (9) | 0.0236 (4) | |
| O3 | 0.26167 (6) | 0.5664 (2) | 0.65217 (9) | 0.0241 (4) | |
| O4 | 0.33056 (7) | 0.2610 (2) | 0.71961 (10) | 0.0267 (4) | |
| C1 | 0.52309 (9) | 0.6378 (3) | 0.60312 (13) | 0.0209 (5) | |
| C2 | 0.58856 (9) | 0.5094 (3) | 0.58841 (12) | 0.0205 (5) | |
| C3 | 0.62441 (10) | 0.3797 (3) | 0.58480 (13) | 0.0246 (6) | |
| H3 | 0.6209 | 0.2451 | 0.5890 | 0.030* | |
| C4 | 0.66571 (10) | 0.4553 (3) | 0.57475 (13) | 0.0251 (6) | |
| H4 | 0.6916 | 0.3717 | 0.5727 | 0.030* | |
| C5 | 0.66959 (10) | 0.6539 (4) | 0.56754 (13) | 0.0261 (6) | |
| H5 | 0.6984 | 0.7025 | 0.5610 | 0.031* | |
| C6 | 0.63306 (10) | 0.7803 (4) | 0.56964 (14) | 0.0275 (6) | |
| H6 | 0.6360 | 0.9145 | 0.5637 | 0.033* | |
| C7 | 0.59119 (9) | 0.7082 (3) | 0.58073 (13) | 0.0223 (5) | |
| C8 | 0.54814 (10) | 0.7870 (3) | 0.59013 (13) | 0.0238 (6) | |
| H8 | 0.5388 | 0.9192 | 0.5877 | 0.029* | |
| C9 | 0.47910 (9) | 0.6394 (3) | 0.61858 (13) | 0.0224 (5) | |
| H9 | 0.4655 | 0.7636 | 0.6198 | 0.027* | |
| C10 | 0.45380 (9) | 0.4932 (3) | 0.63175 (12) | 0.0195 (5) | |
| C11 | 0.47002 (10) | 0.2961 (3) | 0.63316 (13) | 0.0227 (5) | |
| C12 | 0.40551 (9) | 0.5193 (3) | 0.64184 (12) | 0.0192 (5) | |
| C13 | 0.37106 (9) | 0.6807 (3) | 0.61109 (13) | 0.0212 (5) | |
| H13 | 0.3796 | 0.7790 | 0.5863 | 0.025* | |
| C14 | 0.32409 (9) | 0.6977 (3) | 0.61670 (12) | 0.0194 (5) | |
| C15 | 0.31095 (9) | 0.5540 (3) | 0.65306 (12) | 0.0203 (5) | |
| C16 | 0.34635 (9) | 0.3953 (3) | 0.68529 (12) | 0.0208 (5) | |
| C17 | 0.39355 (9) | 0.3767 (3) | 0.68009 (13) | 0.0217 (5) | |
| H17 | 0.4176 | 0.2678 | 0.7024 | 0.026* | |
| C18 | 0.29675 (10) | 0.9924 (3) | 0.54421 (13) | 0.0260 (6) | |
| H18A | 0.3342 | 1.0497 | 0.5790 | 0.039* | |
| H18B | 0.2678 | 1.0926 | 0.5258 | 0.039* | |
| H18C | 0.2949 | 0.9330 | 0.4986 | 0.039* | |
| C19 | 0.26949 (10) | 0.6446 (4) | 0.72368 (14) | 0.0314 (6) | |
| H19A | 0.2982 | 0.5697 | 0.7691 | 0.047* | |
| H19B | 0.2337 | 0.6388 | 0.7211 | 0.047* | |
| H19C | 0.2819 | 0.7787 | 0.7296 | 0.047* | |
| C20 | 0.36866 (10) | 0.1064 (3) | 0.75978 (14) | 0.0260 (6) | |
| H20A | 0.3713 | 0.0270 | 0.7216 | 0.039* | |
| H20B | 0.3549 | 0.0278 | 0.7865 | 0.039* | |
| H20C | 0.4061 | 0.1578 | 0.7990 | 0.039* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0334 (13) | 0.0259 (13) | 0.0429 (13) | 0.0016 (10) | 0.0257 (11) | −0.0004 (10) |
| O1 | 0.0229 (9) | 0.0234 (9) | 0.0289 (9) | 0.0020 (7) | 0.0170 (8) | −0.0005 (7) |
| O2 | 0.0240 (9) | 0.0228 (9) | 0.0273 (9) | 0.0060 (7) | 0.0161 (8) | 0.0043 (7) |
