Abstract
Purpose
To determine the frequency of DNMT3A mutations, their associations with clinical and molecular characteristics and outcome, and the associated gene- and microRNA-expression signatures in primary cytogenetically normal acute myeloid leukemia (CN-AML).
Patients and Methods
Four hundred fifteen previously untreated adults were analyzed for DNMT3A mutations and established prognostic gene mutations and expression markers. Gene- and microRNA-expression profiles were derived using microarrays.
Results
Younger (< 60 years; n = 181) and older (≥ 60 years; n = 234) patients had similar frequencies of DNMT3A mutations (35.3% v 33.3%). Missense mutations affecting arginine codon 882 (R882-DNMT3A) were more common (n = 92; 62%) than those affecting other codons (non–R882-DNMT3A). DNMT3A-mutated patients did not differ regarding complete remission rate, but had shorter disease-free survival (DFS; P = .03) and, by trend, overall survival (OS; P = .07) than DNMT3A–wild-type patients. In multivariable analyses, DNMT3A mutations remained associated with shorter DFS (P = .01), but not with shorter OS. When analyzed separately, the two DNMT3A mutation types had different significance by age group. Younger patients with non–R882-DNMT3A mutations had shorter DFS (P = .002) and OS (P = .02), whereas older patients with R882-DNMT3A mutations had shorter DFS (P = .005) and OS (P = .002) after adjustment for other clinical and molecular prognosticators. Gene- and microRNA-expression signatures did not accurately predict DNMT3A mutational status.
Conclusion
DNMT3A mutations are frequent in CN-AML, and their clinical significance seems to be age dependent. DNMT3A-R882 mutations are associated with adverse prognosis in older patients, and non–R882-DNMT3A mutations are associated with adverse prognosis in younger patients. Low accuracy of gene- and microRNA-expression signatures in predicting DNMT3A mutation status suggested that the role of these mutations in AML remains to be elucidated.
INTRODUCTION
Acute myeloid leukemia (AML) is a genetically heterogeneous disease characterized by nonrandom cytogenetic aberrations1,2 and, at the submicroscopic level, recurrent gene mutations and changes in gene expression.3 Cytogenetic and molecular alterations not only define distinct biologic entities, but are also relevant for disease classification and treatment guidance.4
Cytogenetically normal (CN) AML, comprising 45% to 50% of adults with primary disease,5 is one of the best molecularly characterized cytogenetic groups. Some gene mutations recurrent in CN-AML are strong, independent prognosticators (eg, FLT3 internal tandem duplications [FLT3-ITD],6,7 CEBPA,8,9 and WT110,11 mutations), whereas others affect outcome in distinct molecular or clinical subsets of CN-AML (eg, NPM1,12,13TET2,14 and IDH1/IDH215,16 mutations) or are of uncertain significance (eg, FLT3-tyrosine kinase domain mutations [FLT3-TKD]17,18). More intense treatment may modify the prognostic weight of some molecular markers in CN-AML, such as MLL partial tandem duplication (MLL-PTD)19,20 or FLT3-ITD.21 Additionally, altered expression of genes (eg, high BAALC,22,23ERG,23,24 and MN125–27 levels) and microRNAs (eg, low miR-181a level28) identify high-risk CN-AML patients.
The DNMT3A gene encodes one of the three DNA methyltransferase (DNMT) isoforms. Among these, DNMT1 is the most abundant and preferentially replicates existing DNA methylation patterns, whereas DNMT3A and DNMT3B are responsible for establishing de novo DNA methylation. The process of DNA methylation consists of an enzymatic addition of a methyl group at the carbon 5 position of cytosine in the context of cytosine-guanine dinucleotides. When occurring in the promoter region of a coding gene, it generally results in gene silencing. In AML, all three DNMT enzymes are reportedly overexpressed in malignant blasts compared with normal bone marrow (BM) cells and contribute to leukemogenesis by mediating tumor suppressor gene silencing.29 Somatic DNMT3A mutations in AML were first described by Yamashita et al30 and subsequently by other groups.31,32 Ley et al31 first reported that DNMT3A mutations conferred worse outcome in AML. However, the patients analyzed were heterogeneous for biologic and clinical characteristics and treatment received, and the prognostic value of DNMT3A mutations was not fully evaluated within the context of other known molecular prognosticators.31 Recently, Thol et al33 reported that DNMT3A mutations are associated with shorter overall survival (OS) in cytogenetically diverse patients with AML who are younger than 60 years and with lower complete remission (CR) rates and shorter OS in a CN-AML subset. However, this study included patients with secondary disease and those who received allogeneic stem-cell transplantation (SCT) and did not analyze the prognostic impact of different types of DNMT3A mutations.33
To our knowledge, our study is the first to investigate the prognostic impact of DNMT3A mutations in a large population of patients diagnosed exclusively with primary CN-AML, comprehensively characterized for other molecular prognosticators, and receiving intensive chemotherapy. Additionally, we analyzed the differential impact of DNMT3A mutations by age group (younger [< 60 years] v older [≥ 60 years]) and mutation type (missense mutations at codon R882 [hereafter called R882-DNMT3A] v mutations at other locations [denoted non–R882-DNMT3A]). Furthermore, to gain insights into the biologic role of DNMT3A mutations in CN-AML, we derived genome-wide DNMT3A mutation-associated gene- and microRNA-expression signatures.
PATIENTS AND METHODS
Patients, Treatment, and Cytogenetic Studies
Pretreatment BM or blood samples were obtained from 415 patients with primary CN-AML, 18 to 83 years of age (181 younger and 234 older), who received intensive first-line therapy on Cancer and Leukemia Group B trials.34–42 Patients received cytarabine-daunorubicin-based induction chemotherapy; most younger patients received consolidation with high-dose chemotherapy and autologous SCT. Per protocol, no patient received allogeneic SCT during first CR. For details regarding treatment protocols and sample collection, see the Data Supplement. The diagnosis of normal cytogenetics was based on centrally reviewed analysis of ≥ 20 metaphases in BM specimens.43 All patients provided written informed consent; study protocols were in accordance with the Declaration of Helsinki and approved by local institutional review boards.
