Abstract
This communication reports gelation of lambda-carrageenan, for the first time, in the presence of trivalent iron ions. Kappa-, iota- and lambda-carrageenans are sulfated polysaccharides used extensively in food, pharmaceutical and medical applications. Kappa- and iota-carrageenans show gelation in the presence of mono- and di-valent ions, but lambda-carrageenan yields only viscous solutions. Our results show that gelation in lambda-carrageenan indeed is possible, but with trivalent ions. X-ray fiber diffraction patterns of iron (III)-lambda-carrageenan are characteristic of highly oriented and polycrystalline fibers containing well resolved Bragg reflections. The elastic modulus (G') of the product is far greater than the loss modulus (G") indicating the thermal stability of lambda-carrageenan in the presence of iron (III) ions. This novel finding has potential to expand lambda-carrageenan’s current utility beyond a viscosifying agent.
Keywords: Gelation, Lambda-Carrageenan, Trivalent Cations, X-ray Diffraction, Sulfated Polysaccharide
1. Introduction
Carrageenans are sulfated polysaccharides composed of alternating 3-linked β-d-galactopyranosyl and 4-linked α-d-galactopyranosyl residues. They constitute the major structural cell wall components of certain species of red seaweeds. Various hydroxyl groups in their polymeric chains are commonly substituted with sulfate ester groups. These biopolymers have long been used in food and pharmaceutical applications as thickeners, viscosifiers, gelling agents and stabilizers (Stanley, 1990). Carrageenans ability to substitute for fat and interact with other hydrocolloids and proteins increases their utility in a variety of food applications. For example, carrageenans are often used in yogurt, salad dressings and infant formulas, to name a few, to thicken and improve the texture. They display positive effects on human health as well, especially towards reducing blood cholesterol and triglyceride levels (Panlasigui, Baello, Dimatangal, & Dumelod, 2003). They are being explored as possible topical microbicides against human immunodeficiency virus (HIV) (Coggins et al., 2000) and human papilloma virus (HPV) (Roberts et al., 2007) infections. Anticoagulant (Farias, Valente, Pereira, & Mourao, 2000), antiherpetic (Carlucci, Ciancia, Matulewicz, Cerezo, & Damonte, 1999), and antitumor (Zhou et al., 2004) activities of carrageenans further aid in the development of potential medicinal drugs.
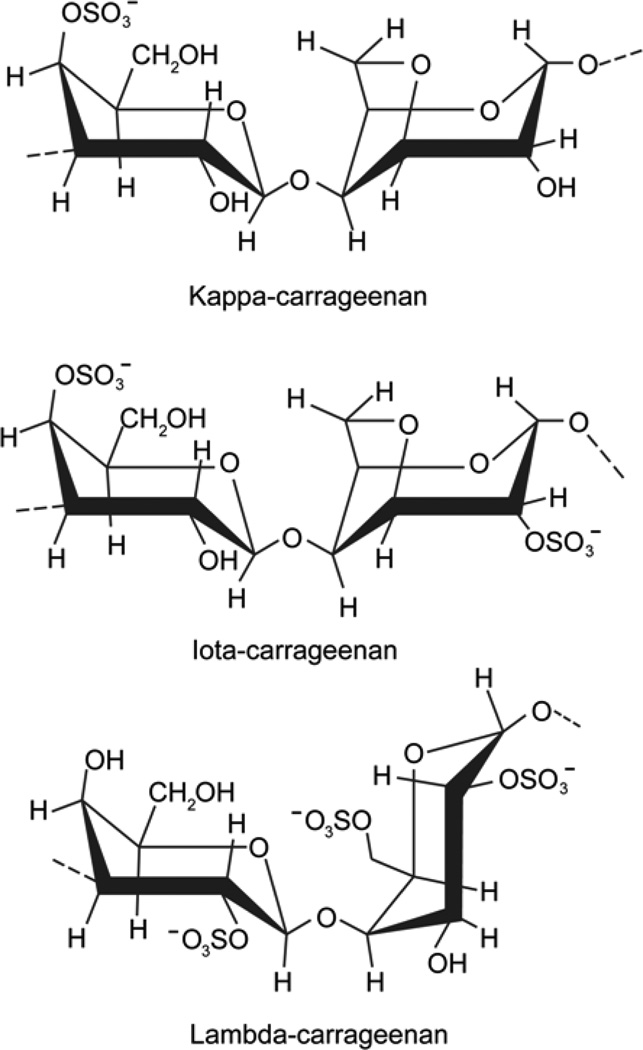
One of the major commercial uses of carrageenans stems from their intrinsic cation-dependent aqueous gelation. The disaccharide repeat units of three common types of carrageenans– kappa-, iota-, and lambda-carrageenan– are shown in Figure 1. Kappa- and iota-carrageenan exhibit gelation in the presence of mono- and di-valent cations, respectively. In contrast, lambda-carrageenan does not gel with either mono- or di-valent cations and displays only viscous behavior. Although excess amounts of cations can promote association among these hydrocolloid chains, gelation of lambda-carrageenan has not been achieved to date. It has been reasoned that kappa- and iota-carrageenans each form ordered three-dimensional networks comprising double helices resulting from “crosslinking” of the adjacent chains in which the sulfate groups are oriented externally. On the other hand, sulfate groups at the 2-postion in lambda-carrageenan face inward precluding “crosslinking” and formation of an ordered network (Campo, Kawano, da Silva, & Carvalho, 2009).