| O3 | 0.0194 (9) | 0.0304 (10) | 0.0251 (9) | 0.0007 (7) | 0.0136 (7) | −0.0019 (7) |
| O4 | 0.0247 (9) | 0.0252 (10) | 0.0334 (9) | 0.0018 (7) | 0.0178 (8) | 0.0077 (7) |
| C1 | 0.0202 (12) | 0.0174 (12) | 0.0225 (12) | 0.0031 (10) | 0.0100 (10) | −0.0011 (10) |
| C2 | 0.0174 (12) | 0.0239 (13) | 0.0198 (12) | −0.0016 (10) | 0.0097 (10) | −0.0023 (10) |
| C3 | 0.0281 (14) | 0.0211 (13) | 0.0274 (13) | 0.0042 (11) | 0.0168 (12) | −0.0002 (10) |
| C4 | 0.0224 (13) | 0.0326 (15) | 0.0208 (12) | 0.0045 (11) | 0.0121 (11) | 0.0009 (11) |
| C5 | 0.0229 (13) | 0.0344 (15) | 0.0248 (13) | 0.0004 (11) | 0.0155 (11) | 0.0016 (11) |
| C6 | 0.0293 (14) | 0.0260 (14) | 0.0326 (14) | −0.0035 (11) | 0.0203 (12) | −0.0019 (11) |
| C7 | 0.0207 (12) | 0.0231 (13) | 0.0229 (12) | 0.0009 (10) | 0.0118 (10) | −0.0008 (10) |
| C8 | 0.0234 (13) | 0.0187 (13) | 0.0285 (13) | 0.0028 (10) | 0.0136 (11) | 0.0001 (10) |
| C9 | 0.0206 (12) | 0.0205 (13) | 0.0258 (13) | 0.0027 (10) | 0.0123 (11) | −0.0033 (10) |
| C10 | 0.0204 (12) | 0.0186 (12) | 0.0193 (11) | 0.0021 (10) | 0.0107 (10) | −0.0001 (10) |
| C11 | 0.0218 (13) | 0.0237 (14) | 0.0273 (13) | 0.0000 (11) | 0.0163 (11) | −0.0001 (11) |
| C12 | 0.0180 (12) | 0.0195 (12) | 0.0184 (11) | −0.0021 (10) | 0.0086 (10) | −0.0035 (10) |
| C13 | 0.0247 (13) | 0.0192 (13) | 0.0224 (12) | −0.0006 (10) | 0.0144 (11) | 0.0004 (10) |
| C14 | 0.0200 (12) | 0.0184 (12) | 0.0181 (11) | 0.0013 (10) | 0.0092 (10) | −0.0016 (9) |
| C15 | 0.0183 (12) | 0.0229 (13) | 0.0193 (12) | −0.0008 (10) | 0.0100 (10) | −0.0024 (10) |
| C16 | 0.0229 (13) | 0.0195 (13) | 0.0193 (12) | −0.0038 (10) | 0.0109 (11) | 0.0001 (10) |
| C17 | 0.0215 (12) | 0.0190 (13) | 0.0204 (12) | 0.0029 (10) | 0.0086 (10) | 0.0027 (10) |
| C18 | 0.0267 (13) | 0.0260 (14) | 0.0256 (13) | 0.0037 (11) | 0.0142 (11) | 0.0023 (11) |
| C19 | 0.0293 (14) | 0.0412 (16) | 0.0279 (14) | 0.0019 (12) | 0.0181 (12) | −0.0012 (12) |
| C20 | 0.0320 (14) | 0.0211 (13) | 0.0271 (13) | 0.0000 (11) | 0.0173 (12) | 0.0045 (10) |
Geometric parameters (Å, º)
| N1—C11 | 1.154 (3) | C8—H8 | 0.9500 |
| O1—C2 | 1.376 (3) | C9—C10 | 1.346 (3) |
| O1—C1 | 1.391 (3) | C9—H9 | 0.9500 |
| O2—C14 | 1.371 (3) | C10—C11 | 1.440 (3) |
| O2—C18 | 1.431 (3) | C10—C12 | 1.487 (3) |
| O3—C15 | 1.377 (3) | C12—C13 | 1.390 (3) |
| O3—C19 | 1.438 (3) | C12—C17 | 1.402 (3) |
| O4—C16 | 1.370 (3) | C13—C14 | 1.388 (3) |
| O4—C20 | 1.421 (3) | C13—H13 | 0.9500 |
| C1—C8 | 1.356 (3) | C14—C15 | 1.400 (3) |