Mutational Analyses
For DNMT3A mutational analysis, the sequences of exons 18, 19, 21, 22, and 24 to 26 (GenBank reference NM_175629) were analyzed from genomic DNA by polymerase chain reaction and direct sequencing. Patients were also characterized for FLT3-ITD,7,44FLT3-TKD,17MLL-PTD,20,45 mutations in NPM1,13CEBPA,8WT1,10,11TET214 and IDH1/IDH2,15 and expression levels of ERG23,24 and BAALC,22,23 as previously reported. Molecular analyses were performed at The Ohio State University.
Microarray Experiments
Gene-expression profiling was performed using oligonucleotide microarrays (Affymetrix, Santa Clara, CA), and microRNA-expression profiling was performed using a custom microarray, as previously reported.13,14,46 Expression signatures were identified by comparing DNMT3A-mutated and DNMT3A–wild-type (DNMT3A-wt) patients, and analyses to predict DNMT3A mutation status were performed (Data Supplement).
Statistical Analyses
Baseline characteristics were compared between DNMT3A-mutated and DNMT3A-wt patients using Fisher's exact test for categorical and the Wilcoxon rank sum test for continuous variables. Clinical end points were defined according to published recommendations (Data Supplement).47 For time-to-event analyses, survival estimates were calculated using the Kaplan-Meier method, and groups were compared using the log-rank test. In addition to analyzing all DNMT3A-mutated cases as a combined group, we also evaluated the prognostic significance of R882-DNMT3A and non–R882-DNMT3A mutations separately, in the entire cohort and in the younger and older groups.
In models considering both age groups, we adjusted for an age-group effect (≥ 60 years v < 60 years). We constructed multivariable logistic regression models to analyze factors influencing achievement of CR and multivariable Cox proportional hazards models for factors associated with survival end points (Data Supplement). All analyses were performed by the Alliance for Clinical Trials in Oncology Statistics and Data Center.
RESULTS
Prevalence and Spectrum of DNMT3A Mutations in Primary CN-AML
Excluding known single-nucleotide polymorphisms, 148 nonsynonymous sequence variations (mutations) in DNMT3A were found in 142 (34.2%) of 415 patients (Data Supplement). The frequencies of these mutations were similar in younger (35.3%) and older (33.3%) patients. Six patients had two mutations each, and four mutations appeared homozygous. Ninety-two mutations (62%) were missense changes in codon R882, leading to an amino acid exchange from arginine to histidine (R882H, n = 49), cysteine (R882C, n = 36), proline (R882P, n = 3), serine (R882S, n = 3), or glycine (R882G, n = 1). R882-DNMT3A missense mutations were the most common mutation type among both younger (26%) and older (19%) patients. The remaining non–R882-DNMT3A mutations (n = 56; 38%) included 22 nonsense, frameshift, and splice-site mutations found in 22 different patients. These mutations are predicted to either trigger nonsense-mediated RNA decay or result in a truncated protein and thus are likely to impair protein function.48 Two of these 22 patients concomitantly had an R882-DNMT3A mutation (for outcome analyses, these patients were included in the R882-DNMT3A mutation group), and two others concomitantly had another non–R882-DNMT3A missense mutation. Furthermore, there were 32 missense mutations not affecting codon R882 and two short in-frame deletions. All 32 non-R882 missense mutations were predicted to be “disease causing” by the MutationTaster software,49 a computational algorithm that evaluates the disease-causing potential of gene mutations on the basis of evolutionary conservation and structural protein features.
Associations of DNMT3A Mutations With Pretreatment Clinical and Molecular Characteristics
No differences in age, sex, or race were observed between patients with and without DNMT3A mutations. However, DNMT3A-mutated patients had higher WBC counts (P < .001) and BM blasts percentages (P = .03) and harbored NPM1 mutations (P < .001) and FLT3-ITD (P = .01) more often and CEBPA mutations (P < .001) less often than those with DNMT3A-wt (Table 1).
Table 1.
Clinical and Molecular Characteristics of 415 Patients With Primary Cytogenetically Normal Acute Myeloid Leukemia According to DNMT3A Mutation Status
| Characteristic |
DNMT3A Mutated (n = 142) |
DNMT3A Wild Type (n = 273) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .46 | ||||
| Median | 61 | 62 | |||
| Range | 22-82 | 18-83 | |||
| Age, years | .68 | ||||
| < 60 | 64 | 45 | 117 | 43 | |
| ≥ 60 | 78 | 55 | 156 | 57 | |
| Female sex | 72 | 51 | 135 | 49 | .84 |
| Race | .49 | ||||
| White | 124 | 89 | 247 | 91 | |
| Nonwhite | 16 | 11 | 25 | 9 | |
| Hemoglobin, g/dL | .80 | ||||
| Median | 9.4 | 9.4 | |||
| Range | 4.8-14.5 | 4.6-15 | |||
| Platelet count, ×109/L | .55 | ||||
| Median | 66 | 61 | |||
| Range | 4-481 | 7-850 | |||
| WBC, ×109/L | < .001 | ||||
| Median | 43.4 | 22.4 | |||
| Range | 0.9-434.1 | 0.9-450 | |||
| Percentage of blood blasts | .83 | ||||
| Median | 58 | 57 | |||
| Range | 0-97 | 0-99 | |||
| Percentage of bone marrow blasts | .03 | ||||
| Median | 70 | 66 | |||
| Range | 4-97 | 7-96 | |||
| FAB category | < .001 | ||||
| M0 | 1 | 1 | 7 | 4 | |
| M1 | 29 | 27 | 54 | 27 | |
| M2 | 18 | 17 | 71 | 36 | |
| M4 | 33 | 31 | 41 | 21 | |
| M5 | 26 | 24 | 21 | 11 | |
| M6 | 0 | 0 | 3 | 2 | |
| NPM1 | < .001 | ||||
| Mutated | 107 | 75 | 146 | 53 | |
| Wild type | 35 | 25 | 127 | 47 | |
| FLT3-ITD | .01 | ||||
| Present | 62 | 44 | 85 | 31 | |
| Absent | 80 | 56 | 188 | 69 | |
| CEBPA | < .001 | ||||
| Mutated | 7 | 5 | 58 | 21 | |
| Single mutated | 4 | 26 | |||
| Double mutated | 3 | 32 | |||
| Wild type | 135 | 95 | 215 | 79 | |
| ELN genetic group† | .10 | ||||
| Favorable | 60 | 42 | 140 | 51 | |
| Intermediate-I | 82 | 58 | 133 | 49 | |
| FLT3-TKD | 1.00 | ||||
| Present | 10 | 7 | 21 | 8 | |
| Absent | 128 | 93 | 245 | 92 | |
| WT1 | .37 | ||||
| Mutated | 10 | 7 | 28 | 10 | |
| Wild type | 132 | 93 | 245 | 90 | |
| TET2 | .54 | ||||
| Mutated | 31 | 22 | 67 | 25 | |
| Wild type | 110 | 78 | 202 | 75 | |
| MLL-PTD | 1.00 | ||||
| Present | 7 | 6 | 15 | 6 | |
| Absent | 116 | 94 | 228 | 94 | |
| IDH1 | .07 | ||||
| R132 | 22 | 16 | 26 | 10 | |
| Wild type | 118 | 84 | 246 | 90 | |
| IDH2 | |||||
| Mutated | 24 | 17 | 51 | 19 | .79 |
| Codon R140 | 18 | 44 | |||
| Codon R172 | 6 | 7 | |||
| Wild type | 116 | 83 | 221 | 81 | |
| ERG expression group‡ | .39 | ||||
| High | 56 | 55 | 92 | 49 | |
| Low | 45 | 45 | 94 | 51 | |
| BAALC expression group‡ | .38 | ||||
| High | 45 | 47 | 104 | 53 | |
| Low | 51 | 53 | 93 | 47 | |
Abbreviations: FAB, French-American-British classification; ELN, European LeukemiaNet; FLT3-ITD, internal tandem duplication of the FLT3 gene; FLT3-TKD, tyrosine kinase domain mutation in the FLT3 gene; MLL-PTD, partial tandem duplication of the MLL gene.
