Abstract
Purpose
The interim analysis of the National Cancer Institute of Canada Clinical Trials Group MA.17 trial showed that letrozole was significantly better than placebo in disease-free survival (DFS) for postmenopausal women with hormone receptor–positive breast cancer following about 5 years of tamoxifen therapy. When patients were unblinded, those on placebo were offered letrozole. Longer-term efficacy of letrozole, especially survival, was of particular interest because the median follow-up of the first interim analysis was only 2.5 years. Efficacy was difficult to assess because more than 60% of placebo patients crossed over to letrozole after being unblinded.
Patients and Methods
Two statistical approaches were used to adjust for the potential effects of treatment crossover: one was based on the inverse probability of censoring weighted (IPCW) Cox model and the other on a Cox model with time-dependent covariates.
Results
With a median follow-up of 64 months, the hazard ratios (HRs) of letrozole and placebo from the IPCW analyses were HR of 0.52 (95% CI, 0.45 to 0.61; P < .001) for DFS, HR of 0.51 (95% CI, 0.42 to 0.61; P < .001) for distant disease-free survival (DDFS), and HR of 0.61 (95% CI, 0.52 to 0.71; P < .001) for overall survival (OS). The results from the analyses based on the Cox model with time-dependent covariates were similar for letrozole and placebo: HR of 0.58 (95% CI, 0.47 to 0.72; P < .001) for DFS, HR of 0.68 (95% CI, 0.52 to 0.88; P = .004) for DDFS, and HR of 0.76 (95% CI, 0.60 to 0.96; P = .02) for OS.
Conclusion
Exploratory analyses based on longer follow-up and adjusting for treatment crossover suggest that extended adjuvant letrozole was superior to placebo in DFS, DDFS, and OS.
INTRODUCTION
MA.17 was a double-blind, placebo-controlled trial conducted by the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) that evaluated the use of letrozole in the extended adjuvant setting following 5 years of treatment with tamoxifen in postmenopausal women with hormone receptor–positive early-stage breast cancer. Between 1998 and 2002, 5,187 women were enrolled onto the study. The first protocol-specified interim analysis was conducted in August 2003 after 40% of the events needed for final analysis were observed. It showed that letrozole significantly improved disease-free survival (DFS) compared with placebo.1,2 Taking into consideration the crossing of the O'Brien-Fleming stopping boundary for DFS and a trend toward an overall survival (OS) advantage, the independent data and safety monitoring committee recommended stopping the trial and unblinding the study participants. Patients randomly assigned to placebo were offered the option of receiving letrozole for a period of 5 years after they were unblinded. Those on letrozole were provided the balance of their 5-year treatment and were observed from that point onward.
The median follow-up of all patients at the time of unblinding was 30 months. Longer-term effects of letrozole, especially its benefit in terms of OS, was one of the major questions left unanswered by stopping the trial at the first interim analysis. Although follow-up of all patients continued after being unblinded, assessment of long-term effects of letrozole is difficult because of selective crossover of patients. An analysis of intent-to-treat (ITT) data3 performed on a database that included data after patients were unblinded, which had a median follow-up of 64 months from random assignment, showed that the difference in DFS was still highly significant between the two treatment arms: the hazard ratio (HR) of letrozole compared with placebo was 0.68 (95% CI, 0.55 to 0.83; P < .001), but no significant difference was found in distant disease-free survival (DDFS; HR, 0.80; 95% CI, 0.63 to 1.03; P = .08) and OS (HR, 0.98; 95% CI, 0.78 to 1.22; P = .85). Results from this ITT analysis are difficult to interpret because almost two thirds of the patients originally randomly assigned to receive placebo chose to take letrozole after unblinding of their treatment assignment. Two additional analyses were performed: one using a landmark approach, which excluded patients randomly assigned to placebo who had had events before crossing over to active treatment and another based on the Cox model with treatment before and after switching as a time-dependent covariate. These two analyses compared patients randomly assigned to placebo who had crossed over to letrozole after unblinding with those who remained on placebo, and the analyses used data from the database after unblinding.4 The latter analyses indicated that patients randomly assigned to placebo who crossed over to letrozole, even after a substantial period of time since discontinuation of prior adjuvant tamoxifen, had improved DFS and DDFS compared with those who did not cross over. However, the question of longer-term efficacy of adjuvant letrozole, defined as if no patient on placebo crossed over to letrozole,5 could not be answered by these analyses.
