Abstract
Purpose
Women with BRCA1 or BRCA2 (BRCA1/2) mutations must choose between prophylactic surgeries and screening to manage their high risks of breast and ovarian cancer, comparing options in terms of cancer incidence, survival, and quality of life. A clinical decision tool could guide these complex choices.
Methods
We built a Monte Carlo model for BRCA1/2 mutation carriers, simulating breast screening with annual mammography plus magnetic resonance imaging (MRI) from ages 25 to 69 years and prophylactic mastectomy (PM) and/or prophylactic oophorectomy (PO) at various ages. Modeled outcomes were cancer incidence, tumor features that shape treatment recommendations, overall survival, and cause-specific mortality. We adapted the model into an online tool to support shared decision making.
Results
We compared strategies on cancer incidence and survival to age 70 years; for example, PO plus PM at age 25 years optimizes both outcomes (incidence, 4% to 11%; survival, 80% to 83%), whereas PO at age 40 years plus MRI screening offers less effective prevention, yet similar survival (incidence, 36% to 57%; survival, 74% to 80%). To characterize patients' treatment and survivorship experiences, we reported the tumor features and treatments associated with risk-reducing interventions; for example, in most BRCA2 mutation carriers (81%), MRI screening diagnoses stage I, hormone receptor-positive breast cancers, which may not require chemotherapy.
Conclusion
Cancer risk-reducing options for BRCA1/2 mutation carriers vary in their impact on cancer incidence, recommended treatments, quality of life, and survival. To guide decisions informed by multiple health outcomes, we provide an online tool for joint use by patients with their physicians (http://brcatool.stanford.edu).
INTRODUCTION
Women with BRCA1 and BRCA2 (BRCA1/2) mutations face substantially elevated lifetime risks of developing breast and ovarian cancer.1,2 The last decade of research with this high-risk population has identified preventive strategies that save lives, notably early bilateral salpingo-oophorectomy,3–6 and screening regimens incorporating magnetic resonance imaging (MRI) that diagnose most breast cancers early.7–12 Although randomized trials have not been performed, studies have reported on breast and ovarian cancer incidence, tumor biologic and clinical features, and quality of life with prophylactic and surveillance interventions for BRCA1/2 mutation carriers.1,2,13–23
Despite substantial progress in managing the cancer risks owing to a BRCA1/2 mutation, patients and their physicians struggle with choices about interventions, such as whether to replace breast screening with bilateral prophylactic mastectomy (PM) and when to pursue PM and/or prophylactic bilateral salpingo-oophorectomy (PO). Evidence-based practice guidelines recommend PO by age 40 years, but advise physicians and patients to discuss the options of PM versus MRI-based breast screening.24 Guiding decisions about these interventions is difficult, because no trials have compared them directly. We and others have used decision analysis to compare screening and prophylactic surgery in terms of survival and cost-effectiveness.25–33 However, prior studies have not fully characterized the patient experience with different interventions—for example, the likelihood that a woman who chooses breast screening will develop a cancer requiring adjuvant chemotherapy—although cancer treatments significantly impact quality of life and survivorship34–36 and may inform choices between risk-reduction strategies. Moreover, there is no practical way to compare multiple clinically relevant options, such as immediate PM and PO versus screening plus immediate PO and delayed PM, for an individual patient in real time.
We adapted a previously developed Monte Carlo simulation model to compare breast and ovarian cancer incidence, tumor prognostic features, recommended treatments, overall survival, and cause-specific mortality for BRCA1/2 mutation carriers. We translated this model into an online clinical decision support tool, enabling personalized cancer risk management for women with BRCA1/2 mutations.
METHODS
We developed a computer simulation model that integrates published data (Table 1) to estimate breast and ovarian cancer incidence and tumor prognostic features, probability of survival to ages 70 and 80 years, and causes of death for women with a BRCA1 or BRCA2 mutation, starting from age 25 years.29 Risk-reducing interventions were modeled alone and in combination, at ages specified by practice guidelines24,51: breast screening consisting of mammography plus MRI started at age 25 years and continued annually to age 69 years, and PM and PO were modeled at ages 25, 40, and 50 years.
Table 1.
Computer Simulation Model Input Parameters on Cancer Incidence, RR, Screening, and Treatment
| BRCA1 | BRCA2 | Range for Sensitivity Analyses | Source | |
|---|---|---|---|---|
| Breast Cancer Incidence and RR | ||||
| Cumulative breast cancer incidence by age 70 years* | 0.65 | 0.45 | 0.47-0.85 (BRCA1); 0.4-0.85 (BRCA2) | 1,2,37–39 |
| 10-year incidence of second primary breast tumor | 0.43 | 0.35 | Not varied | 18 |
| RR for breast cancer with PM† | 0.9 | 0.9 | Not varied | 19 |
| RR for breast cancer with PO by age at PO, years‡ | 0, 0.9‡ | 4,40,41 | ||
| 25 | 0.36 | 0.36 | ||
| 40-50 | 0.50 | 0.50 | ||
| ≥ 50 | None | None | ||
| Duration of RR for breast cancer after PM and PO | Lifelong | Lifelong | Not varied | 40,41 |
| Ovarian cancer incidence and RR | ||||
| Cumulative ovarian cancer incidence by age 70 years | 0.39 | 0.11 | 0.39-0.46 (BRCA1); 0.11-0.27 (BRCA2) | 1,2,37–39 |
| RR for ovarian cancer from PO | 0.8 | 0.8 | Not varied | 4,6,42 |
| Breast cancer characteristics at symptomatic detection (no screening) | ||||
| Distribution of tumor grade | Not varied | 1,5,23 | ||
| 1-2 | 0.22 | 0.53 | ||
| 3 | 0.78 | 0.47 | ||
| Distribution of ER positivity, conditional on grade | Not varied | 15,23 | ||
| 1-2 | 0.91 | 0.94 | ||
| 3 | 0.18 | 0.61 | ||
| Distribution of tumor size, cm | Not varied | Estimated§ | ||
| < 2 | 0.29 | 0.33 | ||
| 2-5 | 0.55 | 0.54 | ||
| > 5 | 0.16 | 0.13 | ||
| Distribution of tumor stage | Not varied | Estimated§ | ||
| Local | 0.43 | 0.47 | ||
| Regional | 0.49 | 0.46 | ||
| Distant | 0.08 | 0.07 | ||
| Mean TVDT,∥ months | 5.7 | 6.8 | Not varied | 43 |
| Screening test and protocol characteristics | ||||
| Screening interval, years | 1 | 1 | Not varied | Assumed |
| Ages of annual mammography screening, years | 25-69 | 25-69 | Not varied | Assumed |
| Ages of annual MRI screening, years | 25-69 | 25-69 | Not varied | Assumed |
| Sensitivity of MRI screening for cancer detection | 85% | 85% | 50%, 90% | 9,10,12,44,45 |
| MRI tumor size detection threshold, cm | 0.5 | 0.5 | 0.3 cm, 1.53 cm | 12,31 |
| Mammography median tumor size detection threshold, cm¶ | 1 | 1 | Not varied | 10,31 |
| Proportion of tumors undetectable by mammography, age in years | Not varied | Estimated# | ||
| < 50 | 0.66 | 0.66 | ||
| ≥ 50 | 0.3 | 0.3 | ||
| RR for breast cancer death after adjuvant systemic therapy | ||||
| Adjuvant multiagent chemotherapy, by age in years | Not varied | 46 | ||
| < 50 | 0.47 | 0.47 | ||
| ≥ 50 | 0.31 | 0.31 | ||
| Adjuvant hormonal therapy, for ER-positive breast cancers | 0.31 | 0.31 | Not varied | 46 |
| Relative risk for other-cause mortality after PO | ||||
| Death resulting from cardiovascular disease | 2.0 | 2.0 | Not varied | 47 |
| Death related to hip fracture | 1.5 | 1.5 | Not varied | 48 |
| Death related to dementia | 1.5 | 1.5 | Not varied | 49,50 |
NOTE. Adapted from Kurian et al.29
Abbreviations: ER, estrogen receptor; MRI, magnetic resonance imaging; PM, prophylactic mastectomy; PO, prophylactic oophorectomy; RR, risk reduction; SEER, Surveillance, Epidemiology, and End Results; TVDT, tumor volume doubling time.