Fig. 1.
Disaccharide repeat units of kappa-, iota- and lambda-carrageenan. The 3-linked β-d-galactopyranosyl residue on the left of all three structures and 4-linked α-d-galactopyranosyl residue on the right in lambda-carrageenan are in 4C1 conformation, respectively. The 4-linked α-d-galactopyranosyl residue on the right in kappa- and iota-carrageenan takes a 1C4 conformation.
A close examination of disaccharide repeat units reveals that kappa- and iota-carrageenans have very similar chemical structures, varying only in the presence (iota) or absence (kappa) of a sulfate group at the 2 position of the 4-linked galactopyranosyl unit. Both kappa-and iota-carrageenans have a 1C4 conformation due to the 3,6-anhydro bridge. Lambda-carrageenan does not contain a 3,6-anhydro-bridge and consequently its d-galactopyranosyl unit assumes a 4C1 conformation. The sulfate group on the 3-linked galactopyranosyl unit is at the 2 position in lambda-carrageenan compared to the 4 position in kappa- and iota-carrageenans. Overall, lambda-carrageenan has three negatively charged sulfate groups per every disaccharide repeating unit, whereas kappa- and iota-carrageenans have only one and two of them, respectively (Fig. 1).
In general, kappa-carrageenan, with one negatively charged sulfate group, shows selectivity for monovalent potassium ions, and iota-carrageenan, with two sulfate groups, prefers divalent calcium ions. Thus, we endeavored to test if lambda-carrageenan, with three sulfate groups, would favor trivalent cations. Our results reveal that iron (III) ions indeed promote favorable interactions between lambda-carrageenan chains leading to junction zone formation and gelation.
2. Experimental Section
2.1 Materials
The sodium salt of lambda-carrageenan, obtained from tetrasporophytic specimens of Gigartina lanceata collected from Aramoana, Dunedin, New Zealand (WELT A21034), was kindly supplied by Industrial Research Ltd, Lower Hutt, New Zealand. Details of the extraction, purification, and chemical and spectroscopic characterization were reported earlier by Falshaw and Furneaux (Falshaw, & Furneaux, 1998).
An aqueous solution of the sodium salt of lambda-carrageenan (1% w/w) was prepared in distilled deionized water and heated in a boiling water bath for 45 minutes with periodic vortexing until all the polysaccharide was dissolved and the solution was homogeneous. Its iron (III) salt form was obtained by adding aqueous FeCl3·6H2O solution (0.4% w/w) to the carrageenan solution. Instantaneously, a yellow-orange colored fibrous and gel-like substance precipitated out (Fig. 2). The coagulum was separated from the solution for further characterization.
Fig. 2.
Sodium salt of lambda-carrageenan (1% w/w) solution (A), and iron (III) salt of lambda-carrageenan gel (B).
