Abstract
Purpose
The primary objective was to establish noninferiority of laparoscopy compared with laparotomy for recurrence after surgical staging of uterine cancer.
Patients and Methods
Patients with clinical stages I to IIA disease were randomly allocated (two to one) to laparoscopy (n = 1,696) versus laparotomy (n = 920) for hysterectomy, salpingo-oophorectomy, pelvic cytology, and pelvic and para-aortic lymphadenectomy. The primary study end point was noninferiority of recurrence-free interval defined as no more than a 40% increase in the risk of recurrence with laparoscopy compared with laparotomy.
Results
With a median follow-up time of 59 months for 2,181 patients still alive, there were 309 recurrences (210 laparoscopy; 99 laparotomy) and 350 deaths (229 laparoscopy; 121 laparotomy). The estimated hazard ratio for laparoscopy relative to laparotomy was 1.14 (90% lower bound, 0.92; 95% upper bound, 1.46), falling short of the protocol-specified definition of noninferiority. However, the actual recurrence rates were substantially lower than anticipated, resulting in an estimated 3-year recurrence rate of 11.4% with laparoscopy and 10.2% with laparotomy, or a difference of 1.14% (90% lower bound, −1.28; 95% upper bound, 4.0). The estimated 5-year overall survival was almost identical in both arms at 89.8%.
Conclusion
This study previously reported that laparoscopic surgical management of uterine cancer is superior for short-term safety and length-of-stay end points. The potential for increased risk of cancer recurrence with laparoscopy versus laparotomy was quantified and found to be small, providing accurate information for decision making for women with uterine cancer.
INTRODUCTION
Uterine cancer is common, with 43,470 patient cases and 7,950 deaths in the United States projected for 2011.1 Common sites of metastasis include pelvic and para-aortic lymph nodes, adnexa, peritoneal surfaces, and omentum and are identified during primary surgical treatment. Staging is undertaken according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) staging system.2 Historically, comprehensive surgical staging in endometrial cancer, including hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic and para-aortic lymphadenectomy, and peritoneal cytology was accomplished via open laparotomy.3,4 Complete cytoreduction of all metastatic tumor with adjuvant treatment including radiation and/or chemotherapy has been reported to improve survival in advanced disease.5–7 Adjuvant therapy has been tailored to the pathologic findings at primary surgery.8–10 Postoperative treatment recommendations have not been standardized; they include radiation and/or chemotherapy tailored to histologic cell type, grade, depth of myometrial and cervical invasion, lymphovascular space invasion, and stage of disease, with an effort to avoid toxicity of overtreatment.6–10 Accurate surgical staging is the first step toward making adjuvant treatment recommendations.
The Gynecologic Oncology Group determined that a prospective randomized trial was indicated to compare the perioperative morbidity and mortality between laparotomy and laparoscopy for the surgical staging of uterine cancer. In April 2001, the study was amended to also assess noninferiority of recurrence rates between the two treatments. There were concerns about increasing the rate of cancer recurrence with laparoscopy because of the loss of tactile senses during laparoscopy, which may result in failure to detect metastatic tumor otherwise palpable at laparotomy, failure to identify high left para-aortic lymph nodes just below the renal vein, potential change in patterns of recurrence associated with the high intra-abdominal pressures resulting from carbon dioxide insufflation, and potential for tumor spill secondary to the use of an intrauterine manipulator. The perioperative and surgical staging outcomes associated with this study were published in 2009. Laparoscopy was associated with shorter hospital stays, fewer moderate-to-severe postoperative adverse events, and improved body image. There was a significant decrease in histologic identification of any pelvic and para-aortic lymph nodes; however, this did not translate into a significant difference in final stage or identification of metastatic disease when comparing laparoscopy with laparotomy.11 In this report, we compare recurrence rates and overall survival (OS) for women randomly assigned to the two surgical techniques used to stage patients with uterine cancer.