Reported lifetime breast cancer risks were assumed to have incorporated a 30% background rate of PO at the age of 45 years42; time to second breast cancer was modeled with a Weibull distribution.
We assumed that the reduction in the probability of developing breast cancer after PM was 0.95 (a 95% reduction) per tumor; given the high risk of multiple primary tumors in BRCA1/2 mutation carriers, the overall reduction in probability of developing breast cancer after PM was 0.9 (a 90% reduction).18
In the base case, we assumed a hazard ratio of 0.5 (a proportional hazard reduction of 50%) for subsequent breast cancer in women undergoing PO between ages 40 and 50 years.40 In sensitivity analyses, we evaluated the assumptions that PO had no effect on subsequent breast cancer risk (hazard ratio of 1.0 for women of all ages) and that PO conveyed a hazard ratio of 0.1 for all women undergoing the procedure before age 50 years (a proportional hazard reduction of 90%). We assumed no reduction in the hazard ratio of breast cancer for women undergoing PO at or after age 50 years.
Derived from our breast cancer natural history model using SEER registry data from 1975 to 1981. Tumor stage categories are derived from SEER and defined as follows: local (tumor is confined to breast and does not involve regional lymph nodes), regional (tumor involves breast and regional lymph nodes), distant (tumor has metastasized to distant organs).
The mean TVDT was estimated by calibrating to approximately 85% sensitivity of screening breast MRI in the population with BRCA1 mutations,9,10,12,44,45 based on the condition that the mean TVDT of grade 3 tumors is approximately 0.54 times the mean TVDT of grade 1 to 2 tumors, which we derived analytically.
The median mammography threshold applies only among women whose tumor is detectable by mammography.
Estimated by calibrating to mammographic screening sensitivity, which was assumed to be 0.25 under age 50 years,10 and 0.5 at age ≥ 50 years. Tumors ≥ 5 cm were assumed always to be detectable by mammography.
Monte Carlo Simulation Model
We initially built and validated a Monte Carlo model to analyze the effects of screening and treatment on the outcomes of patients with breast cancer, working within the Cancer Intervention and Surveillance Modeling Network.52,53 We then modified this model to simulate breast and ovarian cancer incidence, tumor characteristics, and prognosis under treatments recommended by practice guidelines (specific to tumor stage, size, and hormone receptors),1,13,18,20,46,54–56 and the performance of screening mammography and MRI,9,10,44,45 for BRCA1/2 mutation carriers.29,31 In sensitivity analyses, we varied parameters about which significant uncertainty exists, within CIs specified by published literature or more broadly (Table 1).
Patient Characteristics
The model simulates life histories of a 1980 birth cohort of 1,000,000 female BRCA1/2 mutation carriers from age 25 years until age 100 years or death. We extrapolated BRCA1/2-associated cancer risks from meta-analyses.1,2 Because approximately 30% of BRCA1/2 mutation carriers undergo PO at a mean age of 45 years,42,57,58 and because premenopausal PO reduces breast cancer incidence by approximately 50%,4,5,40–42 we assumed that the incidence results from meta-analyses were affected by an unreported PO use of approximately 30%. To estimate breast cancer incidence in the absence of PO, we back-calculated the effect of a 50% reduction in subsequent breast cancer risk for 30% of the cohort as a result of PO performed by age 45 years.
Tumor Characteristics and Screen Detection
We assumed a tumor grade distribution for BRCA1/2-associated breast tumors consistent with published reports; estrogen receptor (ER) expression was modeled as a function of grade and mutation.15,23 Tumor size and stage at clinical diagnosis were estimated as a function of grade, informed by the Surveillance Epidemiology and End Results (SEER) registry from the years before population-wide mammographic screening (1975 to 1981).52,53 We assumed that BRCA1/2-associated breast and ovarian cancers are treated with standard therapies based on tumor size, stage, grade, ER expression, and other prognostic features and that treatment efficacy and prognosis equal those in the general population.20,24,46,54,56,59,60 We assumed a median size detection threshold of 0.5 cm for MRI and that MRI has 85% sensitivity.9,10,12,44 For mammography, we assumed a distribution of detection thresholds, with a median of 1 cm, and that a proportion of tumors are undetectable until they grow to 5 cm; we derived this proportion separately according to age and BRCA mutation, calibrating to published mammographic detection rates in this population (Table 1).9,10,44,45
Efficacy of Prophylactic Surgery
We assumed that PM reduces overall breast cancer incidence by 90%18,19 and PO reduces annual ovarian cancer incidence by 80%.5,6,42 We modeled the impact of PO as the annual probability of breast cancer detection at age i (Pi,PO) as Pi,PO = Pαi,NoPO, where i ≥ age at PO and α is the hazard ratio (HR). For ages less than 40 years, HR was 0.36, for ages 40 to 49 years, HR was 0.5, and for ages ≥ 50 years, HR was 1.0 (Table 1).6,40 We assumed that the breast cancer risk reduction from PM at any age, and from PO before age 50 years, persists indefinitely.41 We assumed that PO has no effect on breast cancer stage or ER expression.
Hormonal Exposures and Other-Cause Mortality
We did not explicitly model use of menopausal hormone therapy, oral contraceptive pills, or prophylactic tamoxifen or raloxifene, given uncertainty about their effects on cancer risks for BRCA1/2 mutation carriers,61–69 but we did explore their impact through sensitivity analyses of the effect of PO on breast cancer incidence. After PO performed at age less than 50 years, we conservatively assumed that cardiovascular disease doubles47 and that osteoporotic hip fracture and dementia increase by 50%.48–50 We computed other-cause mortality using the Berkeley Mortality database,70 adjusting death rates from breast and ovarian cancer, cardiovascular disease, dementia, and hip fracture according to assumed relative risks (Table 1).71,72 Previously, we found that reducing the incidence of cardiovascular disease, osteoporosis, and dementia (as would be anticipated with menopausal hormone therapy) had only a small (2% to 3%) impact on other-cause mortality,29 so those sensitivity analyses were not repeated here.
Development of Online Decision Tool
We collaborated with software developers (L.C., P.R., M.S.) to build a model interface suitable for use as an online decision support tool. After obtaining human subjects approval from the Stanford University institutional review board and informed consent, we initiated pilot-testing of the tool with BRCA1/2 mutation carriers accrued through the Stanford Clinical Cancer Genetics Program and the Facing Our Risk of Cancer Empowered advocacy group73 and with clinicians from Stanford University Hospital and surrounding community practices.