2.2 Fiber Preparation and Intensity Data
To make fibers suitable for X-ray diffraction, approximately 20 µL of iron (III) salt of lambda-carrageenan precipitate was placed in between two glass rods in a fiber puller at 66% relative humidity. After allowing the sample to dry for approximately two hours, the fiber was stretched in regular intervals to 2 times the original length for a final length of approximately 2–3 mm.
Synchrotron X-ray diffraction data were obtained at Argonne National Laboratory (ANL), Chicago, IL. The wavelength of the X-ray beam was set to 0.979 Å and the exposure lasted for 5 seconds. Calcite powder (3.035 Å characteristic spacing) was used for internal calibration. FibreFix (Rajkumar, Al-Khayat, Eakins, Knupp, & Squire, 2007) version 1.3.1 from CCP13 was used to estimate the pattern center, detector to fiber distance, tilt, and rotation. Reflection positions in each quadrant were measured and corresponding ρ (the distance between the origin and reflection point in the reciprocal space) was estimated. The relationship between ρ, and the cylindrical radius (ξ) and vertical component (ζ) is given by: ρ2 = ξ2 + ζ2, where ξ = a*(h2+hk+k2)1/2 for a trigonal system (a = b ≠ c, γ = 120°) and ζ = lc*. The dimensions of the reciprocal unit cell, a* and c*, as well as the Miller indices (h, k, l) for each reflection were estimated and the unit cell parameters calculated using in-house programs.
2.3 Rheology
Viscoelastic behavior of the solutions (1% w/w) of the sodium and iron (III) salts of lambda-carrageenan were analyzed with an ARG2 Rheometer from TA Instruments, New Castle (DE). The instrument was equipped with a cone and plate geometry (40 mm steel 2° cone) along with a solvent trap to minimize water evaporation from the sample during experimentation. Changes in the storage modulus G' and loss modulus G" were measured at 2% strain (within the linear viscoelastic range) as a function of (i) temperature from 1 to 70 °C with a heating rate of 2 °C/min at 1 Hz, and (ii) frequency in the range 0.1 to 100 Hz at 25 °C. The measurements were done in duplicate and average values are reported.
3. Results and Discussion