PATIENTS AND METHODS
Study Design
Patients with clinical stages I to IIA uterine carcinoma/sarcoma were randomly allocated (2:1) to laparoscopy versus laparotomy for hysterectomy, salpingo-oophorectomy, pelvic cytology, and pelvic and para-aortic lymphadenectomy. The study was originally designed to compare perioperative adverse events and quality of life (QOL) between laparoscopy and laparotomy over an 8-week postsurgical follow-up period. In 2001, the protocol was amended to extend follow-up to 5 years and add the primary study end point of noninferiority of recurrence-free interval, defined as a hazard ratio of 1.4 for laparoscopy relative to laparotomy. Other end points included: conversion from laparoscopy to laparotomy, operative time, postoperative length of hospital stay, sites of recurrence, and OS. Study accrual, eligibility, procedures, short-term outcomes, and QOL results were previously published.11,12
Statistical Methods
The target sample size was 2,550 patients to test the null hypothesis of noninferiority of laparoscopy when compared with laparotomy for the surgical staging of uterine cancer. Despite the removal of all disease, approximately 15% of women with clinical stage I or II endometrial cancer were expected to experience a recurrence of disease within 3 years of diagnosis. Investigators expected a short-term benefit from the less invasive laparoscopic procedure but were concerned about the potential for an adverse oncologic outcome of laparoscopy and potentially an increase in the risk of recurrence. All patients signed a locally approved informed consent and authorization permitting release of personal health information.
Specifically, laparoscopy would be considered inferior to laparotomy if the hazard ratio for laparoscopy relative to laparotomy were greater than 1.4. With an expected recurrence rate of 15% with laparotomy, this translated to an acceptable recurrence rate of no more than 20.3% with laparoscopy or, equivalently, to no more than a 5.3% increase in recurrence with laparoscopy after 3 years. The study was designed to have sufficient precision in the estimated relative risk to exclude the region of clinically inferior values with a high degree of confidence. Two one-sided CIs would be used to construct asymmetric noninferiority bounds. With α1 = 0.10 and α2 = 0.05, lower 100(1 − α1)% and upper 100(1 − α2)% confidence limits for the log hazard ratio at interim analysis i, βi, are defined as:
where α1 = 0.10 and α2 = 0.05 and i = 1, 2, and 3 to accommodate interim analyses at approximately one third and two thirds through the full information time. The critical values Ζα1(i) and Ζα2(i) used to construct the CIs were determined by the alpha spending function α*t2, as described by Lan et al.13 At each interim analysis, stopping accrual would be considered if either the lower 90% confidence limit excluded δ = 1.0, indicating that laparotomy was preferred, or the upper 95% confidence limit excluded δ = 1.4, indicating that laparoscopy was preferred. The total required number of recurrences for the final analysis was determined to be 384 and was expected to be observed with 2,550 patients enrolled with 36 months of additional follow-up.
Planned interim analyses were performed after 147 and 269 recurrences and were presented to the GOG Data Monitoring Committee (DMC), with no resulting change to the follow-up plan. However, it was noted at each of these interim time points that the estimated recurrence rates were substantially lower than those projected at the time of study design and that as a result, fewer recurrences than anticipated had been observed. As of November 2009, 301 recurrences had been observed, and because of the lower than expected recurrence rates, it was deemed unlikely that the targeted number of events (ie, 384) would be reached even with extensive additional follow-up. Therefore, an unscheduled interim analysis was performed and presented to the DMC in January 2010. The DMC approved final analysis and release of the clinical trial results.