RESULTS
Cancer Incidence Versus Survival in BRCA1/2 Mutation Carriers
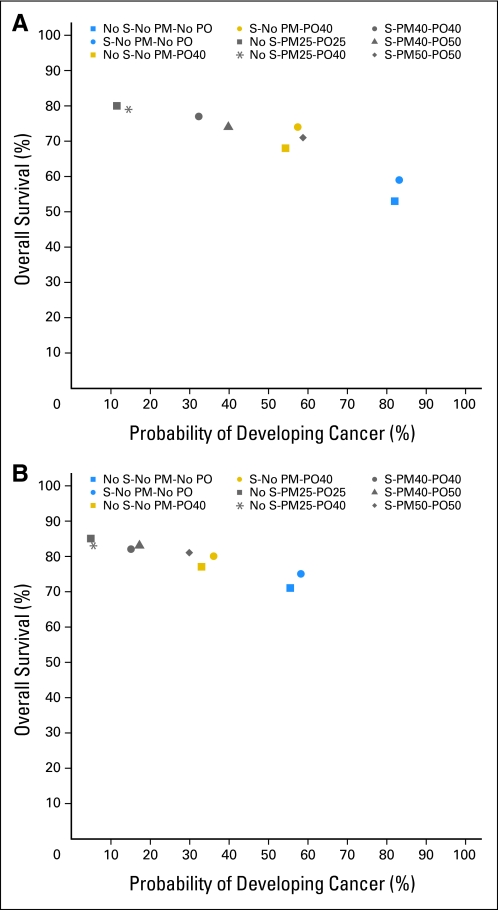
In prior work, we reported survival with combinations of PM, PO, and screening29; we now expand our comparison of intervention strategies along the dual axes of incidence and survival. Figure 1 plots the probability of developing breast or ovarian cancer (combined) versus the probability of survival to age 70 years for BRCA1/2 mutation carriers. For BRCA1 mutation carriers, survival is maximized (80%) and cancer incidence minimized (11%) by the combination of PM and PO (PM + PO) at age 25 years. By comparison, PM + PO at age 40 years yields 3% lower survival probability (77%) with a 21% increase in incidence (32%), whereas breast screening plus PO at age 40 years, without PM, yields 6% lower survival probability (74%) with a 46% increase in incidence (57%). For BRCA2 mutation carriers, PM + PO at age 25 years maximizes survival (83%) and minimizes incidence (4%); by comparison, PM + PO at age 40 years reduces survival probability by 1% (82%), with an 11% increase in incidence (15%), whereas breast screening plus PO at age 40, without PM, yields 3% lower survival probability (80%) with a 22% increase in incidence (36%). For BRCA1/2 mutation carriers, PM, PO, and PM + PO reduce breast cancer incidence, with a magnitude inversely related to age at surgery. The same is true for PO and ovarian cancer incidence, whereas screening slightly increases breast cancer incidence (Table 2). Results were most sensitive to assumptions about the efficacy of PO for breast cancer risk reduction and BRCA1/2 mutation penetrance (Appendix Table A1, online only).
Fig 1.
(A) Probability of developing cancer (combining breast and ovarian cancers) versus survival by age 70 years for BRCA1 mutation carriers choosing different risk-reducing strategies, including prophylactic mastectomy (PM), prophylactic oophorectomy (PO), and/or annual breast screening (S) with mammography and magnetic resonance imaging, performed at various ages (age in years at time of surgery indicated by number after PM or PO). (B) Probability of developing cancer (combining breast and ovarian cancers) versus survival by age 70 years for BRCA2 mutation carriers choosing different risk-reducing strategies, including PM, PO, and/or S, performed at various ages (age in years at time of surgery indicated by number after PM or PO).
Table 2.
Breast and Ovarian Cancer Incidence at Specific Ages for BRCA1/2 Mutation Carriers Under Risk-Reduction Strategies*
| Mutation and Cancer Type | No Breast Screening |
Annual Breast Screening: Mammography and MRI |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Incidence (%) at Specific Ages in Years | ||||||||||||||||||||
| No PM or PO | PM Only |
PO Only |
PM + PO |
No PM or PO | PM Only |
PO Only |
PM + PO |
|||||||||||||
| At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | |||
| BRCA1 mutation carriers | ||||||||||||||||||||
| Breast cancer incidence | ||||||||||||||||||||
| Age 30 years | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 3 | 2 | 3 | 3 | 1 | 3 | 3 | 2 | 3 | 3 |
| Age 40 years | 12 | 3 | 12 | 12 | 5 | 12 | 12 | 2 | 12 | 12 | 17 | 3 | 17 | 17 | 7 | 17 | 17 | 2 | 17 | 17 |
| Age 50 years | 39 | 4 | 24 | 43 | 17 | 27 | 39 | 3 | 23 | 43 | 43 | 4 | 24 | 45 | 19 | 32 | 43 | 3 | 23 | 45 |
| Age 60 years | 56 | 6 | 25 | 47 | 27 | 39 | 56 | 3 | 24 | 47 | 59 | 6 | 25 | 47 | 29 | 43 | 59 | 3 | 24 | 47 |
| Age 70 years | 68 | 7 | 26 | 48 | 36 | 49 | 68 | 4 | 25 | 48 | 70 | 7 | 26 | 48 | 38 | 52 | 70 | 4 | 25 | 48 |
| Age 80 years | 78 | 8 | 27 | 49 | 45 | 58 | 78 | 5 | 25 | 49 | 78 | 8 | 27 | 49 | 45 | 60 | 78 | 5 | 25 | 49 |
| Ovarian cancer incidence | ||||||||||||||||||||
| Age 30 years | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age 40 years | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 3 | 3 | 1 | 3 | 3 |
| Age 50 years | 13 | 13 | 13 | 13 | 3 | 5 | 13 | 3 | 5 | 13 | 13 | 13 | 13 | 13 | 3 | 5 | 13 | 3 | 5 | 13 |
| Age 60 years | 22 | 22 | 22 | 22 | 5 | 7 | 15 | 5 | 7 | 15 | 22 | 22 | 22 | 22 | 5 | 7 | 15 | 5 | 7 | 15 |
| Age 70 years | 38 | 38 | 38 | 38 | 8 | 10 | 18 | 8 | 10 | 18 | 38 | 38 | 38 | 38 | 8 | 10 | 18 | 8 | 10 | 18 |
| Age 80 years | 52 | 52 | 52 | 52 | 10 | 13 | 21 | 10 | 13 | 21 | 52 | 52 | 52 | 52 | 10 | 13 | 21 | 10 | 13 | 21 |
| BRCA2 mutation carriers | ||||||||||||||||||||
| Breast cancer incidence | ||||||||||||||||||||
| Age 30 years | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 |
| Age 40 years | 6 | 2 | 6 | 6 | 2 | 6 | 6 | 2 | 6 | 6 | 9 | 2 | 9 | 9 | 4 | 9 | 9 | 2 | 9 | 9 |
| Age 50 years | 17 | 2 | 12 | 22 | 7 | 12 | 17 | 2 | 12 | 22 | 22 | 2 | 12 | 23 | 9 | 16 | 22 | 2 | 12 | 23 |
| Age 60 years | 34 | 4 | 13 | 26 | 14 | 21 | 34 | 2 | 12 | 26 | 38 | 4 | 13 | 26 | 16 | 25 | 38 | 2 | 12 | 26 |
| Age 70 years | 50 | 5 | 14 | 27 | 22 | 31 | 50 | 3 | 13 | 27 | 53 | 5 | 14 | 27 | 24 | 34 | 53 | 3 | 13 | 27 |
| Age 80 years | 63 | 6 | 15 | 28 | 30 | 41 | 63 | 3 | 14 | 28 | 63 | 6 | 15 | 28 | 30 | 42 | 63 | 3 | 14 | 28 |
| Ovarian cancer incidence | ||||||||||||||||||||
| Age 30 years | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age 40 years | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Age 50 years | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| Age 60 years | 7 | 7 | 7 | 7 | 2 | 2 | 3 | 2 | 2 | 3 | 7 | 7 | 7 | 7 | 2 | 2 | 3 | 2 | 2 | 3 |
| Age 70 years | 11 | 11 | 11 | 11 | 2 | 2 | 3 | 2 | 2 | 3 | 11 | 11 | 11 | 11 | 2 | 2 | 3 | 2 | 2 | 3 |
| Age 80 years | 15 | 15 | 15 | 15 | 3 | 3 | 4 | 3 | 3 | 4 | 15 | 15 | 15 | 15 | 3 | 3 | 4 | 3 | 3 | 4 |
Abbreviations: MRI, magnetic resonance imaging; PM, prophylactic mastectomy; PO, prophylactic oophorectomy.
Risk-reduction strategies included PM or PO done at various ages, and breast screening with mammogram and MRI starting at age 25 years.