3.1 X-ray Diffraction Patterns
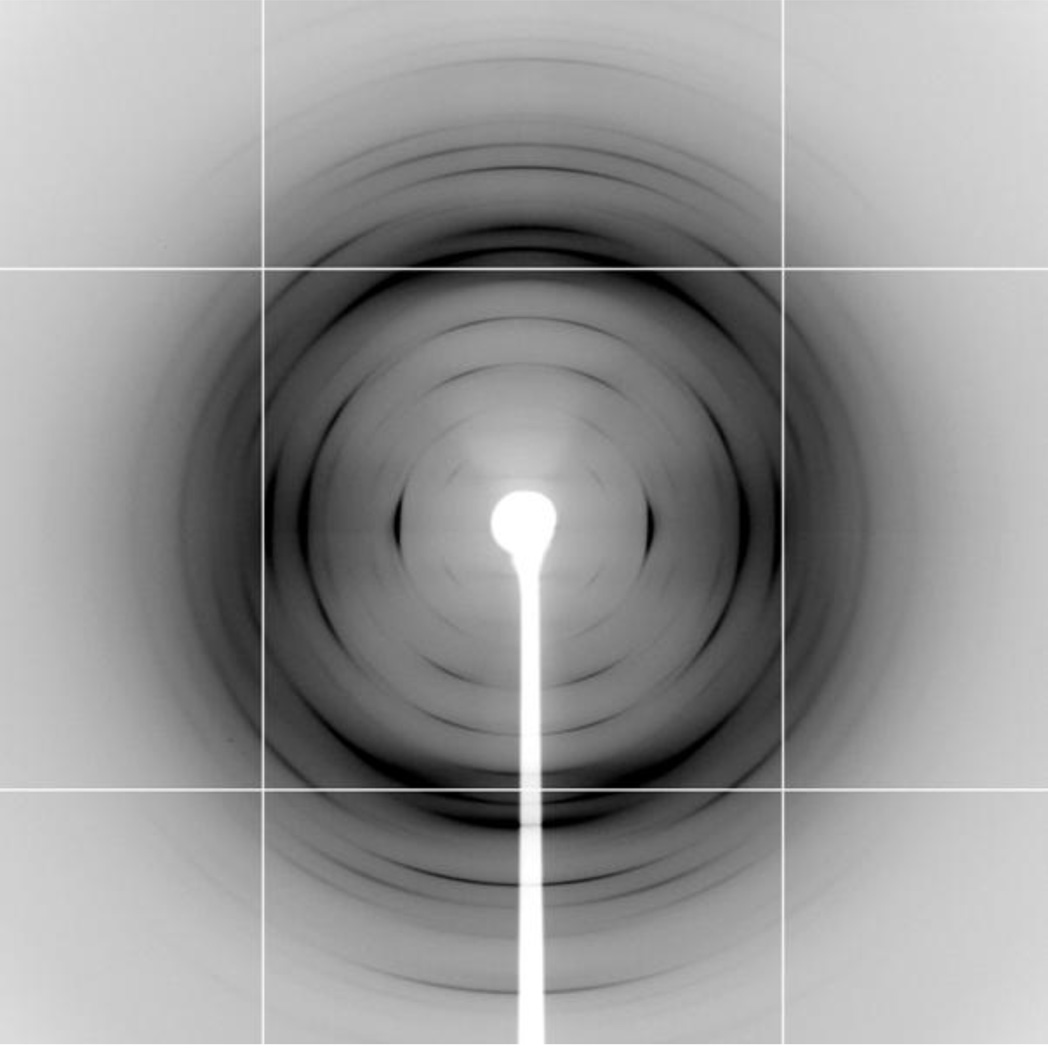
Attempts to prepare good quality fibers from the starting lambda-carrageenan (Na+ form) material were unsuccessful as their diffraction patterns mostly contained either concentric rings or diffused arcs (results not shown). On the other hand, the precipitate of iron (III) salt of lambda-carrageenan (Fe-lambda hereafter) yielded good fibers, and Figure 3 illustrates one of the best diffraction patterns obtained to date from lambda-carrageenan. It shows that the specimen is well oriented and polycrystalline. The first meridional reflection is on the 6th layer line and there are 19 Bragg reflections extending out to 3.7 Å resolution. These are sharp arcs whose length increases toward the edge of the pattern. This observation indicates that larger crystallites are formed within the fiber, but their alignment along the fiber axis is rather short.
Fig. 3.
X-ray diffraction pattern from well oriented and polycrystalline fiber of iron (III) salt of lambda-carrageenan. The fiber is almost perpendicular to the incident X-ray beam. The well resolved Bragg reflections indicate structural ordering in the fiber.
3.2 Unit Cell Dimensions
Reflections with lowest ξ value are seen on the first through fourth layer lines and their average, 0.059 Å−1, has been used as the smallest reciprocal vector (ξs) for further calculations (distribution of ξ for the Bragg reflections on each layer line is shown in Figure SP1 in the Supporting Information). Reflections with similar ξ values across the layer lines are grouped together and subsequent analysis revealed that these higher order ξ values are in multiples of √3, √4, √7, √9, √12, √13, and √16 of ξs. Assigning indices (1, 0) as (h, k) for the smallest reciprocal vector ξs, the indices for higher order reflections are (1, 1), (2, 0), (2, 1), (3, 0), (2, 2), (3, 1) and (4, 0), suggesting a trigonal lattice arrangement. Final analysis yielded unit cell dimensions as a = b = 19.30(1) Å, c = 25.08(4) Å, γ = 120° (Table 1 in the Supporting Information lists the observed and calculated ρ values, and Miller indices of the individual reflections).