Because noncancer deaths might occur before recurrence, cumulative incidence methods were used to obtain estimates of disease recurrence rates in the presence of competing risks.4 The primary analysis comparing hazard rates for recurrence with laparoscopy relative to laparotomy was performed using a Cox proportional hazards model.14 Deaths resulting from unknown causes and noncancer deaths were treated as competing risks for estimation of recurrence probabilities.15 Under the assumption of noninformative censoring, deaths resulting from unknown causes were treated as censored observations in analyses comparing hazard rates, whereas recurrences and treatment-related deaths were treated as recurrences.16,17 Estimates of loss to follow-up rates and OS were calculated using the Kaplan-Meier method.18 Associations between factors known or suspected of influencing the risk of recurrence were also assessed using Cox proportional hazards models, with deaths resulting from unknown causes and noncancer deaths treated as censored observations.14 Factors considered for these analyses included: age, body mass index, performance status, race, surgical stage, cell type, cytology, adnexal involvement, lymph node metastasis, myometrial invasion, invasion of lymphatic or vascular space, and endocervical involvement. FIGO 1988 stages, which were used throughout the duration of this study, were mapped to the new 2009 categories using available data from central pathology review. Cell types were categorized into clear cell, endometrioid, mixed, serous, and sarcoma. Initially, individual models were fitted to data including each factor, randomized treatment group, and an interaction term to assess potential differential treatment effects among subgroups. All factors found to be significantly associated with risk of recurrence were then assessed together through multivariable models, resulting in a final multivariable model containing all factors influencing recurrence. First-order interactions were also assessed. Because of the multiplicity of statistical tests in these exploratory analyses, P values of .01 or lower were used to define statistical significance, and 99% CIs were constructed for each estimate. Graphic displays of treatment effect within subgroup levels of each factor found to be associated with recurrence are presented. However, this study was not designed to have sufficient power to statistically detect a treatment effect within any of the subgroups. Continuous measures are described by the median and interquartile range (IQR), defined as the 25th to 75th percentile, and categorical measures are described as frequency counts and percentages. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
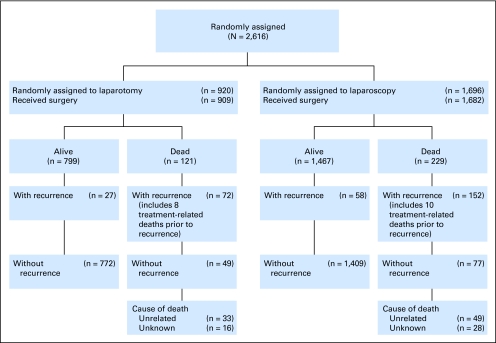
Figure 1 is a consort diagram of the randomly assigned treatment groups and outcomes. The estimated median follow-up times were 59.3 months (IQR, 38.0 to 62.9) for laparoscopy patients and 59.3 months (IQR, 37.9 to 63.0) for laparotomy patients (P = .885). At the time of analysis, there were 309 recurrences (210 laparoscopy; 99 laparotomy) and 350 deaths (229 laparoscopy; 121 laparotomy).
Fig 1.
CONSORT diagram for survival and recurrence in all randomly assigned patients.
As expected, the randomly assigned groups were approximately balanced on age, body mass index, race, and performance status. There was also a similar distribution of 2009 FIGO surgical stage between the two groups.
Recurrence-Free Survival
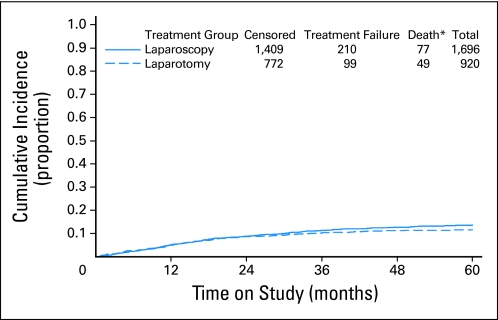
The intent-to-treat analysis includes all randomly assigned participants; there were 210 recurrences in the laparoscopy arm and 99 in the laparotomy arm (with a two-to-one laparoscopy to laparotomy ratio). The estimated hazard ratio for laparoscopy relative to laparotomy is 1.14 (lower 90% confidence limit, 0.92; upper 95% confidence limit, 1.46). This CI includes the inferiority lower bound of 1.0 as well as the noninferiority upper bound of 1.4, indicating that the protocol-specified criteria for concluding noninferiority of laparoscopy relative to laparotomy were not met. However, the 3-year estimated cumulative incidence of recurrence (Fig 2) for patients in the laparotomy arm is 10.24%, compared with 11.39% for patients in the laparoscopy arm, and the estimated difference between groups at the 3-year time point is 1.14% (90% lower bound, −1.278; 95% upper bound, 3.996). Note that this difference is less than the 5.3–percentage point difference at 3 years thought to represent noninferiority at the time of study design. The estimated 5-year recurrence rate in the laparotomy arm is 11.61% and 13.68% for laparoscopy.