Breast Tumor Features and Treatments in BRCA1/2 Mutation Carriers
To characterize patients' treatment and survivorship experiences, we compared the outcomes of risk-reducing strategies in terms of breast tumor features at diagnosis: stage, size, ER expression, and recommended systemic treatments according to guidelines of the National Comprehensive Cancer Network.54 Without risk-reducing interventions, BRCA1 mutation carriers are most likely to develop an ER-negative tumor involving axillary lymph nodes (34%), corresponding to American Joint Committee on Cancer stage II to III74; next most likely diagnoses are a lymph node-negative, ER-negative tumor larger than 2 cm in size (mostly stage II; 16%) or a lymph node-positive, ER-positive tumor (stage II to III; 16%). These three most common scenarios require adjuvant chemotherapy; ER-positive tumors also require adjuvant hormonal therapy.54 Adding MRI-based screening shifts the tumor stage and ER distribution: screened BRCA1 mutation carriers have the greatest probability of a stage I, ER-negative tumor (42%, usually requiring chemotherapy), followed by a stage I ER-positive tumor (25%, requiring hormonal therapy and possibly chemotherapy) and a stage II to III, lymph node-positive, ER-negative tumor (14%, requiring chemotherapy; Table 3).54
Table 3.
Stage and ER Expression (%) of BCs Diagnosed in BRCA1/2 Mutation Carriers Under Risk Reduction With PM, PO, and/or Screening at Various Ages
| BC Stage and ER Expression (%) | No Breast Screening |
Annual Breast Screening: Mammography and MRI |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No PM/ PO | PM Only |
PO Only |
PM + PO |
No PM/ PO | PM Only |
PO Only |
PM + PO |
|||||||||||||
| At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | At 25 | At 40 | At 50 | |||
| BRCA1 mutation carriers | ||||||||||||||||||||
| Local stage (lymph node negative)* | ||||||||||||||||||||
| Tumor ≤ 2 cm (stage I)* | ||||||||||||||||||||
| ER positive | 8 | 21 | 19 | 11 | 8 | 8 | 8 | 25 | 19 | 11 | 25 | 21 | 29 | 28 | 24 | 25 | 25 | 25 | 30 | 28 |
| ER negative | 10 | 21 | 24 | 15 | 10 | 10 | 10 | 24 | 24 | 15 | 42 | 21 | 43 | 45 | 39 | 40 | 41 | 24 | 44 | 45 |
| Tumor > 2 cm (majority stage II)* | ||||||||||||||||||||
| ER positive | 9 | 6 | 5 | 8 | 9 | 9 | 9 | 5 | 5 | 8 | 3 | 6 | 2 | 3 | 4 | 4 | 3 | 5 | 2 | 3 |
| ER negative | 16 | 10 | 10 | 14 | 16 | 16 | 16 | 8 | 10 | 14 | 8 | 10 | 6 | 7 | 9 | 8 | 8 | 8 | 5 | 7 |
| Regional stage (lymph node positive, stage II-III)* | ||||||||||||||||||||
| ER positive | 16 | 12 | 12 | 14 | 16 | 16 | 16 | 11 | 12 | 14 | 5 | 12 | 5 | 5 | 6 | 6 | 5 | 11 | 5 | 5 |
| ER negative | 34 | 25 | 25 | 31 | 34 | 34 | 34 | 22 | 25 | 31 | 14 | 25 | 13 | 12 | 16 | 15 | 15 | 22 | 12 | 12 |
| Distant stage (metastatic, stage IV)* | ||||||||||||||||||||
| ER positive | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| ER negative | 6 | 4 | 4 | 6 | 6 | 6 | 6 | 3 | 4 | 6 | 1 | 4 | 1 | 1 | 2 | 2 | 1 | 3 | 1 | 1 |
| BRCA2 mutation carriers | ||||||||||||||||||||
| Local stage (lymph node negative)* | ||||||||||||||||||||
| Tumor ≤ 2 cm (stage I)* | ||||||||||||||||||||
| ER positive | 18 | 37 | 38 | 33 | 18 | 18 | 18 | 46 | 40 | 33 | 55 | 37 | 59 | 63 | 52 | 54 | 54 | 46 | 62 | 63 |
| ER negative | 4 | 6 | 7 | 6 | 4 | 4 | 4 | 8 | 7 | 6 | 13 | 6 | 13 | 14 | 12 | 13 | 13 | 8 | 13 | 14 |
| Tumor > 2 cm (majority stage II)* | ||||||||||||||||||||
| ER positive | 20 | 14 | 13 | 15 | 20 | 20 | 20 | 10 | 12 | 15 | 9 | 14 | 7 | 6 | 10 | 9 | 9 | 10 | 6 | 6 |
| ER negative | 5 | 4 | 3 | 4 | 5 | 5 | 5 | 3 | 3 | 4 | 3 | 4 | 2 | 2 | 3 | 3 | 3 | 3 | 2 | 2 |
| Regional stage (lymph node positive, stage II-III)* | ||||||||||||||||||||
| ER positive | 36 | 27 | 26 | 29 | 36 | 36 | 36 | 22 | 25 | 29 | 14 | 27 | 13 | 10 | 15 | 14 | 14 | 22 | 12 | 10 |
| ER negative | 11 | 8 | 8 | 9 | 11 | 11 | 11 | 7 | 8 | 9 | 5 | 8 | 4 | 4 | 5 | 5 | 5 | 7 | 4 | 4 |
| Distant stage (metastatic, stage IV)* | ||||||||||||||||||||
| ER positive | 5 | 3 | 3 | 4 | 5 | 5 | 5 | 3 | 3 | 4 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 |
| ER negative | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
Abbreviations: BC, breast cancer; ER, estrogen receptor; MRI, magnetic resonance imaging; PM, prophylactic mastectomy; PO, prophylactic oophorectomy; SEER, Surveillance, Epidemiology, and End Results.
Without intervention, BRCA2 mutation carriers are most likely to develop a stage II to III, lymph node-positive, ER-positive tumor (36%, requiring chemotherapy and hormonal therapy), followed by a stage II, lymph node-negative, ER-positive tumor larger than 2 cm (20%, requiring hormonal therapy and probably chemotherapy) or a stage I, lymph node-negative, ER-positive tumor (18%, requiring hormonal therapy and possibly chemotherapy; Table 3).54 With MRI-based screening, BRCA2 mutation carriers are most likely to have a stage I, ER-positive cancer (55%, requiring hormonal therapy and possibly chemotherapy), followed by a stage II to III lymph node-positive, ER-positive tumor (14%, requiring chemotherapy and hormonal therapy), and a stage I, ER-negative tumor (13%, usually requiring chemotherapy).54 Results were most affected by our assumptions about the sensitivity of screening MRI (Appendix Table A1). Figure 2 presents risks of developing breast cancer to age 70 years, stratified by likely systemic treatments,54 for BRCA1/2 mutation carriers under various strategies.
Fig 2.
(A) Absolute risk of developing breast cancer by age 70 years, stratified by guideline-recommended systemic treatments according to stage, size, and estrogen receptor expression, for BRCA1 mutation carriers who choose risk-reducing strategies including annual breast screening (S) with mammography and magnetic resonance imaging, prophylactic mastectomy (PM), and/or prophylactic oophorectomy (PO) performed at various ages (age in years indicated by number after PM or PO). (B) Absolute risk of developing breast cancer by age 70 years, stratified by guideline-recommended systemic treatments according to stage, size, and estrogen receptor expression, for BRCA2 mutation carriers who choose risk-reducing strategies including S, PM, and/or PO performed at various ages (age in years indicated by number after PM or PO).
Model Runs Underlying the Online Decision Tool
To inform an age- and mutation-specific decision tool, we performed 2,130 model runs, considering cancer-free women with BRCA1 and BRCA2 mutations, in the age intervals of 25 to 29, 30 to 34, 35 to 39, 40 to 44, 45 to 49, 50 to 54, 55 to 59, 60 to 64, and 65 to 69 years. Because each run simulates the individual histories of 1,000,000 women, our estimates incorporate outcomes of more than 1,000,000,000 women, stratified by age at BRCA1/2 mutation testing and risk-reducing intervention. Results of all runs are presented in the online decision tool.
