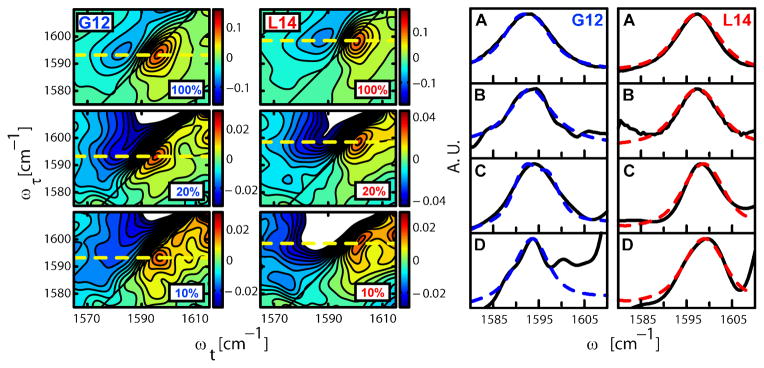
Fig. 2. 2D IR correlation spectra and FTIR spectra with different concentrations of 13C=18O isotopic substitution on residues G12 and L14.
The mixtures of 13C=18O labeled and unlabeled (12C=16O) helices are given as the percentage of the sample that is 13C=18O. The figure on the left shows the 2D IR correlation spectra of peptides substituted to the extent indicated (10 to 100%) with 13C=18O at the G12 (left) and L14 (right) residues. The spectra were taken at T=300fs and a τ range of −2800 fs to 3200 fs. The dashed line indicates the peak assigned to the 13C=18O amide I transition. The corresponding 2D IR diagonal traces for the 100 and 10% samples are shown in (C) and (D) respectively. The subtracted FTIR spectra of (A) 100% and (B) 10% 13C=18O substituted samples at G12 (left) and L14 (right) are shown on the right. The normalized experimental results (black, full) were fitted with the model explained in the text (blue, dotted). For G12 the 100% sample peaks are broader than the 10% sample peaks in the FTIR (A vs. B) and in the 2D IR (C vs. D) spectra, whereas the data for L14 show no significant difference in peak widths for different isotopic dilutions. The peak at ca. 1601 cm−1 in panel D of G12 is a weak transition that becomes evident in the 10% samples where the signal to background ratio is reduced. It is definitely not related to the 13C=18O amide I transition so it was readily subtracted and removed from the analysis (see SOM and Fig. S5).