Fig 2.
Cumulative incidence of recurrence by randomly assigned treatment group. (*) Deaths prior to recurrence.
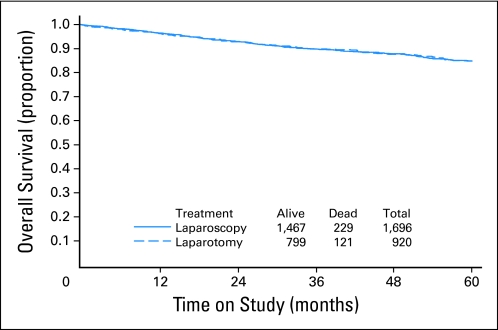
OS
The estimated 5-year OS is 89.8% for patients randomly assigned to laparoscopy and 89.8% for patients randomly assigned to laparotomy (Fig 3). There have been a total of 350 deaths (229 laparoscopy; 121 laparotomy), of which 224 deaths resulted from disease; 152 occurred in the laparoscopy arm, and 72 in the laparotomy arm (Fig 3).
Fig 3.
Overall survival by randomly assigned treatment group.
Site of Recurrence
Sites of first recurrence for the recurrences observed at the time of analysis were recorded and then retrospectively categorized into vagina, pelvis, abdomen, liver, lung, bone, nodal, multiple sites, or no recurrence and were similar between the two treatment arms (P = .470). Postoperative adjuvant therapy was recorded and included radiation, chemotherapy, or a combination of both and was similar between the two treatment arms (P = .607; Table 1).
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Laparoscopy Arm (n = 1,696) |
Laparotomy Arm (n = 920) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 62.8 | 62.7 | ||
| IQR | 55.4-71.6 | 54.9-70.6 | ||
| Minimum | 23.9 | 25.6 | ||
| Maximum | 92.8 | 94.2 | ||
| Age group, years | ||||
| ≤ 39 | 45 | 2.7 | 19 | 2.1 |
| 40-49 | 141 | 8.3 | 96 | 10.4 |
| 50-59 | 513 | 30.3 | 273 | 29.7 |
| 60-69 | 504 | 29.7 | 283 | 30.8 |
| 70-79 | 382 | 22.5 | 194 | 21.1 |
| ≥ 80 | 111 | 6.5 | 55 | 6.0 |
| BMI | ||||
| Median | 28.4 | 28.5 | ||
| IQR | 24.4-34.0 | 24.2-34.2 | ||
| Minimum | 14.9 | 15.0 | ||
| Maximum | 65.3 | 68.0 | ||
| BMI category | ||||
| < 25 | 543 | 32.2 | 300 | 32.9 |
| 26-30 | 495 | 29.4 | 260 | 28.5 |
| 31-35 | 325 | 19.3 | 159 | 17.4 |
| > 35 | 322 | 19.1 | 192 | 21.1 |
| Race | ||||
| White | 1,495 | 88.6 | 785 | 85.7 |
| Asian | 54 | 3.2 | 34 | 3.7 |
| Black | 61 | 3.6 | 37 | 4.0 |
| Hispanic | 67 | 4.0 | 45 | 4.9 |
| Other | 10 | < 1.0 | 15 | 1.6 |
| Performance status | ||||
| 0 | 1,527 | 90.1 | 821 | 89.2 |
| 1 | 160 | 9.4 | 89 | 9.7 |
| 2 | 5 | < 1.0 | 9 | 1.0 |
| 3 | 2 | < 1.0 | 1 | < 1.0 |
| 2009 FIGO surgical stage | ||||
| IA | 1,128 | 69.6 | 604 | 68.6 |
| IB | 204 | 12.6 | 110 | 12.5 |
| II | 65 | 4.0 | 34 | 3.9 |
| IIIA | 42 | 2.6 | 22 | 2.5 |
| IIIC1 | 77 | 4.8 | 40 | 4.5 |
| IIIC2 | 66 | 4.1 | 43 | 4.9 |
| IVB | 39 | 2.4 | 28 | 3.2 |
| Site of first recurrence | ||||
| Vagina | 27 | 1.6 | 14 | 1.5 |
| Pelvis | 22 | 1.3 | 9 | 1.0 |
| Abdomen | 23 | 1.4 | 11 | 1.2 |
| Liver | 11 | 0.7 | 5 | 0.5 |
| Lung | 34 | 2.0 | 14 | 1.5 |
| Bone | 1 | 0.1 | 4 | 0.4 |
| Nodal | 22 | 1.3 | 9 | 1.0 |
| Multiple | 30 | 1.8 | 16 | 1.7 |
| Unknown | 40 | 2.4 | 17 | 1.9 |
| No recurrence | 1,486 | 87.6 | 821 | 89.2 |
| Postoperative therapy | ||||
| Chemotherapy | 89 | 5.7 | 40 | 4.7 |
| Radiation | 284 | 18.1 | 168 | 19.7 |
| Both | 112 | 7.2 | 58 | 6.8 |
| None | 1,081 | 69.0 | 589 | 68.9 |
Abbreviations: BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; IQR, interquartile range.