P values for categorical variables are from Fisher's exact test; P values for continuous variables are from the Wilcoxon rank sum test.
The ELN favorable genetic group includes patients with mutated CEBPA and/or mutated NPM1 without FLT3-ITD.4 The ELN intermediate-I risk group comprises the remaining patients with CN-AML who had wild-type CEBPA and wild-type NPM1 with or without FLT3-ITD or mutated NPM1 with FLT3-ITD.
The median expression value was used as a cut point.
Because AML biology and treatment regimens differ between younger and older patients, and it is unclear whether R882-DNMT3A and non–R882-DNMT3A mutations are functionally and clinically equivalent, we performed subgroup analyses taking age and DNMT3A mutation types into account. Younger R882-DNMT3A–mutated patients more often had NPM1 mutations (P = .02) and FLT3-ITD (P = .03) and less often had CEBPA mutations (P < .001), WT1 mutations (P = .02), and low ERG expression (P = .04) than DNMT3A-wt patients (Data Supplement). Younger patients with non–R882-DNMT3A mutations were more frequently NPM1-mutated (P = .02) and showed trends toward a higher frequency of FLT3-ITD (P = .11) and lower frequency of CEBPA mutations (P = .07; Data Supplement). Among older patients, those with R882-DNMT3A mutations showed trends toward a higher frequency of NPM1 mutations (P = .09) and FLT3-ITD (P = .15) and lower frequency of CEBPA mutations (P = .14; Data Supplement). Older patients with non–R882-DNMT3A mutations were more likely NPM1-mutated (P = .003) and, by trend, WT1-mutated (P = .07) and less likely CEBPA-mutated (P = .05; Data Supplement).
Association of DNMT3A Mutation Status With Clinical Outcome
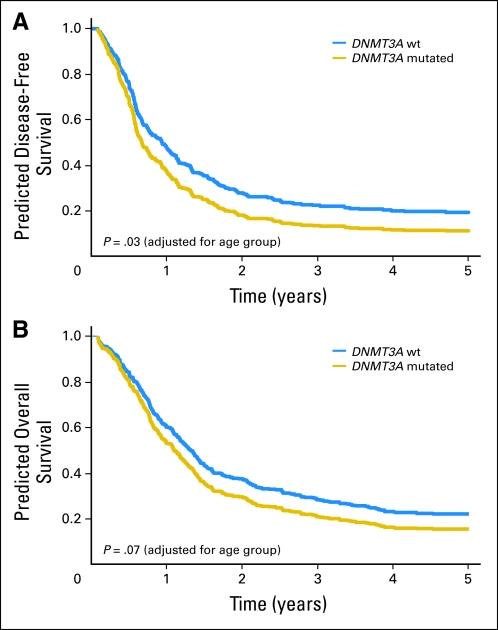
When younger and older patients were considered together in analyses adjusted for age group, DNMT3A mutations were not associated with the probability of CR attainment (P = .42; Table 2). With a median follow-up of 7.5 years (range, 2.3 to 12.4 years) for patients alive, those harboring DNMT3A mutations had shorter disease-free survival (DFS; P = .03) and a trend toward shorter OS (P = .07) than DNMT3A-wt patients (Table 2; Fig 1). In a multivariable analysis for DFS (Table 3), DNMT3A mutations were associated with a 47% increased risk of relapse or death (P = .01), once adjusted for FLT3-ITD, WT1 mutations, MLL-PTD status, and age group. In contrast, once adjusted for other clinical and molecular prognosticators, there was no association of DNMT3A mutation status with OS.
Table 2.
Age Group–Adjusted Analysis of Outcomes of Patients With Primary Cytogenetically Normal Acute Myeloid Leukemia According to DNMT3A Mutation Status
| End Point | DNMT3-mut (n = 142) | R882-DNMT3A (n = 92) | non–R882-DNMT3A (n = 50) | DNMT3A-wt (n = 273) | P (DNMT3A-mut vDNMT3A-wt) | P (R882-DNMT3AvDNMT3A-wt) | P (non–R882-DNMT3AvDNMT3A-wt) |
|---|---|---|---|---|---|---|---|
| Complete remission | .42 | .85 | .21 | ||||
| Odds ratio | 1.22 | 1.05 | 1.62 | Reference group | |||
| 95% CI | 0.75 to 1.96 | 0.61 to 1.83 | 0.76 to 3.43 | ||||
| Disease-free survival | .03 | .03 | .19 | ||||
| Hazard ratio | 1.34 | 1.42 | 1.30 | Reference group | |||
| 95% CI | 1.02 to 1.75 | 1.03 to 1.96 | 0.88 to 1.90 | ||||
| Overall survival | .07 | .05 | .39 | ||||
| Hazard ratio | 1.25 | 1.32 | 1.16 | Reference group | |||
| 95% CI | 0.98 to 1.57 | 1.01 to 1.74 | 0.83 to 1.63 |
Abbreviations: mut, mutated; wt, wild type.