Development and Features of the Online Decision Tool
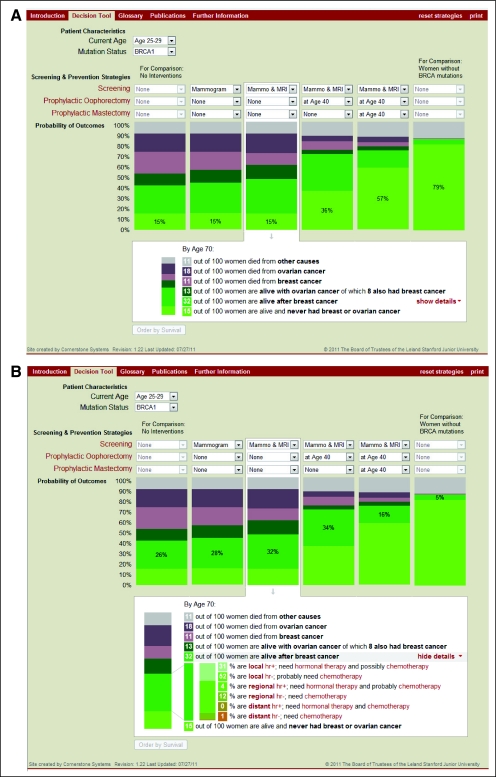
We developed an online user interface with a vertical bar-graph display, adapting figures from our prior survival analysis29 and from our current work on cancer incidence, features, and treatments (Fig 2). We selected a bar-graph format by analogy to the successful Adjuvant! model for breast cancer treatment.75,76 User-selected variables are patient characteristics (age and mutation) and intervention strategies (annual screening mammogram, annual screening MRI, PM, and PO, alone or in combination at different ages). Each vertical bar represents a selected strategy showing the following outcomes: survival probability to age 70 years, causes of death, breast and ovarian cancer incidence, and breast tumor features influencing treatment recommendations (stage and hormone receptor expression). Of six vertical bars, the middle four can be customized to report specific strategies; for comparison, the left-most anchoring bar shows outcomes if no risk-reducing interventions are undertaken and the right-most anchoring bar shows the outcomes of an age-matched woman with no BRCA1/2 mutation. The four user-customized bars can be ranked by survival, and the display can be printed. Figure 3 presents the main screen of the tool; the user can choose to display survival and causes of death only (Fig 3A) or to include features and guideline-recommended treatments of breast cancers (Fig 3B). Additional screens include (1) an introduction, specifying the intended use conditions under physician supervision, the intended population, and the modeling assumptions; (2) a glossary; (3) contact information; and (4) publication links. The decision tool is available online.77
Fig 3.
(A) Display screen of online decision tool (brcatool.stanford.edu) comparing user-selected scenarios for cancer risk reduction. Screening options include yearly mammography (Mammo) and/or magnetic resonance imaging (MRI); preventive options include prophylactic mastectomy or salpingo-oophorectomy at various ages. Each bar shows the probability of outcomes by age 70 years under the selected strategy in terms of cancer-free survival (light green), survival after breast cancer (darker green), survival with ovarian cancer (darkest green), and death resulting from breast cancer (pink), ovarian cancer (purple), and other causes (gray). Terms in red font are linked to definitions in the glossary. (B) Expanded display screen of decision tool, showing additional details of each user-selected scenario (vertical bars) in terms of breast cancer stage (local, regional, distant), hormone receptor (hr) expression (negative – or positive +), and recommended systemic therapies according to practice guidelines.
DISCUSSION
We built a simulation model to estimate and compare multiple health outcomes for BRCA1/2 mutation carriers under various cancer risk-reduction strategies and converted it into an online decision tool for use by physicians and patients. We found that early prophylactic mastectomy and salpingo-oophorectomy most effectively prevent cancer, but alternatives that reduce cancer incidence far less substantially can offer comparable survival. MRI-based breast screening yields this benefit through a diagnostic stage shift, increasing the proportion of stage I tumors from 18% to 22% up to 67% to 68%; treatment recommendations vary by hormone receptor expression, which is correlated with the type of BRCA mutation. A BRCA2 mutation carrier who elects MRI screening may well escape a recommendation for adjuvant chemotherapy, because 81% of breast cancers diagnosed in this setting are smaller than 2 cm, node-negative, and hormone receptor-positive. The online decision tool77 enables direct comparison of many possible strategies for an individual patient, combining various screening methods with prophylactic surgeries undertaken at different ages and weighing their impact on cancer incidence, treatment experiences, and survival.
Decisions about cancer risk reduction are complex and highly personal. For most women in the population, the greatest challenge is estimating risk accurately.78 Imprecision in communicating risk-benefit ratios may contribute to under-utilization of effective strategies such as chemoprevention.79 For BRCA1/2 mutation carriers, cancer risks are higher and better defined, driving greater uptake of prophylactic surgeries and intensive surveillance.57,58 Nonetheless, a significant number of women with BRCA1/2 mutations never develop cancer, given variations in penetrance which are incompletely understood80–82; removing organs at risk thus remains a gamble. Use of prophylactic surgeries varies by country, age, and prior cancer diagnosis, and aspects of personal experience such as family cancer history and parity play a role.56,57,83,84 Prior decision analyses have assigned a set value to life after prophylactic surgery or with screening and reported results in quality-adjusted life years.25,27,31,85,86 Given our experience of substantial variation in patient preferences, we elected against ranking health states under different risk-reducing interventions. Instead, our estimates across multiple outcomes aim to guide patients in optimizing their quality of life, depending on their individual values: for one woman this could entail retaining her breasts for decades, with eventual diagnosis of an early-stage breast cancer that might require chemotherapy; for another, this could entail maximal cancer prevention by removing her breasts and ovaries early in life.
With rapid growth in therapeutic and diagnostic technology, decision aids are increasingly used in oncology practice. They synthesize diverse data sources and integrate comparisons across disparate (and often conflicting) scales of benefit, such as efficacy, toxicity, and cost. Trials have demonstrated improvements in decisional conflict and satisfaction with the use of decision aids in breast, colorectal, and thoracic oncology.76,87–92 Decision aids weigh the absolute magnitude of an intervention's benefit against competing risks and may align choices more closely with expected therapeutic gains.93 The online format of our decision tool facilitates access and personalization; results are customized for a patient's age, allowing women to revisit their decisions over time should their health status, life circumstances, or priorities change. Additionally, the online tool can be readily adapted to accommodate new data from emerging studies. No decision aid can replace any aspect of the physician–patient relationship; our tool aims rather to channel the discussion toward choices that more fully realize a patient's personal preferences.
Our work has some limitations. The results of any simulation model depend on its assumptions. We initially developed and validated our model with SEER registry data, modeling tumor growth and ER expression as a function of grade52,53,94; we subsequently applied this same strategy to BRCA1/2-associated breast cancers, using appropriate tumor grade and ER distributions.13,15,23,55,95,96 Although justified by reports that patients with breast cancer with and without BRCA1/2 mutations have similar outcomes,20,59,60,97 this fundamental approach is difficult to validate. The model lacks data on breast tumor progesterone receptor and human epidermal growth factor receptor 2 expression, given their absence in SEER; because human epidermal growth factor receptor 2 overexpression is rare in BRCA1/2-associated tumors,13,55,95,96 this limitation is unlikely to change our conclusions. An additional limitation is our use of average BRCA1/2 mutation penetrance estimates, derived from meta-analyses1,2; our model does not incorporate factors that may mediate individual risk variation, such as birth cohort, family history, lifestyle and environmental exposures, or single-nucleotide polymorphisms in other genes.81,82,98,99 We did not assign a separate prognostic category to carcinoma in situ or consider emerging treatments such as poly (ADP-ribose) polymerase inhibitors. We varied input parameters widely in sensitivity analyses and found that our assumptions about BRCA1/2-associated cancer risks, the sensitivity of MRI, and the effect of PO on breast cancer incidence were most influential. If MRI-based breast screening detects preinvasive cancers,9,45,100 which have optimal survival and no requirement for chemotherapy,74 or if BRCA1/2 mutation-targeted cancer therapies improve survival with few adverse effects,101–104 then breast screening may provide a better outcome than we estimate; conversely, if mutation penetrance is higher or cancer prognosis worse than we estimate, prophylactic surgeries would appear more favorable. The online decision tool focuses on cancer-free women; it does not report second primary cancer risks, mortality from a prior cancer, or benefit from a procedure performed in the past. We have not measured the tool's impact on decision outcomes in a clinical trial, but pilot-testing among 60 patients and providers yielded high rankings on clinical relevance and ease of use, with a full analysis underway. Future work is warranted to address these limitations of the model and decision tool.