Specific interest in abdominal wall (trocar site) recurrences resulted in retrospective review and specific notation of such recurrences. The four abdominal wall recurrences were potentially trocar recurrence sites, because all were identified in patients undergoing laparoscopy. Assuming these were associated with trocar site placement, the incidence rate is four per 1,696 randomly assigned patients, or 0.24%. Three of these cases were grade 2 endometrioid adenocarcinomas (one each of stage IB, stage IIIA, and stage IIIC), and one additional case was stage IVB carcinosarcoma. Of note, three of four presumed trocar site recurrences occurred in patients with advanced disease.
Assessment of Factors Associated With Treatment Effect
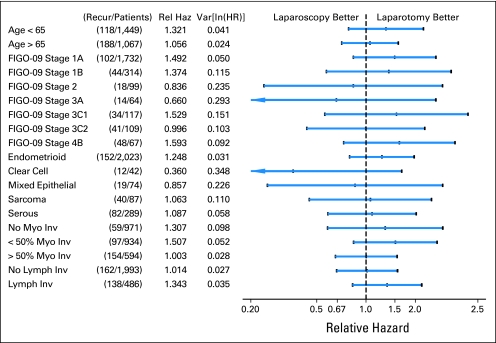
Age, 2009 FIGO stage, histologic cell type, positive cytology, adnexal involvement, nodal status, myometrial invasion, lymphatic/vascular space invasion, and endocervical involvement were significantly involved with recurrence on univariate analysis. A multivariable model confirmed age, surgical stage, cell type, myometrial invasion, and lymphatic/vascular space involvement as influencing recurrence. The treatment effects within each level of these factors are shown graphically in Figure 4. There were 2,023 patients (77.3%) with endometrioid histology. Of these, 534 (26.4%) were well differentiated, 1,136 (56.2%) were moderately differentiated, and 353 (17.5%) were poorly differentiated. Outcomes in this subgroup will be the subject of a future report.
Fig 4.
Treatment effect associated with recurrence by subgroup. FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; Inv, invasion; ln, natural log; Lymph Inv, lymphatic invasion; Myo, myometrial; Recur, recurrence; Rel Haz, relative hazard; Var, variance.
DISCUSSION
This prospective, multi-institutional randomized trial sought to assess whether laparoscopic surgical treatment and staging were noninferior to open laparotomy, in terms of time to recurrence, for the surgical staging of uterine cancer. The a priori statistical boundaries for noninferiority, based on the assumption of a 15% recurrence rate with laparotomy, were not reached. However, the absolute percentage difference in recurrence rates between the two treatment arms at 3 years was 1.14% (90% lower bound, −1.278; 95% upper bound, 3.996). This trial previously confirmed the feasibility and improved short-term surgical safety profile associated with laparoscopic staging for uterine cancer when compared with the same procedures undertaken via laparotomy.11 The 5-year OS of 84.8% in both arms is excellent for this population, with 13.6% demonstrating histologic evidence of metastatic disease (stages III and IV) including: 9% with positive lymph node metastasis, 2.4% with adnexal metastasis, and 2.6% with intraperitoneal spread of tumor. There is controversy worldwide regarding the survival benefit of comprehensive staging.18,19 However, as morbidity and cost associated with these procedures decrease, the added information results in individualized treatment and improvement in the overall quality of cancer care in the United States.