There have been several approaches proposed in the statistical literature that could be used to estimate longer-term clinical outcomes of an experimental treatment in randomized clinical trials in the presence of substantial treatment crossover. Korn and Freidlin6 have commented on some of the existing approaches. Recently, an approach using an inverse probability of censoring weighted (IPCW) Cox proportional hazard model was used to adjust for selective crossover in assessing longer-term clinical efficacy of letrozole versus tamoxifen in the adjuvant setting based on data from the Breast International Group BIG 1-98 early-stage breast cancer adjuvant trial.7,8 This approach was discussed in some detail in a recent editorial in the Journal of Clinical Oncology.9 In this study, we determined the longer-term clinical efficacy of letrozole in MA.17 by using the IPCW method. In addition, sensitivity analyses based on an approach that models the treatment crossover effect in a Cox model with latent hazard rate10 were also performed to verify the robustness of our results from this IPCW approach.
PATIENTS AND METHODS
Study Design
The details of the study design for MA.17 have been published previously.2 In brief, MA.17 was a phase III, randomized, double-blind, placebo-controlled clinical trial designed to investigate the efficacy of letrozole in postmenopausal women with hormone receptor–positive primary breast cancer who were disease-free and within 3 months of completing approximately 5 years (range, 4.5 to 6 years) of adjuvant tamoxifen. Patients were randomly assigned to letrozole (2.5 mg orally daily) or placebo for a planned 5 years. In all, 5,187 women were enrolled but 17 patients were excluded from analysis because of noncompliance with good clinical practice guidelines. The MA.17 trial was unblinded in October 2003 on the recommendation of the Data Safety Monitoring Committee after a protocol-specified interim analysis demonstrated superiority of letrozole over placebo in DFS. All patients randomly assigned to the study were informed of the results from the interim analysis and of their treatment allocation, and those receiving placebo were offered letrozole for a planned period of 5 years. Follow-up of all patients continued after unblinding, and a database that included data after unblinding was locked on July 28, 2006, for assessment of long-term effects of letrozole on clinical outcomes. This database was used for all analyses presented herein.
Statistical Considerations
The clinical end points for this analysis are as defined in the original MA.17 trial. Specifically, DFS, the primary end point, was defined as the time from random assignment to the time of recurrence of the primary disease (in breast, chest wall, nodal, or metastatic sites) or to the development of new contralateral breast cancer. Secondary end points included DDFS defined as the time from random assignment to the time of recurrence in metastatic sites and OS defined as time from random assignment to death from any cause.
For each time to an event end point, analyses based on two approaches that adjust for treatment crossover were performed. The first approach was based on an IPCW Cox regression model.11 This approach accounts for selective treatment crossover by first censoring the original time to an event for women randomly assigned to placebo but who crossed over to letrozole after unblinding at the time of crossover and then recreates their new time to an event by weighting the time to an event of the women in the placebo group who had similar demographic and disease characteristics but who remained on placebo. The baseline factors that were significantly associated with the probability of treatment crossover4 and also the specific end point at the 0.1 level were used to calculate the weights. A pooled logistic regression model,12 weighted on the basis of estimated conditional probabilities of having remained on placebo for women who crossed over, was used to estimate the HR of letrozole to placebo as if there was no treatment crossover. This approach is denoted as the IPCW approach.
The second approach was proposed by Shao et al10 (referred to as the SCC approach on the basis of the first letters of the last names of three authors) and was based on a Cox model with a time-dependent treatment covariate: 1 for women randomly assigned to letrozole and 0 for women randomly assigned to placebo until the time when they crossed over to letrozole and 1 afterward. Additional time-dependent covariates defined as the quadratic functions of times when treatment was switched were also included in the model. Time of crossover was defined as infinity if women never switched. These additional covariates measure crossover effect caused by the selective nature of the treatment crossover. Baseline factors used for the IPCW analysis were also included in the model as fixed covariates. HRs of letrozole to placebo, as if there was no treatment crossover, were derived from the coefficient of the time-dependent treatment covariate.