Fig 1.
Age group–adjusted clinical outcome for patients with and without DNMT3A mutations. (A) Disease-free survival. (B) Overall survival. The curves are adjusted for age group. wt, wild type.
Table 3.
Multivariable Analyses for Outcome in Patients With Primary Cytogenetically Normal Acute Myeloid Leukemia
| Group | Disease-Free Survival |
Overall Survival |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| All patients | ||||||
| DNMT3A, mutated v wild type | 1.47 | 1.08 to 2.00 | .01 | DNMT3A mutation status was not significantly associated with OS upon adjusting for other variables | ||
| FLT3-ITD, ITD v no ITD | 1.82 | 1.34 to 2.48 | < .001 | |||
| WT1, mutated v wild type | 2.17 | 1.26 to 3.72 | .005 | |||
| MLL-PTD, present v absent | 1.95 | 1.12 to 3.39 | .02 | |||
| Age group, older v younger | 2.53 | 1.89 to 3.39 | < .001 | |||
| Patients, age < 60 years | ||||||
| DNMT3A, non-R882-mutated v wild type | 2.78 | 1.45 to 5.36 | .002 | 2.24 | 1.17 to 4.30 | .02 |
| NPM1, mutated v wild type | 0.52 | 0.29 to 0.95 | .03 | 0.38 | 0.23 to 0.64 | < .001 |
| FLT3-ITD, ITD v no ITD | 1.79 | 1.07 to 3.02 | .03 | 1.70 | 1.06 to 2.76 | .03 |
| CEBPA, double-mutated v single-mutated or wild type | 0.21 | 0.09 to 0.50 | < .001 | 0.15 | 0.06 to 0.35 | < .001 |
| WT1, mutated v wild type | 4.88 | 2.37 to 10.04 | < .001 | 5.91 | 3.20 to 10.90 | < .001 |
| Patients, age ≥ 60 years | ||||||
| DNMT3A, R882-mutated v wild type | 1.85 | 1.20 to 2.84 | .005 | 1.76 | 1.24 to 2.49 | .002 |
| NPM1, mutated v wild type | 0.54 | 0.36 to 0.80 | .002 | 0.48 | 0.35 to 0.66 | < .001 |
| FLT3-ITD, ITD v no ITD | 2.00 | 1.34 to 2.98 | < .001 | 1.80 | 1.31 to 2.47 | < .001 |
| Age, each 10 year increase | 0.96 | 0.93 to 0.99 | .02 | |||
Abbreviations: FLT3-ITD, internal tandem duplication of the FLT3 gene; HR, hazard ratio; MLL-PTD, partial tandem duplication of the MLL gene.
Association of Different DNMT3A Mutation Types With Clinical Outcome
We tested the association of the two types of DNMT3A mutations with outcome of younger and older patients separately because these age groups were treated on Cancer and Leukemia Group B protocols that differ in chemotherapy intensity (Data Supplement). Neither type of DNMT3A mutation had an impact on the probability of achieving CR in younger or older patients.
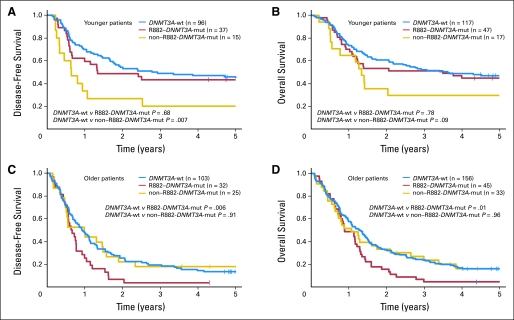
In younger patients, R882-DNMT3A mutations were not significantly associated with DFS or OS (Table 4, Figs 2A and 2B). In contrast, patients harboring non–R882-DNMT3A mutations had a significantly shorter DFS (P = .007; 3-year rates, 20% v 49%; Fig 2A) and a trend toward shorter OS (P = .09; 3-year rates, 29% v 52%; Fig 2B) than DNMT3A-wt patients (Table 4). In a multivariable analysis for DFS (Table 3), patients with non–R882-DNMT3A mutations had an almost three-fold increased risk of relapse or death (P = .002), once adjusted for FLT3-ITD and mutations in NPM1, CEBPA, and WT1. Likewise, in a multivariable model for OS (Table 3), the risk of death of non–R882-DNMT3A–mutated patients was more than twice that of DNMT3A-wt patients (P = .02) after adjustment for FLT3-ITD, NPM1, CEBPA, and WT1 mutation status.
Table 4.
Outcome of Patients With Primary Cytogenetically Normal Acute Myeloid Leukemia, According to Age Group and Type of DNMT3A Mutation
| End Point | DNMT3A-mut | R882-DNMT3A | non–R882-DNMT3A | DNMT3A-wt | P (DNMT3A-mut vDNMT3A-wt) | P (R882-DNMT3AvDNMT3A-wt) | P (non–R882-DNMT3AvDNMT3A-wt) |
|---|---|---|---|---|---|---|---|
| Patients < 60 years of age, no. | 64 | 47 | 17 | 117 | |||
| Complete remission rate, % | 81 | 79 | 88 | 96 (82) | 1.00 | .66 | .74 |
| Disease-free survival | .16 | .68 | .007 | ||||
| Median, years | 1.1 | 1.3 | 0.7 | 2.9 | |||
| % Disease-free at 3 years | 37 | 43 | 20 | 49 | |||
| 95% CI | 24 to 49 | 27 to 58 | 5 to 42 | 39 to 58 | |||
| Overall survival | .36 | .78 | .09 | ||||
| Median, years | 1.4 | 3.5 | 1.3 | 3.6 | |||
| % Alive at 3 years | 45 | 51 | 29 | 52 | |||
| 95% CI | 33 to 57 | 36 to 64 | 11 to 51 | 43 to 61 | |||
| Patients ≥ 60 years of age, no. | 78 | 45 | 33 | 156 | |||
| Complete remission rate,% | 73 | 71 | 76 | 66 | .30 | .59 | .31 |
| Disease-free survival | .11 | .006 | .91 | ||||
| Median, years | 0.7 | 0.7 | 1.0 | 1.0 | |||
| % Disease-free at 3 years | 11 | 3 | 20 | 21 | |||
| 95% CI | 4 to 20 | 1 to 14 | 7 to 37 | 14 to 30 | |||
| Overall survival | .10 | .01 | .96 | ||||
| Median, years | 1.0 | 0.9 | 1.1 | 1.3 | |||
| % Alive at 3 years | 12 | 4 | 24 | 24 | |||
| 95% CI | 6 to 21 | 1 to 13 | 11 to 39 | 17 to 31 |
Abbreviations: mut, mutated; wt, wild type.