Improved follow-up in the initial postsurgical period of 3 years would have strengthened the study and enhanced its ability to achieve a more definitive finding, partially because of the late addition of the long-term end point, approximately 23% of patients had fewer than 3 years of follow-up. However, the fact that this study did not demonstrate statistical noninferiority as originally planned should be considered together with the fact that the initial assumption of a 15% recurrence rate with laparotomy resulted in establishment of noninferiority boundaries that were not met when actual recurrence rates were substantially lower. This, along with the resulting finding that the estimated difference in recurrence rates at 3 years was only 1.14% (90% lower bound, −1.278; 95% upper bound, 3.996), should be considered as strong evidence that laparoscopy may be an acceptable alternative to the more invasive laparotomy. The importance of demonstrating that laparoscopic staging does not adversely affect survival in patients with uterine cancer cannot be overstated. This clinical trial should alleviate concerns about missing metastatic disease or laparoscopic surgery altering recurrence rates or patterns of recurrence. Thorough surgical staging and histologic evaluation of expected metastatic sites were performed in this trial, and the differences observed were not clinically or statistically different. Evaluating the appropriateness of using laparoscopy in low-risk patients (ie, those with grades 1 to 2 endometrioid tumors), but not in those with high-risk histologic cell types, was not a study end point. This concern was examined, and we observed no significant differences in treatment effect related to histologic cell type or nuclear grading of endometrioid cell types. Because this study was not adequately powered to assess differences in recurrence rates within any subgroups, these observations warrant further investigation. Our experience suggests that neither serous papillary histology nor grade 3 endometrioid cancers involve a higher failure rate from laparoscopy because of poor detection of intraperitoneal disease or high left para-aortic lymph nodes. The concern that using laparoscopy could result in new sites of recurrent disease at the trocar sites was investigated. The low rate of port site recurrences (0.24%), three fourths of which were identified in patients with metastatic disease, has now been systematically documented and is reassuring.
These results do not demonstrate a survival decrement from laparoscopy, which allows patients and surgeons comfort in choosing the less morbid procedure. The conversion to laparotomy when adequate surgical staging cannot be completed laparoscopically allows for completion of surgical staging without compromising the patient. The results of this trial cannot be generalized to the use of laparoscopic hysterectomy without lymphadenectomy, because thorough surgical staging was required in both arms of this trial, and conversion was required when lymphadenectomy could not be completed using laparoscopy. Improved surgical training and technology may make minimally invasive surgery safer and improve success rates in the increasingly more common obese population. Two other major prospective clinical trials in the Netherlands and Australia evaluating minimally invasive treatment of endometrial cancer should not be compared to this trial because of their exclusion of nonendometrioid cell types and the comprehensive staging requirement for all of the participants in this trial.20,21 These laparoscopic surgery trials included only endometrioid histology and required only hysterectomy and bilateral salpingoophorectomy,20 and the LACE (Laparoscopic Approach to Cancer of the Endometrium) trial in Austrailia excluded nonendometrioid cell types, did not require lymphadenectomy (only recommended this), and did not mandate conversion to laparotomy for completion of staging.21
Examination of a large variety of pathologic subsets (cell type, grade, node status, stage) did not reveal any evidence of a particular subgroup that should not be treated with laparoscopy. Endometrial cancer is an ideal cancer for minimally invasive surgery. Combined with the previously published results from this study, patients treated by laparoscopy had a superior QOL through the first 6 postoperative weeks when compared with those treated by laparotomy, with fewer complications, less pain, faster recovery, and significantly reduced length of hospital stay without compromising OS.11,12
This study demonstrates that comprehensive surgical staging of endometrial cancer can be performed laparoscopically with relatively small differences in recurrence rates (estimated difference at 3 years, 1.14%; 90% lower bound, −1.278; 95% upper bound, 3.996). These results, combined with previous findings from this study of improved QOL and decreased complications associated with laparoscopy, are reassuring to patients and allow surgeons to reasonably suggest this method as a means to surgically treat and stage patients with presumed early-stage uterine cancers.