The cell constants of Fe-lambda are similar to those of iota- and kappa-carrageenans. Iota-carrageenan adopts a trigonal cell with dimensions a = b = 24.02 and c = 12.96 Å in the sodium form (Janaswamy, & Chandrasekaran, 2001), and a = b = 23.61 and c = 13.21 Å in the calcium form (Janaswamy, & Chandrasekaran, 2002). Similarly, sodium salt of kappa-carrageenan also prefers a trigonal unit cell of a = b = 26.7 and c = 25.2 Å (our unpublished results). Tertiary structure analysis reveals that iota-carrageenan prefers a half-staggered, parallel, three-fold double helix of pitch 2c (~ 26 Å). Though the precise structural details of kappa-carrageenan are still not available, molecular modeling suggests a three-fold double helix of pitch c (~ 25 Å) consisting of a non-half-staggered parallel or anti-parallel arrangement as a possible structure (Millane, Chandrasekaran, & Arnott, 1988). The present observation of 25.08 Å as a fiber repeat for Fe-lambda clearly points out that a helix pitch of around 25 Å is more or less maintained in the carrageenan family. However, depending on the system as well as the nature and type of cation (mono-, di- or tri-valent) used for balancing charge on the polysaccharide chain, the preferred molecular arrangement could be half-staggered double helix, non-half-staggered double helix or an anti-parallel double helix.
Although single helical structures, in the solid state, are not evidenced for iota- and kappa-carrageenans such an arrangement cannot be ruled out in the case of lambda-carrageenan. More than five decades ago, a 3-fold single helix was proposed for lambda-carrageenan from a sparsely oriented and non-crystalline diffraction pattern (Bayley, 1955). Subsequent molecular modeling suggested both left- and right-handed helices as probable structures and ruled out a double helix due to steric hindrance among the pairing lambda-carrageenan chains (Millane, Nzewi, & Arnott, 1989). Computer modeling studies also favored the single helical arrangement (Le Questel, Cros, Mackie, & Perez, 1995). However, no