Fig 2.
Kaplan-Meier survival curves according to DNMT3A mutation type (R882-DNMT3A v non–R882-DNMT3A mutations v DNMT3A wild type). (A) Disease-free survival and (B) overall survival of younger (< 60 years) patients. (C) Disease-free survival and (D) overall survival of older (≥ 60 years) patients. mut, mutated; wt, wild type.
In older patients, R882-DNMT3A mutations were associated with significantly shorter DFS (P = .006; 3-year rates, 3% v 21%; Fig 2C) and OS (P = .01; 3-year rates, 4% v 24%; Fig 2D), whereas non–R882-DNMT3A mutations were not (Table 4, Figs 2C and 2D). In a multivariable model for DFS (Table 3), R882-DNMT3A mutations remained associated with an 85% increased risk of relapse or death (P = .005) after adjustment for NPM1 mutation and FLT3-ITD status and age. Similarly, in a multivariable model for OS (Table 3), R882-DNMT3A mutations were associated with a 76% increased risk of death (P = .002) once adjusted for NPM1 mutation and FLT3-ITD status.
Gene- and microRNA-Expression Signatures Associated With DNMT3A Mutations
To gain insights into the biology of DNMT3A-mutated CN-AML, we studied mutation-associated gene-expression signatures in a subset of patients (n = 278) with available material. Clinical and molecular characteristics and outcome of this subset were similar to those of patients not analyzed.
A gene-expression signature associated with DNMT3A mutations comprised 1,886 differentially expressed probe sets: 1,323 were upregulated and 563 downregulated in DNMT3A-mutated patients (Data Supplement). The most upregulated known gene was VCAN, encoding a protein involved in cell adhesion, proliferation, migration, and angiogenesis; the most downregulated gene was ALAS2, involved in the heme biosynthetic pathway. However, the signature had an overall cross-validated accuracy of only 67% for predicting DNMT3A mutation status (62% sensitivity; 70% specificity), thereby suggesting the contributing effect of other associated molecular aberrations.
When we attempted to derive gene-expression signatures associated with specific types of DNMT3A mutations, no significant signature separated patients harboring non–R882-DNMT3A mutations (n = 32) from those with DNMT3A-R882 mutation (n = 60).
For microRNA profiling, younger and older patients were analyzed separately to avoid confounding batch effects. Testing for differentially expressed microRNAs revealed no signature associated with DNMT3A mutations in the younger group. In contrast, we derived a signature consisting of 12 microRNAs associated with DNMT3A mutations in older patients (Data Supplement), with four microRNA probes upregulated, including a member of the miR-10 family reportedly associated with NPM1 mutations,13 and eight microRNA probes downregulated, including miR-181c, a member of the miR-181 family associated with CEBPA mutations.8 However, these features might reflect confounding as a result of the significant positive association of DNMT3A mutations with NPM1 mutations and the negative association with CEBPA mutations. This microRNA-expression signature had an overall accuracy of only 58% for predicting DNMT3A mutation status (49% sensitivity; 62% specificity).
DISCUSSION
Advanced sequencing technologies have allowed analysis of the whole genome of AML blasts. Application of these technologies has recently identified two novel recurrent gene mutations in CN-AML, first IDH1 mutations50 and, more recently, DNMT3A mutations.31 As this approach becomes broadly used, it is likely that previously unrecognized mutations in AML will continue to emerge. Because these mutations have the potential to contribute to myeloid leukemogenesis and become prognostic factors and/or therapeutic targets, it is imperative to rapidly test their biologic and clinical impact on patients with AML. However, from previously discovered mutated or aberrantly expressed genes in CN-AML, we have learned that only rarely is testing for a single genetic alteration sufficient for accurate outcome prediction and treatment guidance.13 Instead, the clinical impact of most molecular markers is influenced by other, concurrent molecular aberrations.12,14–16,21 Therefore, to fully understand the clinical significance of emerging molecular markers, such as DNMT3A mutations, they need to be evaluated in large series of patients homogeneous for age and type of disease (primary v secondary or treatment-related AML), similarly treated and fully characterized for established prognostic markers. To our knowledge, our study analyzed DNMT3A mutations in the largest CN-AML patient cohort to date and is first to report subgroup analyses and multivariable models considering different types of DNMT3A mutations in distinct age groups.
We found that DNMT3A mutations were among the most common mutations in CN-AML, occurring in 34% of patients, with a similar frequency among younger and older patients, and were significantly associated with NPM1 mutations, FLT3-ITD, and wild-type CEBPA. Regarding prognostic significance, we showed that DNMT3A mutations had worse DFS and OS, after adjustment for age. Moreover, we observed that the prognostic significance of DNMT3A mutations depended both on age and the type of mutation (R882-DNMT3A v non–R882-DNMT3A) considered concurrently (see Fig 2 and also Data Supplement). In younger patients, only non–R882-DNMT3A mutations were associated with worse clinical outcome, whereas R882-DNMT3A mutations had no prognostic significance. Conversely, in older patients, only R882-DNMT3A mutations, not non–R882-DNMT3A mutations, were independently associated with worse outcome. The reasons why the prognostic significance of different DNMT3A mutation types varies in younger and older patients are currently unknown. One could postulate that this is related to their association with other prognosticators. Thus, in older patients, the potentially negative prognostic significance of non–R882-DNMT3A mutations might have been somewhat offset by a high incidence (79%) of accompanying NPM1 mutations, known to favorably affect prognosis of older patients.13 However, two thirds of older patients with the prognostically adverse R882-DNMT3A mutations also harbored NPM1 mutations, and slight differences in frequencies of other molecular markers between patients harboring R882-DNMT3A and non–R882-DNMT3A mutations, both in the older and younger age groups, do not seem sufficient to account for the differential association of the two DNMT3A mutation types on treatment outcome.