We calculated cancer incidence, tumor prognostic features that influence treatment and quality of life, overall survival, and cause-specific mortality under many possible risk-reduction strategies for BRCA1/2 mutation carriers. We customized these results by age and BRCA mutation and adapted them into an online tool to support joint decision making by patients and physicians. By characterizing the multiple health outcomes associated with cancer risk-reduction options, our decision tool aims to clarify a patient's priorities and guide choices that preserve them.
Acknowledgment
We thank Bronislava M. Sigal, PhD, who made important contributions to earlier versions of the simulation model code and consulted on modifications made for this study, and the Facing Our Risk of Cancer Empowered patient advocacy group, who assisted in pilot-testing the online decision tool.
Appendix
Table A1.
Sensitivity Analysis on Outcomes of BC Incidence, Stage (% local), ER Expression (% ER positive), and OC Incidence by Age 70 Years in BRCA1/2 Mutation Carriers
| Parameter | BC Incidence (%) |
BC Stage (% local) |
BC ER (% positive) |
OC Incidence (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | Lower | Upper | Base | Lower | Upper | Base | Lower | Upper | Base | Lower | Upper | |
| BRCA1 mutation carriers | ||||||||||||
| BC by 70 years, BRCA1 | 0.65 | 0.47 | 0.85 | 0.65 | 0.47 | 0.85 | 0.65 | 0.47 | 0.85 | 0.65 | 0.47 | 0.85 |
| No S* or PM or PO | 68 | 53 | 89 | 42 | 42 | 42 | 34 | 34 | 34 | 38 | 38 | 38 |
| S*, no PM or PO | 70 | 55 | 90 | 79 | 75 | 80 | 34 | 34 | 34 | 38 | 38 | 38 |
| S* and PO, no PM† | 52 | 39 | 77 | 77 | 74 | 78 | 34 | 34 | 34 | 10 | 10 | 10 |
| S* and PO and PM† | 25 | 19 | 38 | 81 | 80 | 82 | 37 | 37 | 37 | 10 | 10 | 10 |
| OC by 70 years, BRCA1 | 0.39 | 0.39 | 0.46 | 0.39 | 0.39 | 0.46 | 0.39 | 0.39 | 0.46 | 0.39 | 0.39 | 0.46 |
| No S* or PM or PO | 68 | 68 | 68 | 42 | 42 | 42 | 34 | 34 | 34 | 38 | 38 | 46 |
| S*, no PM or PO | 70 | 70 | 70 | 79 | 79 | 79 | 34 | 34 | 34 | 38 | 38 | 46 |
| S* and PO, no PM† | 52 | 52 | 52 | 77 | 77 | 77 | 34 | 34 | 34 | 10 | 10 | 12 |
| S* and PO and PM† | 25 | 25 | 25 | 81 | 81 | 81 | 37 | 37 | 37 | 10 | 10 | 12 |
| MRI sensitivity‡ | 0.85 | 0.50 | 0.90 | 0.85 | 0.50 | 0.90 | 0.85 | 0.50 | 0.90 | 0.85 | 0.50 | 0.90 |
| No S* or PM or PO | 68 | 68 | 68 | 42 | 42 | 42 | 34 | 34 | 34 | 38 | 38 | 38 |
| S*, no PM or PO | 70 | 69 | 71 | 79 | 59 | 82 | 34 | 34 | 34 | 38 | 38 | 38 |
| S* and PO, no PM† | 52 | 50 | 54 | 77 | 58 | 81 | 34 | 34 | 34 | 10 | 10 | 10 |
| S* and PO and PM† | 25 | 23 | 24 | 81 | 68 | 86 | 37 | 37 | 37 | 10 | 10 | 10 |
| HR for BC after PO | 0.5§ | 0.1 | 1 | 0.5§ | 0.1 | 1 | 0.5§ | 0.1 | 1 | 0.5§ | 0.1 | 1 |
| No S* or PM or PO | 68 | 68 | 68 | 42 | 42 | 42 | 34 | 34 | 34 | 38 | 38 | 38 |
| S*, no PM or PO | 70 | 70 | 70 | 79 | 79 | 79 | 34 | 34 | 34 | 38 | 38 | 38 |
| S* and PO, no PM† | 52 | 27 | 71 | 77 | 77 | 76 | 34 | 35 | 34 | 10 | 10 | 10 |
| S* and PO and PM† | 25 | 23 | 27 | 81 | 84 | 80 | 37 | 37 | 37 | 10 | 10 | 10 |
| BRCA2 mutation carriers | ||||||||||||
| BC by 70 years, BRCA2 | 0.45 | 0.4 | 0.85 | 0.45 | 0.4 | 0.85 | 0.45 | 0.4 | 0.85 | 0.45 | 0.4 | 0.85 |
| No S* or PM or PO | 50 | 45 | 86 | 47 | 47 | 47 | 78 | 78 | 78 | 11 | 11 | 11 |
| S*, no PM or PO | 53 | 48 | 88 | 80 | 77 | 83 | 78 | 78 | 78 | 11 | 11 | 11 |
| S* and PO, no PM† | 34 | 31 | 72 | 79 | 76 | 80 | 78 | 78 | 78 | 2 | 2 | 2 |
| S* and PO and PM† | 13 | 12 | 34 | 83 | 82 | 86 | 81 | 80 | 80 | 2 | 2 | 2 |
| OC by 70 years, BRCA2 | 0.11 | 0.11 | 0.27 | 0.11 | 0.11 | 0.27 | 0.11 | 0.11 | 0.27 | 0.11 | 0.11 | 0.27 |
| No S* or PM or PO | 50 | 50 | 50 | 47 | 47 | 47 | 78 | 78 | 78 | 11 | 11 | 27 |
| S*, no PM or PO | 53 | 53 | 53 | 80 | 80 | 80 | 78 | 78 | 78 | 11 | 11 | 27 |
| S* and PO, no PM† | 34 | 34 | 34 | 79 | 79 | 79 | 78 | 78 | 78 | 2 | 2 | 6 |
| S* and PO and PM† | 13 | 13 | 13 | 83 | 83 | 83 | 81 | 81 | 81 | 2 | 2 | 6 |
| MRI sensitivity‡ | 0.85 | 0.50 | 0.90 | 0.85 | 0.50 | 0.90 | 0.85 | 0.50 | 0.90 | 0.85 | 0.50 | 0.90 |
| No S* or PM or PO | 50 | 50 | 50 | 47 | 47 | 47 | 78 | 78 | 78 | 11 | 11 | 11 |
| S*, no PM or PO | 53 | 50 | 54 | 80 | 63 | 82 | 78 | 78 | 78 | 11 | 11 | 11 |
| S* and PO, no PM† | 34 | 32 | 36 | 79 | 62 | 81 | 78 | 78 | 78 | 2 | 2 | 2 |
| S* and PO and PM† | 13 | 13 | 13 | 83 | 71 | 86 | 81 | 80 | 80 | 2 | 2 | 2 |
| HR for BC after PO | 0.5§ | 0.1 | 1 | 0.5§ | 0.1 | 1 | 0.5§ | 0.1 | 1 | 0.5§ | 0.1 | 1 |
| No S* or PM or PO | 50 | 50 | 50 | 47 | 47 | 47 | 78 | 78 | 78 | 11 | 11 | 11 |
| S*, no PM or PO | 53 | 53 | 53 | 80 | 80 | 80 | 78 | 78 | 78 | 11 | 11 | 11 |
| S* and PO, no PM† | 34 | 15 | 52 | 79 | 79 | 78 | 78 | 79 | 78 | 2 | 2 | 2 |
| S* and PO and PM† | 13 | 12 | 15 | 83 | 86 | 80 | 81 | 81 | 80 | 2 | 2 | 2 |
NOTE. Parameters, varied from base case to lower and upper cases, are BC and OC risks by age 70 years owing to BRCA1/2 mutations (BC by 70 years, OC by 70 years), MRI sensitivity for BC detection, and HR for BC development after premenopausal PO; interventions evaluated are S, PM, and/or PO.