definitive results on the molecular structure and packing association are yet available, mainly due to lack of good quality intensity data. Our results are the first to report a well oriented and crystalline diffraction patterns containing only Bragg reflections. Precise structural details of lambda-carrageenan’s tertiary structure and its mode of interactions with neighboring helices, however, require further analysis.
3.3 Gelation
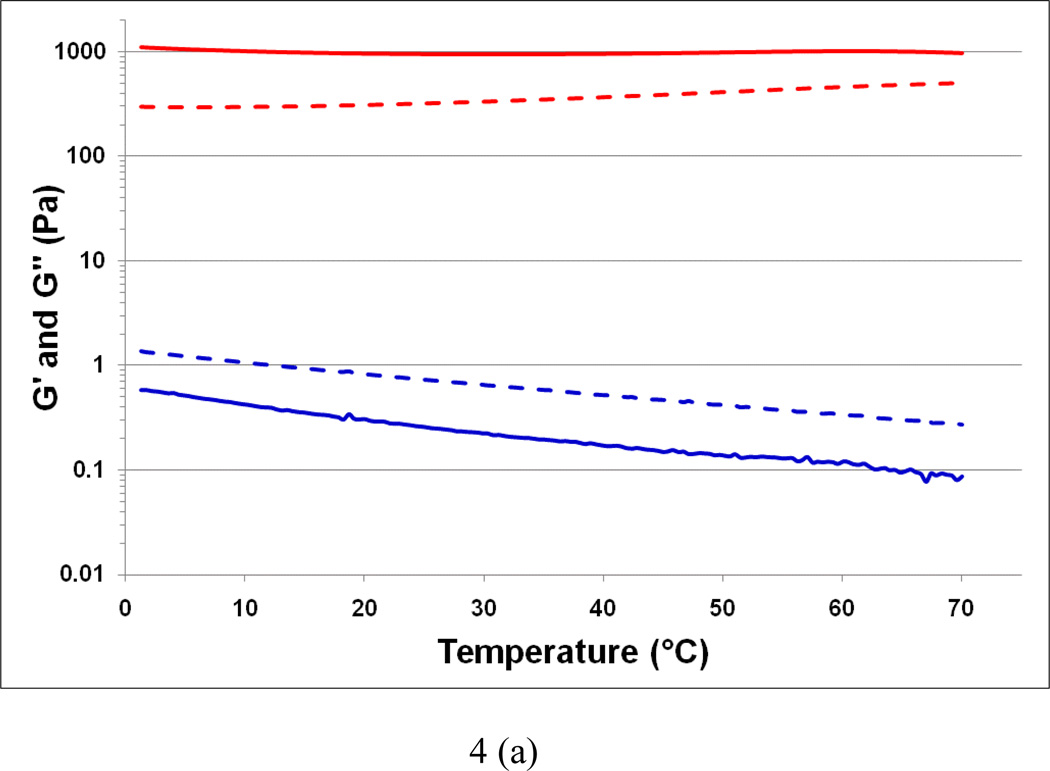
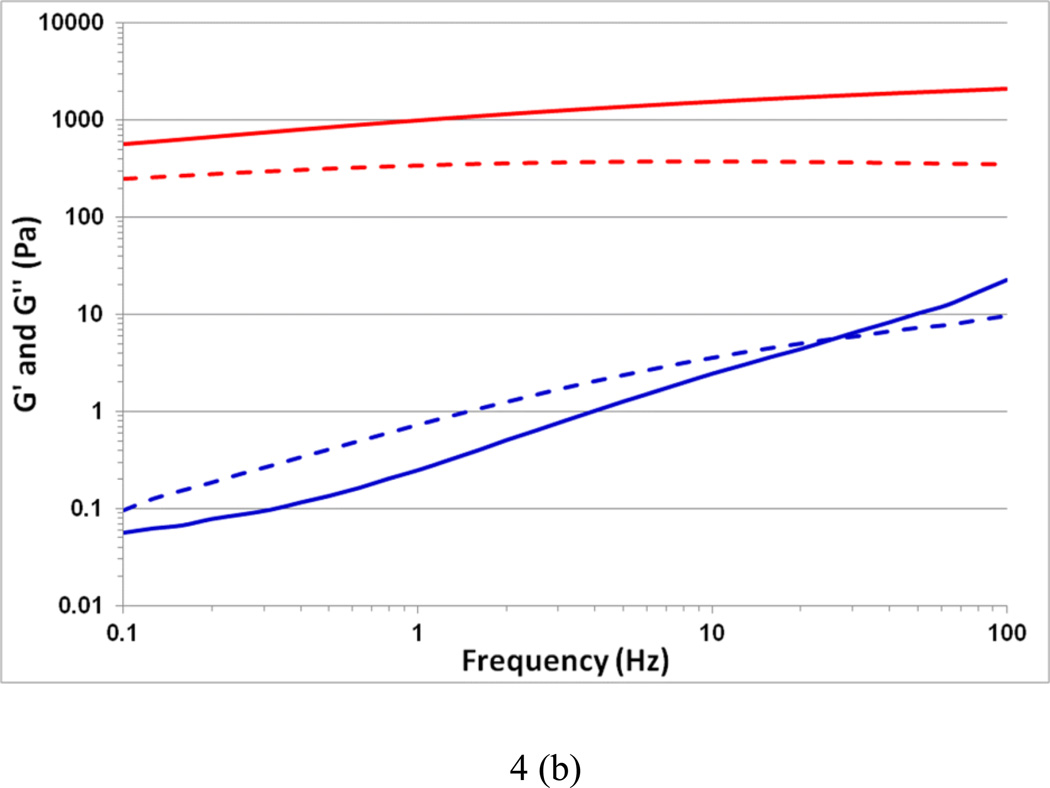
The variation in elastic (G') and loss (G") moduli as a function of temperature for sodium lambda-carrageenan without (control) and with iron (III) salt are shown in Figure 4a. In the control solution, the values of G' and G" at 5 °C are 0.6 and 1.2 Pa, respectively. The trend of G' less than G" continues throughout the temperature range 1 – 70 °C signifying the viscous nature of the control sample. This observation is consistent with previously reported results (van de Velde, & Ruiter, 2002). On the other hand, the Fe-lambda shows G' and G" values of 1200 and 150 Pa, respectively, at 5 °C, indicating a strong gelation behavior. Further, this gel is thermally stable as there is no crossover between G' and G" with a rise in temperature. The increase in G' is more than 3 orders of magnitude for the iron (III) salt form compared to the control. The frequency dependence of G' and G" are shown in Figure 4b. Throughout the 0.1 to 100 Hz range, Fe-lambda displayed G' > G" further confirming its gelation behavior. In the control, G' < G" is observed corroborating the temperature scan results. Interestingly, at higher frequencies elastic character with G' over G" is noticed. This occurrence can be attributed to the temporary association of lambda-carrageenan chains during short oscillation periods. Overall, trivalent iron ions appear to be more suitable than monovalent sodium ions for balancing the three negative sulfate charges, per disaccharide repeat unit, of lambda-carrageenan and promoting cooperative inter-chain interactions.