| C1—C9 | 1.428 (3) | C15—C16 | 1.390 (3) |
| C2—C3 | 1.383 (3) | C16—C17 | 1.391 (3) |
| C2—C7 | 1.397 (3) | C17—H17 | 0.9500 |
| C3—C4 | 1.384 (3) | C18—H18A | 0.9800 |
| C3—H3 | 0.9500 | C18—H18B | 0.9800 |
| C4—C5 | 1.400 (3) | C18—H18C | 0.9800 |
| C4—H4 | 0.9500 | C19—H19A | 0.9800 |
| C5—C6 | 1.369 (3) | C19—H19B | 0.9800 |
| C5—H5 | 0.9500 | C19—H19C | 0.9800 |
| C6—C7 | 1.403 (3) | C20—H20A | 0.9800 |
| C6—H6 | 0.9500 | C20—H20B | 0.9800 |
| C7—C8 | 1.430 (3) | C20—H20C | 0.9800 |
| C2—O1—C1 | 105.54 (17) | C13—C12—C10 | 120.4 (2) |
| C14—O2—C18 | 116.95 (17) | C17—C12—C10 | 119.6 (2) |
| C15—O3—C19 | 113.60 (17) | C14—C13—C12 | 119.8 (2) |
| C16—O4—C20 | 116.92 (17) | C14—C13—H13 | 120.1 |
| C8—C1—O1 | 111.17 (19) | C12—C13—H13 | 120.1 |
| C8—C1—C9 | 129.5 (2) | O2—C14—C13 | 124.0 (2) |
| O1—C1—C9 | 119.36 (19) | O2—C14—C15 | 115.23 (19) |
| O1—C2—C3 | 125.5 (2) | C13—C14—C15 | 120.8 (2) |
| O1—C2—C7 | 110.68 (19) | O3—C15—C16 | 121.12 (19) |
| C3—C2—C7 | 123.8 (2) | O3—C15—C14 | 119.68 (19) |
| C2—C3—C4 | 116.8 (2) | C16—C15—C14 | 119.1 (2) |
| C2—C3—H3 | 121.6 | O4—C16—C15 | 115.57 (19) |
| C4—C3—H3 | 121.6 | O4—C16—C17 | 123.7 (2) |
| C3—C4—C5 | 120.6 (2) | C15—C16—C17 | 120.7 (2) |
| C3—C4—H4 | 119.7 | C16—C17—C12 | 119.7 (2) |
| C5—C4—H4 | 119.7 | C16—C17—H17 | 120.2 |
| C6—C5—C4 | 122.0 (2) | C12—C17—H17 | 120.2 |
| C6—C5—H5 | 119.0 | O2—C18—H18A | 109.5 |
| C4—C5—H5 | 119.0 | O2—C18—H18B | 109.5 |
| C5—C6—C7 | 118.8 (2) | H18A—C18—H18B | 109.5 |
| C5—C6—H6 | 120.6 | O2—C18—H18C | 109.5 |
| C7—C6—H6 | 120.6 | H18A—C18—H18C | 109.5 |
| C2—C7—C6 | 118.1 (2) | H18B—C18—H18C | 109.5 |
| C2—C7—C8 | 105.4 (2) | O3—C19—H19A | 109.5 |
| C6—C7—C8 | 136.5 (2) | O3—C19—H19B | 109.5 |
| C1—C8—C7 | 107.2 (2) | H19A—C19—H19B | 109.5 |
| C1—C8—H8 | 126.4 | O3—C19—H19C | 109.5 |
| C7—C8—H8 | 126.4 | H19A—C19—H19C | 109.5 |
| C10—C9—C1 | 130.3 (2) | H19B—C19—H19C | 109.5 |
| C10—C9—H9 | 114.8 | O4—C20—H20A | 109.5 |
| C1—C9—H9 | 114.8 | O4—C20—H20B | 109.5 |
| C9—C10—C11 | 121.9 (2) | H20A—C20—H20B | 109.5 |
| C9—C10—C12 | 123.5 (2) | O4—C20—H20C | 109.5 |
| C11—C10—C12 | 114.56 (19) | H20A—C20—H20C | 109.5 |
| N1—C11—C10 | 175.8 (2) | H20B—C20—H20C | 109.5 |
| C13—C12—C17 | 120.0 (2) | ||
| C2—O1—C1—C8 | 0.8 (2) | C9—C10—C12—C17 | −159.1 (2) |
| C2—O1—C1—C9 | −177.95 (18) | C11—C10—C12—C17 | 23.9 (3) |
| C1—O1—C2—C3 | 178.0 (2) | C17—C12—C13—C14 | −1.6 (3) |
| C1—O1—C2—C7 | −0.5 (2) | C10—C12—C13—C14 | 176.09 (19) |
| O1—C2—C3—C4 | −176.8 (2) | C18—O2—C14—C13 | 3.0 (3) |