Abbreviations: BC, breast cancer; ER, estrogen receptor; HR, hazard ratio; MRI, magnetic resonance imaging; OC, ovarian cancer; PM, prophylactic mastectomy; PO, prophylactic oophorectomy; S, screening.
S consists of mammography and MRI annually, according to national practice guidelines24,51; it is initiated at age 25 years and continued through age 69 years unless PM occurs, after which time breast S stops.
Prophylactic surgeries (PM and/or PO) are performed at age 40 years in these scenarios.
MRI sensitivity varied in sensitivity analysis by adjusting the tumor size detection threshold between 0.3 cm (90% sensitivity) and 1.53 cm (50% sensitivity).
In the base case, the HR for BC is 0.50 after PO is performed between ages 40 and 50 years, as described in Table 1.
Footnotes
See accompanying editorial on page 471
Supported by the National Institutes of Health (Grants No. R01 CA829040, U01 CA088248, and R01 CA66785 to S.K.P), the Robert Wood Johnson Foundation (Physician Faculty Scholars Award, Grant No. 64317 to A.W.K.), and a Stanford Cancer Institute Developmental Research Award.
Presented in part at the Robert Wood Johnson Foundation Physician Faculty Scholars Meeting, December 8-10, 2010, Fort Lauderdale, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Allison W. Kurian, Lauren Clarke, Sylvia K. Plevritis
Financial support: Allison W. Kurian, Sylvia K. Plevritis
Provision of study materials or patients: Allison W. Kurian, Elizabeth A. Schackmann, Sylvia K. Plevritis
Collection and assembly of data: All authors
Data analysis and interpretation: Allison W. Kurian, Diego F. Munoz, Elizabeth A. Schackmann, Meredith A. Mills, Sylvia K. Plevritis
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domchek SM, Friebel TM, Neuhausen SL, et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: A prospective cohort study. Lancet Oncol. 2006;7:223–229. doi: 10.1016/S1470-2045(06)70585-X. [DOI] [PubMed] [Google Scholar]
- 4.Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967–975. doi: 10.1001/jama.2010.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauff ND, Domchek SM, Friebel TM, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J Clin Oncol. 2008;26:1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagen AI, Kvistad KA, Maehle L, et al. Sensitivity of MRI versus conventional screening in the diagnosis of BRCA-associated breast cancer in a national prospective series. Breast. 2007;16:367–374. doi: 10.1016/j.breast.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Kriege M, Brekelmans CT, Peterse H, et al. Tumor characteristics and detection method in the MRISC screening program for the early detection of hereditary breast cancer. Breast Cancer Res Treat. 2007;102:357–363. doi: 10.1007/s10549-006-9341-6. [DOI] [PubMed] [Google Scholar]
- 9.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23:8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 10.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS) Lancet. 2005;365:1769–1778. doi: 10.1016/S0140-6736(05)66481-1. [DOI] [PubMed] [Google Scholar]
- 11.Trecate G, Vergnaghi D, Bergonzi S, et al. Breast MRI screening in patients with increased familial and/or genetic risk for breast cancer: A preliminary experience. Tumori. 2003;89:125–131. doi: 10.1177/030089160308900204. [DOI] [PubMed] [Google Scholar]
- 12.Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29:1664–1669. doi: 10.1200/JCO.2009.27.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresser PJ, Seynaeve C, Van Gool AR, et al. Satisfaction with prophylactic mastectomy and breast reconstruction in genetically predisposed women. Plast Reconstr Surg. 2006;117:1675–1682. doi: 10.1097/01.prs.0000217383.99038.f5. [DOI] [PubMed] [Google Scholar]
- 15.Foulkes WD, Metcalfe K, Sun P, et al. Estrogen receptor status in BRCA1- and BRCA2-related breast cancer: The influence of age, grade, and histological type. Clin Cancer Res. 2004;10:2029–2034. doi: 10.1158/1078-0432.ccr-03-1061. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082–1089. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93:1633–1637. doi: 10.1093/jnci/93.21.1633. [DOI] [PubMed] [Google Scholar]
- 18.Metcalfe K, Lynch HT, Ghadirian P, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J Clin Oncol. 2004;22:1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 20.Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 21.Robson M, Hensley M, Barakat R, et al. Quality of life in women at risk for ovarian cancer who have undergone risk-reducing oophorectomy. Gynecol Oncol. 2003;89:281–287. doi: 10.1016/s0090-8258(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 22.Tercyak KP, Peshkin BN, Brogan BM, et al. Quality of life after contralateral prophylactic mastectomy in newly diagnosed high-risk breast cancer patients who underwent BRCA1/2 gene testing. J Clin Oncol. 2007;25:285–291. doi: 10.1200/JCO.2006.07.3890. [DOI] [PubMed] [Google Scholar]
- 23.Tung N, Wang Y, Collins LC, et al. Estrogen receptor positive breast cancers in BRCA1 mutation carriers: Clinical risk factors and pathologic features. Breast Cancer Res. 2010;12:R12. doi: 10.1186/bcr2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: Breast and ovarian. J Natl Compr Canc Netw. 2010;8:562–594. doi: 10.6004/jnccn.2010.0043. [DOI] [PubMed] [Google Scholar]
- 25.Anderson K, Jacobson JS, Heitjan DF, et al. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann Intern Med. 2006;144:397–406. doi: 10.7326/0003-4819-144-6-200603210-00006. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: A decision analysis. J Clin Oncol. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 27.Grann VR, Jacobson JS, Thomason D, et al. Effect of prevention strategies on survival and quality-adjusted survival of women with BRCA1/2 mutations: An updated decision analysis. J Clin Oncol. 2002;20:2520–2529. doi: 10.1200/JCO.2002.10.101. [DOI] [PubMed] [Google Scholar]
- 28.Grann VR, Patel PR, Jacobson JS, et al. Comparative effectiveness of screening and prevention strategies among BRCA1/2-affected mutation carriers. Breast Cancer Res Treat. 2011;125:837–847. doi: 10.1007/s10549-010-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28:222–231. doi: 10.1200/JCO.2009.22.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JM, Kopans DB, McMahon PM, et al. Breast cancer screening in BRCA1 mutation carriers: Effectiveness of MR imaging—Markov Monte Carlo decision analysis. Radiology. 2008;246:763–771. doi: 10.1148/radiol.2463070224. [DOI] [PubMed] [Google Scholar]
- 31.Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295:2374–2384. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]
- 32.Schrag D, Kuntz KM, Garber JE, et al. Decision analysis: Effects of prophylactic mastectomy and oophorectomy on life expectancy among women with BRCA1 or BRCA2 mutations. N Engl J Med. 1997;336:1465–1471. doi: 10.1056/NEJM199705153362022. [DOI] [PubMed] [Google Scholar]
- 33.Schrag D, Kuntz KM, Garber JE, et al. Life expectancy gains from cancer prevention strategies for women with breast cancer and BRCA1 or BRCA2 mutations. JAMA. 2000;283:617–624. doi: 10.1001/jama.283.5.617. [DOI] [PubMed] [Google Scholar]
- 34.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 35.Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18:3302–3317. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 36.Ganz PA, Kwan L, Stanton AL, et al. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29:1101–1109. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans DG, Shenton A, Woodward E, et al. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a clinical cancer genetics service setting: Risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer. 2008;8:155. doi: 10.1186/1471-2407-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families: The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 40.Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: An international case-control study. J Clin Oncol. 2005;23:7491–7496. doi: 10.1200/JCO.2004.00.7138. [DOI] [PubMed] [Google Scholar]
- 41.Kramer JL, Velazquez IA, Chen BE, et al. Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long-term follow-up of BRCA1 mutation carriers. J Clin Oncol. 2005;23:8629–8635. doi: 10.1200/JCO.2005.02.9199. [DOI] [PubMed] [Google Scholar]
- 42.Finch A, Beiner M, Lubinski J, et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA. 2006;296:185–192. doi: 10.1001/jama.296.2.185. [DOI] [PubMed] [Google Scholar]
- 43.Tilanus-Linthorst MM, Obdeijn IM, Hop WC, et al. BRCA1 mutation and young age predict fast breast cancer growth in the Dutch, United Kingdom, and Canadian magnetic resonance imaging screening trials. Clin Cancer Res. 2007;13:7357–7362. doi: 10.1158/1078-0432.CCR-07-0689. [DOI] [PubMed] [Google Scholar]
- 44.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 45.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 46.Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 47.Colditz GA, Willett WC, Stampfer MJ, et al. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 48.Melton LJ, Khosla S, Malkasian GD, et al. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900–905. doi: 10.1359/jbmr.2003.18.5.900. [DOI] [PubMed] [Google Scholar]
- 49.Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 50.Rocca WA, Grossardt BR, de Andrade M, et al. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 51.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 52.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 53.Plevritis SK, Sigal BM, Salzman P, et al. Chapter 12: a stochastic simulation model of U.S. breast cancer mortality trends from 1975 to 2000. J Natl Cancer Inst Monogr. 2006;36:86–95. doi: 10.1093/jncimonographs/lgj012. [DOI] [PubMed] [Google Scholar]
- 54.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer. Clinical practice guidelines in oncology, Version 2. 2011. http://www.nccn.org.