Fig. 4.
Variation in the viscoelastic properties G' (thick line) and G" (dashed line) of the control sodium salt of lambda-carrageenan (bottom) and the iron (III) salt of lambda-carrageenan (top) as a function of (a) temperature at 1 Hz, and (b) frequency at 25 °C.
There are very few studies in the literature about the functional behavior of lambda-carrageenan in the presence of trivalent ions. Oxidation states of ions, including aluminum (III) and iron (III) ions, have been found to affect viscosity (Zabik, & Aldrich, 1967), but with negligible binding activity from yttrium (III) ions (Khotimchenko, Khozhaenko, Khotimchenko, Kolenchenko, & Kovalev, 2010), and observation of turbid solutions upon FeCl3 addition (Jones, Cölfen, & Antoneitti, 2000). However, viscoelastic behaviors have not been reported previously, and to the best of our knowledge this communication is the first to describe gelation of lambda-carrageenan.
4. Conclusions
Our results demonstrate that gelation of lambda-carrageenan is indeed possible, but with trivalent iron ions. This novel finding certainly has the potential to expand lambda-carrageenan’s current utility beyond a viscosifying agent in food, pharmaceutical and medicinal applications. Importantly, lambda-carrageenan displayed potent inhibitory activity against HIV (Nakashima et al., 1987), however, most of the research involving carrageenans has been limited to either iota- or kappa-carrageenan (Coggins et al., 2000; Roberts et al., 2007). One possible reason could be the need of a gelling substance in these biological studies. We strongly foresee that the gelling ability of lambda-carrageenan with trivalent cations will revolutionize the utility of carrageenans in the development of effective as well as inexpensive topical microbicides. In order to reap the complete potential of lambda-carrageenan, an in-depth study about the influence of different trivalent cations on its solution properties and precise knowledge about the structure-function relationships is necessary.
Highlights.
Gelation in lambda-carrageenan is induced by iron (III) ions.
The iron-lambda-carrageenan gels are thermally stable.
Fiber Diffraction patterns are composed of well resolved Bragg reflections suggesting a highly ordered hydrocolloid network.
Supplementary Material
Acknowledgements
We thank Industrial Research Ltd, Lower Hutt, New Zealand for providing the sodium salt of lambda-carrageenan. We also thank Prof. Chandrasekaran and Prof. Campanella for helpful discussions; Whistler Center for Carbohydrate Research for support; and BioCARS scientists at Argonne National Laboratory, Chicago, IL for the synchrotron X-ray intensity data. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract No. DE-AC02-06CH11357. Use of the BioCARS Sector 14 was supported by the National Institutes of Health, National Center for Research Resources, under grant number RR007707.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bayley ST. X-ray and infrared studies on carrageenin. Biochimica et Biophysica Acta. 1955;17:194–204. doi: 10.1016/0006-3002(55)90350-4. [DOI] [PubMed] [Google Scholar]
- Campo VL, Kawano DF, da Silva DB, Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis - A review. Carbohydrate Polymers. 2009;77:167–180. [Google Scholar]
- Carlucci MJ, Ciancia M, Matulewicz MC, Cerezo AS, Damonte EB. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antiviral Research. 1999;43:93–102. doi: 10.1016/s0166-3542(99)00038-8. [DOI] [PubMed] [Google Scholar]