Our results differ somewhat from those reported by Ley et al,31 who found a strong, independent association of DNMT3A mutations with OS, and those by Thol et al,33 who studied only patients younger than 60 years and who found that in the CN-AML subgroup, DNMT3A mutations were associated with a lower CR rate and shorter OS in multivariable analyses. These discrepancies may be related to differences in the patient populations analyzed with respect to their size, cytogenetics, molecular markers, age, disease type, and treatment. Furthermore, previous studies did not include older patients33 or included only a small proportion of older patients and did not present data on CR rates, DFS, or multivariable analyses for patients with CN-AML.31 Therefore, a direct comparison of the findings across studies is not possible.
We also report the first gene- and microRNA-expression signatures associated with DNMT3A mutations. However, the accuracy of the gene-expression signature in predicting DNMT3A mutational status was only 67%. These results are consistent with an unsupervised analysis of gene-expression array data reported by Ley et al,31 where no patient cluster was clearly linked to DNMT3A mutation status. Similarly, a microRNA-expression signature derived in older patients with CN-AML comprised microRNAs strongly associated with other markers (ie, NPM1 mutations and wild-type CEBPA) and was not accurate in predicting DNMT3A mutational status. These results suggest that DNMT3A mutations have no strong impact on genome-wide gene- and microRNA-expression profiles in CN-AML. The signatures we identified might at least partially reflect the association of DNMT3A mutation status with other molecular markers that are themselves associated with characteristic gene- and microRNA-expression signatures.
The mechanisms through which DNMT3A mutations contribute to leukemogenesis are not yet characterized. Although two studies31,33 found no differences in global DNA methylation or changes in gene methylation patterns in DNMT3A-mutated patients, other reports30,32 suggested that most of the DNMT3A mutations decrease the enzymatic activity of the encoded protein. Uncovering how DNMT3A mutations affect DNA methylation and epigenetic regulation of gene expression may have ramifications for treatment selection because DNA hypomethylating agents, such as decitabine, are increasingly used for up-front or salvage therapies in older patients with AML,51 and response to these drugs may be affected by alterations in DNMT3A function.52
In summary, testing for the mutations in DNMT3A may provide a new tool for refining age-related risk classification of CN-AML. The strongest prognostic significance was found in older patients harboring R882-DNMT3A mutations, whereas non–R882-DNMT3A mutations were associated with relapse risk in younger patients. The gene- and microRNA-expression signatures were not accurate in predicting DNMT3A mutational status likely because they are affected by other, concurrent molecular markers. Thus the contribution of the DNMT3A mutations to myeloid leukemogenesis requires further investigation, as does the usefulness of DNMT3A mutations for risk stratification both in patients with CN-AML and in other cytogenetic and molecular subsets of AML.
Supplementary Material
Acknowledgment
We thank Donna Bucci and the Cancer and Leukemia Group B Leukemia Tissue Bank at the The Ohio State University Comprehensive Cancer Center, Columbus, OH, for sample processing and storage services, and Lisa J. Sterling and Colin G. Edwards, PhD, for data management.
Appendix
The following Cancer and Leukemia Group B (CALGB) institutions, principal investigators, and cytogeneticists participated in this study: Wake Forest University School of Medicine, Winston-Salem, NC: David D. Hurd, P. Nagesh Rao, Wendy L. Flejter, and Mark J. Pettenati (Grant No. CA03927); The The Ohio State University Medical Center, Columbus, OH: Clara D. Bloomfield, Karl S. Theil, Diane Minka, and Nyla A. Heerema (Grant No. CA77658); North Shore–Long Island Jewish Health System, Manhasset, NY: Daniel R. Budman and Prasad R.K. Koduru (Grant No. CA35279); University of Iowa Hospitals, Iowa City, IA: Daniel A. Vaena and Shivanand R. Patil (Grant No. CA47642); Roswell Park Cancer Institute, Buffalo, NY: Ellis G. Levine and AnneMarie W. Block (Grant No. CA02599); Duke University Medical Center, Durham, NC: Jeffrey Crawford, Sandra H. Bigner, Mazin B. Qumsiyeh, John Eyre, and Barbara K. Goodman (Grant No. CA47577); Washington University School of Medicine, St. Louis, MO: Nancy L. Bartlett, Michael S. Watson, Eric C. Crawford, and Jaime Garcia-Heras (Grant No. CA77440); University of Chicago Medical Center, Chicago, IL: Hedy L. Kindler, Diane Roulston, Katrin M. Carlson, Yanming Zhang, and Michelle M. Le Beau (Grant No. CA41287); University of North Carolina, Chapel Hill, NC: Thomas C. Shea and Kathleen W. Rao (Grant No. CA47559); University of Massachusetts Medical Center, Worcester, MA: William V. Walsh, Vikram Jaswaney, Michael J. Mitchell, and Patricia Miron (Grant No. CA37135); Dana-Farber Cancer Institute, Boston, MA: Harold J. Burstein, Ramana Tantravahi, Leonard L. Atkins, Paola Dal Cin, and Cynthia C. Morton (Grant No. CA32291); Vermont Cancer Center, Burlington, VT: Steven M. Grunberg, Elizabeth F. Allen, and Mary Tang (Grant No. CA77406); Weill Medical College of Cornell University, New York, NY: John Leonard, Ram S. Verma, Prasad R.K. Koduru, and Susan Mathew (Grant No. CA07968); Ft. Wayne Medical Oncology/Hematology, Ft. Wayne, IN: Sreenivasa Nattam and Patricia I. Bader; Dartmouth Medical School, Lebanon, NH: Konstantin Dragnev, Doris H. Wurster-Hill, and Thuluvancheri K. Mohandas (Grant No. CA04326); Eastern Maine Medical Center, Bangor, ME: Harvey M. Segal and Laurent J. Beauregard (Grant No. CA35406); Mount Sinai School of Medicine, New York, NY: Lewis R. Silverman and Vesna Najfeld (Grant No. CA04457); Minneapolis VA Medical Center, Minneapolis, MN: Vicki A. Morrison and Sugandhi A. Tharapel (Grant No. CA47555); University of Puerto Rico School of Medicine, San Juan, PR: Eileen I. Pacheco, Leonard L. Atkins, Paola Dal Cin, and Cynthia C. Morton; Rhode Island Hospital, Providence, RI: William Sikov, Teresita Padre-Mendoza, Hon Fong L. Mark, Shelly L. Kerman, and Aurelia Meloni-Ehrig (Grant No. CA08025); SUNY Upstate Medical University, Syracuse, NY: Stephen L. Graziano, Larry Gordon, and Constance