| C7—C2—C3—C4 | 1.5 (3) | C18—O2—C14—C15 | −175.70 (18) |
| C2—C3—C4—C5 | −0.9 (3) | C12—C13—C14—O2 | −178.6 (2) |
| C3—C4—C5—C6 | −0.4 (4) | C12—C13—C14—C15 | 0.0 (3) |
| C4—C5—C6—C7 | 1.1 (4) | C19—O3—C15—C16 | 85.9 (2) |
| O1—C2—C7—C6 | 177.78 (19) | C19—O3—C15—C14 | −98.1 (2) |
| C3—C2—C7—C6 | −0.8 (3) | O2—C14—C15—O3 | 4.1 (3) |
| O1—C2—C7—C8 | 0.0 (2) | C13—C14—C15—O3 | −174.67 (19) |
| C3—C2—C7—C8 | −178.5 (2) | O2—C14—C15—C16 | −179.78 (19) |
| C5—C6—C7—C2 | −0.6 (3) | C13—C14—C15—C16 | 1.5 (3) |
| C5—C6—C7—C8 | 176.3 (3) | C20—O4—C16—C15 | −174.09 (19) |
| O1—C1—C8—C7 | −0.8 (3) | C20—O4—C16—C17 | 7.6 (3) |
| C9—C1—C8—C7 | 177.8 (2) | O3—C15—C16—O4 | −3.8 (3) |
| C2—C7—C8—C1 | 0.5 (3) | C14—C15—C16—O4 | −179.84 (19) |
| C6—C7—C8—C1 | −176.6 (3) | O3—C15—C16—C17 | 174.64 (19) |
| C8—C1—C9—C10 | −179.9 (2) | C14—C15—C16—C17 | −1.4 (3) |
| O1—C1—C9—C10 | −1.3 (4) | O4—C16—C17—C12 | 178.18 (19) |
| C1—C9—C10—C11 | 0.9 (4) | C15—C16—C17—C12 | −0.1 (3) |
| C1—C9—C10—C12 | −175.8 (2) | C13—C12—C17—C16 | 1.6 (3) |
| C9—C10—C12—C13 | 23.2 (3) | C10—C12—C17—C16 | −176.07 (19) |
| C11—C10—C12—C13 | −153.8 (2) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG5136).
References
- Choi, H. D., Seo, P. J., Son, B. W. & Lee, U. (2007). Acta Cryst. E63, o521–o522.
- Naruto, S., Mizuta, H., Yoshida, T. & Uno, H. (1983). Chem. Pharm. Bull. 31, 3022–3032. [DOI] [PubMed]
- Nonius (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Ohsumi, K., Nakagawa, R., Fukuda, Y. & Hatanaka, T. (1998). J. Med. Chem. 41, 3022–3032. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Parmar, V. S., Kumar, A., Prasad, A. K. & Singh, S. K. (1988). Bioorg. Med. Chem. 25, 911–914.
- Saczewski, F., Reszka, P., Gdaniec, M., Grunert, R. & Bednarski, P. I. (2004). J. Med. Chem. 47, 3438–3449. [DOI] [PubMed]
- Sanna, P., Carta, A. & Nikookar, M. E. R. (2000). Eur. J. Med. Chem. 35, 535–543. [DOI] [PubMed]
- Sanna, P., Carta, A. & Paglietti, G. (1999). Heterocycles, 51, 2171–2181.
- Seo, P. J., Choi, H. D., Son, B. W. & Lee, U. (2009). Acta Cryst. E65, o2302. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shiba, S. A. (1996). Phosphorus Sulfur Silicon Relat. Elem. 114, 29–37.
- Sonar, V. N., Parkin, S. & Crooks, P. A. (2007). Acta Cryst. C63, o743–o745. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536812005831/hg5136sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536812005831/hg5136Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536812005831/hg5136Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report