Supplementary Material
Acknowledgment
We thank Gregory Spiegel, MD, PhD (deceased September 27, 2010), who was a coauthor of our prior report, for his contributions to pathology and quality assurance for this study.
Presented in part at the Society of Gynecologic Oncologists (SGO) Annual Meeting on Women's Cancer, March 14-17, 2010, San Francisco, CA; in part at the International Gynecologic Cancer Society Meeting, October 14-18, 2006, Santa Monica, CA; at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA; and at the 37th SGO Annual Meeting, March 22-26, 2006, Palm Springs, CA.
Appendix
The following Gynecologic Oncology Group (GOG) institutions participated in the GOG 2222 (LAP2) study: Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, University of Pennsylvania Cancer Center, University of California at San Diego, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, University of California Medical Center at Irvine, Tufts–New England Medical Center, Rush–Presbyterian–St Luke's Medical Center, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Women's Cancer Center, University of Oklahoma, Tacoma General Hospital, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Fletcher Allen Health Care, University of Wisconsin Hospital, Women and Infants Hospital, and Community Clinical Oncology Program.
Footnotes
Processed as a Rapid Communication manuscript. See accompanying editorial on page 681; listen to the podcast by Dr Creutzberg at www.jco.org/podcasts
Supported by National Cancer Institute Grants No. 1CA65221 to the University of Oklahoma, CA 27469 to the Gynecologic Oncology Group (GOG) Administrative Office, and CA 37517 to the GOG Statistical and Data Center.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002706.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Joan L. Walker, Nick M. Spirtos, John B. Schlaerth
Provision of study materials or patients: Joan L. Walker, Nick M. Spirtos, Scott M. Eisenkop, John B. Schlaerth, Robert S. Mannel, Richard Barakat, Michael L. Pearl, and Sudarshan K. Sharma
Collection and assembly of data: Joan L. Walker, Marion R. Piedmonte
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Mutch DG. The New FIGO staging system for cancers of the vulva, cervix, endometrium, and sarcomas. Gynecol Oncol. 2009;115:325–328. [Google Scholar]
- 3.Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.Morrow CP, Bundy BN, Kurman FJ, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group Study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Zerbe MJ, Rosenshein NB, et al. Stage IVB endometrial carcinoma: The role of cytoreductive surgery and determinants of survival. Gynecol Oncol. 2000;78:85–91. doi: 10.1006/gyno.2000.5843. [DOI] [PubMed] [Google Scholar]
- 6.Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 7.Homesley HD, Filiaci V, Gibbons SK, et al. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group Study. Gynecol Oncol. 2009;112:543–552. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 9.Aalders J, Abeler V, Kolstad P, et al. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419–427. [PubMed] [Google Scholar]
- 10.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label non-inferiority, randomized trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 11.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornblith AB, Huang HQ, Walker JL, et al. Quality of life of patients with endometrial cancer undergoing laparoscopic surgical staging compared to laparotomy. J Clin Oncol. 2009;27:5337–5342. doi: 10.1200/JCO.2009.22.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1984;70:659–663. [Google Scholar]
- 14.Cox DR. Regression models and life-tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 15.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11:813–829. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 16.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley and Sons; 1980. pp. 163–178. [Google Scholar]
- 17.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–486. [Google Scholar]
- 19.Kitchener H, Swart AM, Qian Q, et al. Efficacy of systematic pelvic lymphadenectomy I endometrial cancer (MRC ASTEC trial): A randomized study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 21.Janda M, Gebski V, Brand A, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): A randomised trial. Lancet Oncol. 2010;11:772–780. doi: 10.1016/S1470-2045(10)70145-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.