K. Stein (Grant No. CA21060); University of California at San Diego: Barbara A. Parker, Renée Bernstein, and Marie L. Dell'Aquila (Grant No. CA11789); Christiana Care Health Services, Newark, DE: Stephen S. Grubbs, Digamber S. Borgaonkar, and Jeanne M. Meck (Grant No. CA45418); Long Island Jewish Medical Center CCOP, Lake Success, NY: Kanti R. Rai and Prasad R. K. Koduru (Grant No. CA11028); Massachusetts General Hospital, Boston, MA: Jeffrey W. Clark, Leonard L. Atkins, Paola Dal Cin, and Cynthia C. Morton (Grant No. CA 12,449); University of Maryland Cancer Center, Baltimore, MD: Martin J. Edelman, Joseph R. Testa, Maimon M. Cohen, Judith Stamberg, and Yi Ning (Grant No. CA31983); University of Minnesota, Minneapolis, MN: Bruce A. Peterson, Diane C. Arthur, and Betsy A. Hirsch (Grant No. CA16450); University of Missouri/Ellis Fischel Cancer Center, Columbia, MO: Michael C. Perry and Tim H. Huang (Grant No. CA12046); University of Nebraska Medical Center, Omaha, NE: Anne Kessinger and Warren G. Sanger (Grant No. CA77298); Western Pennsylvania Hospital, Pittsburgh, PA: John Lister and Gerard R. Diggans; University of Illinois at Chicago: David J. Peace, Maureen M. McCorquodale, and Kathleen E. Richkind (Grant No. CA74811); Walter Reed Army Medical Center, Washington, DC: David C. Van Echo, Rawatmal B. Surana, and Digamber S. Borgaonkar (Grant No. CA26806); Georgetown University Medical Center, Washington, DC: Minnetta C. Liu and Jeanne M. Meck (Grant No. CA77597); McGill Department of Oncology, Montreal, Quebec: J.L. Hutchison and Jacqueline Emond (Grant No. CA31809); Medical University of South Carolina, Charleston, SC: Mark R. Green, G. Shashidhar Pai, and Daynna J. Wolff (Grant No. CA03927); University of Cincinnati Medical Center, Cincinnati, OH: Orlando J. Martelo and Ashok K. Srivastava (Grant No. CA47515); Columbia-Presbyterian Medical Center, New York, NY: Rose R. Ellison and Dorothy Warburton (Grant No. CA12011); University of Minnesota, Minneapolis, MN: Bruce A. Peterson, Diane C. Arthur, and Betsy A. Hirsch (Grant No. CA16450); Virginia Commonwealth University MB CCOP, Richmond, VA: John D. Roberts and Colleen Jackson-Cook (Grant No. CA52784); SUNY Maimonides Medical Center, Brooklyn, NY: Sameer Rafla and Ram S. Verma (Grant No. CA25119); Southern Nevada Cancer Research Foundation CCOP, Las Vegas, NV: John Ellerton and Marie L. Dell'Aquila (Grant No. CA35421); University of California at San Francisco: Charles J. Ryan and Kathleen E. Richkind (Grant No. CA60138).
Footnotes
Author affiliations appear at the end of this article.
Supported in part by the National Cancer Institute (Grants No. CA101140, CA114725, CA140158, CA31946, CA33601, CA16058, CA77658, CA129657 CA41287, and CA102031), the Coleman Leukemia Research Foundation, and the Deutsche Krebshilfe–Dr Mildred Scheel Cancer Foundation (H.B.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Guido Marcucci, Klaus H. Metzeler, Clara D. Bloomfield
Financial support: Guido Marcucci
Provision of study materials or patients: Guido Marcucci, Bayard L. Powell, Thomas H. Carter, Jonathan E. Kolitz, Meir Wetzler, Andrew J. Carroll, Maria R. Baer, Joseph O. Moore, Michael A. Caligiuri, Richard A. Larson
Collection and assembly of data: Guido Marcucci, Klaus H. Metzeler, Sebastian Schwind, Heiko Becker, Krzysztof Mrózek, Susan P. Whitman, Yue-Zhong Wu, Bayard L. Powell, Thomas H. Carter, Jonathan E. Kolitz, Meir Wetzler, Andrew J. Carroll, Maria R. Baer, Joseph O. Moore, Michael A. Caligiuri, Richard A. Larson, Clara D. Bloomfield
Data analysis and interpretation: Guido Marcucci, Klaus H. Metzeler, Sebastian Schwind, Heiko Becker, Kati Maharry, Krzysztof Mrózek, Michael D. Radmacher, Jessica Kohlschmidt, Deedra Nicolet, Clara D. Bloomfield
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 3.Mrózek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: Are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 6.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 7.Whitman SP, Maharry K, Radmacher MD, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. Blood. 2010;116:3622–3626. doi: 10.1182/blood-2010-05-283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcucci G, Maharry K, Radmacher MD, et al. Prognostic significance of, and gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: A Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:5078–5087. doi: 10.1200/JCO.2008.17.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taskesen E, Bullinger L, Corbacioglu A, et al. Prognostic impact, concurrent genetic mutations, and gene expression features of AML with CEBPA mutations in a cohort of 1182 cytogenetically normal AML patients: Further evidence for CEBPA double mutant AML as a distinctive disease entity. Blood. 2011;117:2469–2475. doi: 10.1182/blood-2010-09-307280. [DOI] [PubMed] [Google Scholar]
- 10.Paschka P, Marcucci G, Ruppert AS, et al. Wilms' tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:4595–4602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker H, Marcucci G, Maharry K, et al. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. Blood. 2010;116:788–792. doi: 10.1182/blood-2010-01-262543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Döhner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood. 2005;106:3740–3746. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 13.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:596–604. doi: 10.1200/JCO.2009.25.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzeler KH, Maharry K, Radmacher MD, et al. TET2 mutations improve the new European LeukemiaNet risk classification of acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2011;29:1373–1381. doi: 10.1200/JCO.2010.32.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcucci G, Maharry K, Wu Y-Z, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 17.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mead AJ, Linch DC, Hills RK, et al. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–1270. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 19.Döhner K, Tobis K, Ulrich R, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: A study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–3261. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 20.Whitman SP, Ruppert AS, Marcucci G, et al. Long-term disease-free survivors with cytogenetically normal acute myeloid leukemia and MLL partial tandem duplication: A Cancer and Leukemia Group B study. Blood. 2007;109:5164–5167. doi: 10.1182/blood-2007-01-069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–1918. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 22.Baldus CD, Tanner SM, Ruppert AS, et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: A Cancer and Leukemia Group B study. Blood. 2003;102:1613–1618. doi: 10.1182/blood-2003-02-0359. [DOI] [PubMed] [Google Scholar]