- Coggins C, Blanchard K, Alvarez F, Brache V, Weisberg E, Kilmarx PH, Lacarra M, Massai R, Mishell D, Jr., Salvatierra A, Witwatwongwana P, Elias C, Ellertson C. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sexually Transmitted Infections. 2000;76:480–483. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falshaw R, Furneaux RH. Structural analysis of carrageenans from the tetrasporic stages of the red algae, Gigartina lanceata and Gigartina chapmanii (Gigartinaceae, Rhodophyta) Carbohydrate Research. 1998;307:325–331. [Google Scholar]
- Farias WR, Valente AP, Pereira MS, Mourão PAS. Structure and anticoagulant activity of sulfated galactans. Isolation of a unique sulfated galactan from the red algae botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. Journal of Biological Chemistry. 2000;275:29299–29307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- Janaswamy S, Chandrasekaran R. Three-dimensional structure of the sodium salt of iota-carrageenan. Carbohydrate Research. 2001;335:181–194. doi: 10.1016/s0008-6215(01)00219-1. [DOI] [PubMed] [Google Scholar]
- Janaswamy S, Chandrasekaran R. Effect of calcium ions on the organization of iota-carrageenan helices: an X-ray investigation. Carbohydrate Research. 2002;337:523–535. doi: 10.1016/s0008-6215(02)00017-4. [DOI] [PubMed] [Google Scholar]
- Jones F, Cölfen H, Antoneitti M. Iron oxyhydroxide colloids stabilized with polysaccharides. Colloid and Polymer Science. 2000;278:491–501. [Google Scholar]
- Khotimchenko YS, Khozhaenko EV, Khotimchenko MY, Kolenchenko EA, Kovalev VV. Carrageenans as a new source of drugs with metal binding properties. Marine Drugs. 2010;8:1106–1121. doi: 10.3390/md8041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Questel JY, Cros S, Mackie W, Perez S. Computer modeling of sulfated carbohydrates - Applications to carrageenans. International Journal of Biological Macromolecules. 1995;17:161–175. doi: 10.1016/0141-8130(95)92682-g. [DOI] [PubMed] [Google Scholar]
- Millane RP, Chandrasekaran R, Arnott S, Dea ICM. The molecular structure of kappa-carrageenan and comparison with iota-carrageenan. Carbohydrate Research. 1988;182:1–17. [Google Scholar]
- Millane RP, Nzewi EU, Arnott S. Molecular structures of carrageenans as determined by x-ray fiber diffraction. In: Millane RP, BeMiller JN, Chandrasekaran R, editors. Frontiers in Carbohydrate Research-1: Food Applications. New York: Elsevier Applied Science; 1989. pp. 104–131. [Google Scholar]
- Nakashima H, Kido Y, Kobayashi N, Motoki Y, Neushul M, Yamamoto N. Purification and characterization of an avian-myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrobial Agents and Chemotherapy. 1987;31:1524–1528. doi: 10.1128/aac.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlasigui LN, Baello OQ, Dimatangal JM, Dumelod BD. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pacific Journal of Clinical Nutrition. 2003;12:209–214. [PubMed] [Google Scholar]
- Rajkumar G, Al-Khayat HA, Eakins F, Knupp C, Squire JM. The CCP13 FibreFix program suite: semi-automated analysis of diffraction patterns from non-crystalline materials. Journal of Applied Crystallography. 2007;40:178–184. doi: 10.1107/S0021889806048643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nature Medicine. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Stanley NF. Carrageenans. In: Harris P, editor. Food Gels. London: Elsevier; 1990. pp. 79–119. [Google Scholar]
- van de Velde F, De Ruiter G. Carrageenan. In: Vandamme EJ, De Baets S, Steinbüchel A, editors. Biopolymers: Polysaccharides II, Polysaccharides from Eukaryotes. Weinheim: Wiley-Vch Verlag GmbH; 2002. pp. 245–273. [Google Scholar]
- Zabik ME, Aldrich PJ. Effect of cations on viscosity of lambda-carrageenan. Journal of Food Science. 1967;32:91–97. [Google Scholar]
- Zhou G, Sun Y, Xin H, Zhang Y, Li Z, Xu Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacological Research. 2004;50:47–53. doi: 10.1016/j.phrs.2003.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.