RESULTS
Patient Population
Characteristics of the women randomly assigned to placebo who switched to letrozole after the trial was unblinded can be found in Goss et al.4 Briefly, among 2,587 patients who were originally randomly assigned to receive placebo, 204 (7.9%) experienced recurrence or death before the date of unblinding, 1,579 (61.0%) were confirmed to have crossed over to letrozole, and 804 (31.1%) elected no further treatment after unblinding and were considered as having remained on placebo. The median time from random assignment to treatment crossover was 2.7 years (range, 1.1 to 7.0 years) for women who crossed over from placebo to letrozole. A higher proportion of women who crossed over to letrozole were white compared with women who did not cross over (91.9% v 90.0%; P = .03) and young (median, 60.7 v 64.5 years; P < .001). Other characteristics more common in those who crossed over to letrozole included an Eastern Cooperative Oncology Group (ECOG) performance status of 0 (92.3% v 86.8%; P < .001), a longer period between initial diagnosis and random assignment (median 64.7 v 63.7 months; P < .001), positive axillary nodes (51.0% v 44.9%; P < .001), positive tumor hormone receptor status at diagnosis (98.3% v 95.5%; P = .001), prior adjuvant chemotherapy (49.2% v 37.1%; P < .001), and axillary node dissection (96.7% v 93.4%; P < .001). Among these characteristics, ethnicity (white v nonwhite), performance status (0 v 1 or 2), time from initial diagnosis to random assignment (< 5 v ≥ 5 years), prior chemotherapy (yes v no), and pathologic N stage (0 v others) were associated with DFS. Performance status (0 v 1 or 2), prior chemotherapy (yes v no), and pathologic N stage (0 v others) were associated with DDFS. Age (< 70 v ≥ 70 years), performance status (0 v 1 or 2), time from initial diagnosis to random assignment (< 5 v ≥ 5 years), pathologic N stage (0 v others), and prior chemotherapy (yes v no) were associated with OS. These characteristics were used as covariates in the IPCW and SCC analyses, respectively, for each end point.
Outcomes
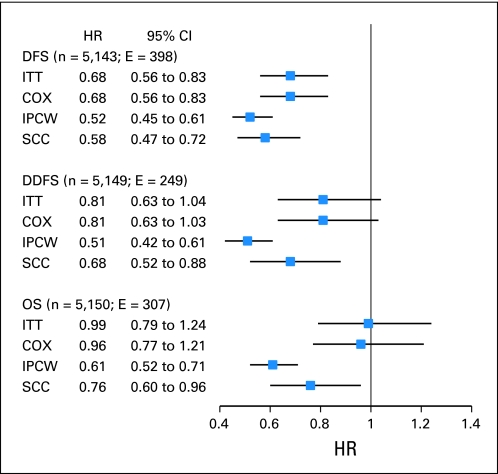
As reported previously, at a median follow-up of 64 months, the adjusted HRs for letrozole versus placebo in our ITT analysis were 0.68 (95% CI, 0.56 to 0.83; P < .001) for DFS, 0.81 (95% CI, 0.63 to 1.04; P = .09) for DDFS, and 0.99 (95% CI, 0.79 to 1.24; P = .83) for OS.3 Adjusting for treatment crossover, both IPCW and SCC analyses showed significant improvements for letrozole versus placebo for all three clinical end points (Fig 1). In the IPCW analyses, the HRs for letrozole and placebo were 0.52 (95% CI, 0.45 to 0.61; P < .001) for DFS, 0.51 (95% CI, 0.42 to 0.61; P < .001) for DDFS, and 0.61 (95% CI, 0.52 to 0.71; P < .001) for OS. By using the SCC approach for analysis, the HRs for letrozole versus placebo were 0.58 (95% CI, 0.47 to 0.72; P < .001) for DFS, 0.68 (95% CI, 0.52 to 0.88; P = .004) for DDFS, and 0.77 (95% CI, 0.61 to 0.97; P = .03) for OS.
Fig 1.
Hazard ratios (HRs) and 95% CIs for letrozole versus placebo for all women randomly assigned in the National Cancer Institute of Canada Clinical Trials Group MA.17 study. Inverse probability of censoring weighted (IPCW) analyses excluded 34 disease-free survival (DFS), 19 distant disease-free survival (DDFS), and 21 overall survival (OS) events observed after the crossover. COX, Cox proportional hazard model; E, number of events; ITT, intent to treat; SCC, approach proposed by Shao, Chang, and Chow.12.
DISCUSSION
Although stopping at the first interim analysis at a median follow-up of 30 months in MA.17 provided clear-cut proof of the strong effect extended letrozole had on improving DFS and DDFS, the effect on OS is still uncertain because the study was only powered to detect the difference in the primary end point, which was DFS. The longer follow-up data with a median duration of 64 months in the database after unblinding are useful for explaining this effect; however, results from a direct and an ITT comparison of women randomly assigned to letrozole and placebo are difficult to interpret because more than 60% of women randomly assigned to placebo elected to cross over and receive letrozole after unblinding. In the analyses described here, we used two approaches that adjusted for crossover from placebo to active treatment by using efficacy data from the post unblinding database. Results from both of these two approaches suggest that letrozole was statistically significantly superior to placebo in all three end points, including OS, which was an important secondary end point in the MA.17 trial but was not found to be significant from either the first interim or post unblinding ITT analyses. IPCW and SCC approaches showed that letrozole potentially reduces the risk of death by 35% and 24%, respectively.