- 23.Schwind S, Marcucci G, Maharry K, et al. BAALC and ERG expression levels are associated with outcome and distinct gene and microRNA expression profiles in older patients with de novo cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. Blood. 2010;116:5660–5669. doi: 10.1182/blood-2010-06-290536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcucci G, Maharry K, Whitman SP, et al. High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2007;25:3337–3343. doi: 10.1200/JCO.2007.10.8720. [DOI] [PubMed] [Google Scholar]
- 25.Heuser M, Beutel G, Krauter J, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108:3898–3905. doi: 10.1182/blood-2006-04-014845. [DOI] [PubMed] [Google Scholar]
- 26.Langer C, Marcucci G, Holland KB, et al. Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2009;27:3198–3204. doi: 10.1200/JCO.2008.20.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwind S, Marcucci G, Kohlschmidt J, et al. Low expression of MN1 associates with better treatment response in older patients with de novo cytogenetically normal acute myeloid leukemia. Blood. 2011;118:4188–4198. doi: 10.1182/blood-2011-06-357764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwind S, Maharry K, Radmacher MD, et al. Prognostic significance of expression of a single microRNA, miR-181a, in cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:5257–5264. doi: 10.1200/JCO.2010.29.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno S, Chijiwa T, Okamura T, et al. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97:1172–1179. doi: 10.1182/blood.v97.5.1172. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita Y, Yuan J, Suetake I, et al. Array-based genomic resequencing of human leukemia. Oncogene. 2010;29:3723–3731. doi: 10.1038/onc.2010.117. [DOI] [PubMed] [Google Scholar]
- 31.Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med. 2010;363:2424–2433. doi: 10.1056/NEJMoa1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan X-J, Xu J, Gu Z-H, et al. Exome sequencing identifies somatic mutations of DNA methyltransferase gene DNMT3A in acute monocytic leukemia. Nat Genet. 2011;43:309–315. doi: 10.1038/ng.788. [DOI] [PubMed] [Google Scholar]
- 33.Thol F, Damm F, Lüdeking A, et al. Incidence and prognostic influence of DNMT3A mutations in acute myeloid leukemia. J Clin Oncol. 2011;29:2889–2896. doi: 10.1200/JCO.2011.35.4894. [DOI] [PubMed] [Google Scholar]
- 34.Kolitz JE, George SL, Marcucci G, et al. P-glycoprotein inhibition using valspodar (PSC-833) does not improve outcomes for patients under age 60 years with newly diagnosed acute myeloid leukemia: Cancer and Leukemia Group B study 19808. Blood. 2010;116:1413–1421. doi: 10.1182/blood-2009-07-229492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: Final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol. 2004;22:4290–4301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 36.Baer MR, George SL, Sanford BL, et al. Escalation of daunorubicin and addition of etoposide in the ADE regimen in acute myeloid leukemia patients aged 60 years and older: Cancer and Leukemia Group B study 9720. Leukemia. 2011;25:800–807. doi: 10.1038/leu.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 38.Stone RM, Berg DT, George SL, et al. Postremission therapy in older patients with de novo acute myeloid leukemia: A randomized trial comparing mitoxantrone and intermediate-dose cytarabine with standard-dose cytarabine. Blood. 2001;98:548–553. doi: 10.1182/blood.v98.3.548. [DOI] [PubMed] [Google Scholar]
- 39.Lee EJ, George SL, Caligiuri M, et al. Parallel phase I studies of daunorubicin given with cytarabine and etoposide with or without the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age or older with acute myeloid leukemia: Results of Cancer and Leukemia Group B study 9420. J Clin Oncol. 1999;17:2831–2839. doi: 10.1200/JCO.1999.17.9.2831. [DOI] [PubMed] [Google Scholar]
- 40.Baer MR, George SL, Dodge RK, et al. Phase 3 study of the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age and older with acute myeloid leukemia: Cancer and Leukemia Group B Study 9720. Blood. 2002;100:1224–1232. [PubMed] [Google Scholar]
- 41.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B study 9720. J Clin Oncol. 2008;26:4934–4939. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a proapoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients > 60 years old. J Clin Oncol. 2007;25:360s. (suppl; abstr 7012) [Google Scholar]
- 43.Mrózek K, Carroll AJ, Maharry K, et al. Central review of cytogenetics is necessary for cooperative group correlative and clinical studies of adult acute leukemia: The Cancer and Leukemia Group B experience. Int J Oncol. 2008;33:239–244. [PMC free article] [PubMed] [Google Scholar]
- 44.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 45.Caligiuri MA, Strout MP, Schichman SA, et al. Partial tandem duplication of ALL1 as a recurrent molecular defect in acute myeloid leukemia with trisomy 11. Cancer Res. 1996;56:1418–1425. [PubMed] [Google Scholar]
- 46.Marcucci G, Radmacher MD, Maharry K, et al. MicroRNA expression in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1919–1928. doi: 10.1056/NEJMoa074256. [DOI] [PubMed] [Google Scholar]
- 47.Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. doi: 10.1200/JCO.1990.8.5.813. [DOI] [PubMed] [Google Scholar]
- 48.Scholzová E, Malík R, Sevcík J, et al. RNA regulation and cancer development. Cancer Lett. 2007;246:12–23. doi: 10.1016/j.canlet.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz JM, Rödelsperger C, Schuelke M, et al. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 50.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plass C, Oakes C, Blum W, et al. Epigenetics in acute myeloid leukemia. Semin Oncol. 2008;35:378–387. doi: 10.1053/j.seminoncol.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metzeler KH, Walker A, Geyer S, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. doi: 10.1038/leu.2011.342. doi: 10.1038/leu.2011.342 [epub ahead of print on November 29, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.