We also performed an analysis that adjusted the characteristics associated with probability of crossover and outcome by including them as covariates in an ordinary Cox regression model with a treatment variable. The results are shown in Figure 1. We may find that these results are almost the same as those from the simple ITT analysis, which implies that simple covariate adjustment with a Cox model is not designed to adjust results to account for selective crossover. Unlike that analysis and another analysis that simply censors women at the time of treatment crossover, our analyses adjust for the effect of treatment crossover and provide a more reliable inference regarding the longer-term outcomes, including survival for extended endocrine therapy with letrozole. But, as pointed out by Korn and Freidlin,6 a major limitation of the statistical approaches used to adjust for treatment crossover is their requirement of some unverifiable assumptions. The three main assumptions mentioned were (1) effect of treatment is the same no matter when the treatment is given, (2) absolute treatment benefit is never greater than the actual treatment times, and (3) all patients receive the same benefit from the treatment. These assumptions may be reasonably satisfied by the data in this study. For example, Goss et al4 showed that women who started letrozole later still benefited from letrozole treatment. Another study13 pointed out another limitation of the SCC approach: it specifically requires an assumption that treatment crossover is independent of prognosis. In our study, although all women randomly assigned to placebo were offered the option of crossing over, those with comorbidities or other contraindications may not be likely to accept this option; as we have shown, the women who crossed over were younger and had better performance status. The consistency of results from the IPCW and SCC approaches suggests, however, a degree of robustness of these results.
In summary, the analyses presented here provide statistical evidence related to longer-term efficacy, especially on survival, of letrozole in the treatment of hormone receptor–positive or hormone receptor–unknown breast cancer following 5 years of tamoxifen. Following the suggestions of Korn and Freidlin,6 results presented here should be considered as exploratory, and readers should be aware there are strong assumptions behind these analyses.
Footnotes
See accompanying editorial on page 684 and articles on pages 709 and 722; listen to the podcast by Dr. Mayer at www.jco.org/podcasts
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003140.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Naiqing Zhao, Bayer Pharmaceuticals (C), Novartis (C); Paul E. Goss, Novartis (C) Stock Ownership: None Honoraria: Paul E. Goss, Novartis Research Funding: Naiqing Zhao, Bayer Pharmaceuticals, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Administrative support: Lois E. Shepherd
Provision of study materials or patients: Paul E. Goss
Collection and assembly of data: Dongsheng Tu, Lois E. Shepherd,Paul E. Goss
Data analysis and interpretation: Huan Jin, Dongsheng Tu, Naiqing Zhao, Paul E. Goss
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 2.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 3.Ingle JN, Tu D, Pater JL, et al. Intent-to-treat analysis of the placebo-controlled trial of letrozole for extended adjuvant therapy in early breast cancer: NCIC CTG MA.17. Ann Oncol. 2008;19:877–882. doi: 10.1093/annonc/mdm566. [DOI] [PubMed] [Google Scholar]
- 4.Goss PE, Ingle JN, Pater JL, et al. Late extended adjuvant treatment with letrozole improves outcome in women with early-stage breast cancer who complete 5 years of tamoxifen. J Clin Oncol. 2008;26:1948–1955. doi: 10.1200/JCO.2007.11.6798. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A, Zeger SL. On estimating efficacy from clinical trials. Stat Med. 1991;10:45–52. doi: 10.1002/sim.4780100110. [DOI] [PubMed] [Google Scholar]
- 6.Korn EL, Freidlin B. Causal inference for definitive clinical end points in a randomized clinical trial with intervening nonrandomized treatments. J Clin Oncol. 2010;28:3800–3802. doi: 10.1200/JCO.2010.30.0442. [DOI] [PubMed] [Google Scholar]
- 7.BIG 1-98 Collaborative Group. Mouridsen H, Giobbie-Hurder A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colleoni M, Giobbie-Hurder A, Regan MM, et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J Clin Oncol. 2011;29:1117–1124. doi: 10.1200/JCO.2010.31.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkelstein DM, Schoenfeld DA. Correcting for discretionary treatment crossover in an analysis of survival in the Breast International Group BIG 1-98 trial by using the inverse probability of censoring weighted method. J Clin Oncol. 2011;29:1093–1095. doi: 10.1200/JCO.2010.33.9374. [DOI] [PubMed] [Google Scholar]
- 10.Shao J, Chang M, Chow SC. Statistical inference for cancer trials with treatment switching. Stat Med. 2005;24:1783–1790. doi: 10.1002/sim.2128. [DOI] [PubMed] [Google Scholar]
- 11.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostino RB, Lee ML, Belanger AJ, et al. Relation of pooled logistic regression to time dependent Cox regression analysis: The Framingham Heart Study. Stat Med. 1990;9:1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]
- 13.White IR. Estimating treatment effects in randomized trials with treatment switching. Stat Med. 2006;25:1619–1622. doi: 10.1002/sim.2453. [DOI] [PubMed] [Google Scholar]