- 55.Chappuis PO, Nethercot V, Foulkes WD. Clinico-pathological characteristics of BRCA1- and BRCA2-related breast cancer. Semin Surg Oncol. 2000;18:287–295. doi: 10.1002/(sici)1098-2388(200006)18:4<287::aid-ssu3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 56.Stolier AJ, Corsetti RL. Newly diagnosed breast cancer patients choose bilateral mastectomy over breast-conserving surgery when testing positive for a BRCA1/2 mutation. Am Surg. 2005;71:1031–1033. [PubMed] [Google Scholar]
- 57.Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, et al. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122:2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wainberg S, Husted J. Utilization of screening and preventive surgery among unaffected carriers of a BRCA1 or BRCA2 gene mutation. Cancer Epidemiol Biomarkers Prev. 2004;13:1989–1995. [PubMed] [Google Scholar]
- 59.Bayraktar S, Gutierrez-Barrera AM, Liu D, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat. 2011;130:145–153. doi: 10.1007/s10549-011-1711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee LJ, Alexander B, Schnitt SJ, et al. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer. 2011;117:3093–3100. doi: 10.1002/cncr.25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eisen A, Lubinski J, Gronwald J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361–1367. doi: 10.1093/jnci/djn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rebbeck TR, Friebel T, Wagner T, et al. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J Clin Oncol. 2005;23:7804–7810. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- 63.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 64.Brohet RM, Goldgar DE, Easton DF, et al. Oral contraceptives and breast cancer risk in the International BRCA1/2 Carrier Cohort Study: A report from EMBRACE, GENEPSO, GEO-HEBON, and the IBCCS Collaborating Group. J Clin Oncol. 2007;25:3831–3836. doi: 10.1200/JCO.2007.11.1179. [DOI] [PubMed] [Google Scholar]
- 65.Gronwald J, Tung N, Foulkes WD, et al. Tamoxifen and contralateral breast cancer in BRCA1 and BRCA2 carriers: An update. Int J Cancer. 2006;118:2281–2284. doi: 10.1002/ijc.21536. [DOI] [PubMed] [Google Scholar]
- 66.Jernström H, Loman N, Johannsson OT, et al. Impact of teenage oral contraceptive use in a population-based series of early-onset breast cancer cases who have undergone BRCA mutation testing. Eur J Cancer. 2005;41:2312–2320. doi: 10.1016/j.ejca.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 67.King MC, Wieand S, Hale K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286:2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 68.Milne RL, Knight JA, John EM, et al. Oral contraceptive use and risk of early-onset breast cancer in carriers and noncarriers of BRCA1 and BRCA2 mutations. Cancer Epidemiol Biomarkers Prev. 2005;14:350–356. doi: 10.1158/1055-9965.EPI-04-0376. [DOI] [PubMed] [Google Scholar]
- 69.Modan B, Hartge P, Hirsh-Yechezkel G, et al. Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235–240. doi: 10.1056/NEJM200107263450401. [DOI] [PubMed] [Google Scholar]
- 70.University of California, Berkeley. Berkeley Mortality Database. http://www.demog.berkeley.edu/∼bmd/States/ssa/life.tables/ufgen.lt.1x1.
- 71.Centers for Disease Control and Prevention. National Vital Statistics System, Worktable 292R: Death rates for 358 selected causes by 5-year age groups, race, and sex—United States, 1999-2004. http://www.cdc.gov/nchs.
- 72.Parker WH, Broder MS, Liu Z, et al. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219–226. doi: 10.1097/01.AOG.0000167394.38215.56. [DOI] [PubMed] [Google Scholar]
- 73.FORCE. Facing Our Risk of Cancer Empowered. http://www.facingourrisk.org.
- 74.Singletary SE, Connolly JL. Breast cancer staging: Working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin. 2006;56:37–47. doi: 10.3322/canjclin.56.1.37. [DOI] [PubMed] [Google Scholar]
- 75.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 76.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 77.Stanford University Cancer Institute. BRCA Decision Tool. http://brcatool.stanford.edu.
- 78.Amir E, Freedman OC, Seruga B, et al. Assessing women at high risk of breast cancer: A review of risk assessment models. J Natl Cancer Inst. 2010;102:680–691. doi: 10.1093/jnci/djq088. [DOI] [PubMed] [Google Scholar]
- 79.Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res (Phila) 2010;3:686–688. doi: 10.1158/1940-6207.CAPR-10-0100. [DOI] [PubMed] [Google Scholar]
- 80.Begg CB, Haile RW, Borg A, et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA. 2008;299:194–201. doi: 10.1001/jama.2007.55-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simchoni S, Friedman E, Kaufman B, et al. Familial clustering of site-specific cancer risks associated with BRCA1 and BRCA2 mutations in the Ashkenazi Jewish population. Proc Natl Acad Sci U S A. 2006;103:3770–3774. doi: 10.1073/pnas.0511301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tryggvadottir L, Sigvaldason H, Olafsdottir GH, et al. Population-based study of changing breast cancer risk in Icelandic BRCA2 mutation carriers, 1920-2000. J Natl Cancer Inst. 2006;98:116–122. doi: 10.1093/jnci/djj012. [DOI] [PubMed] [Google Scholar]
- 83.Haroun I, Graham T, Poll A, et al. Reasons for risk-reducing mastectomy versus MRI-screening in a cohort of women at high hereditary risk of breast cancer. Breast. 2011;20:254–258. doi: 10.1016/j.breast.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 84.Staton AD, Kurian AW, Cobb K, et al. Cancer risk reduction and reproductive concerns in female BRCA1/2 mutation carriers. Fam Cancer. 2008;7:179–186. doi: 10.1007/s10689-007-9171-7. [DOI] [PubMed] [Google Scholar]
- 85.Grann VR, Jacobson JS, Sundararajan V, et al. The quality of life associated with prophylactic treatments for women with BRCA1/2 mutations. Cancer J Sci Am. 1999;5:283–292. [PubMed] [Google Scholar]
- 86.van Roosmalen MS, Verhoef LC, Stalmeier PF, et al. Decision analysis of prophylactic surgery or screening for BRCA1 mutation carriers: A more prominent role for oophorectomy. J Clin Oncol. 2002;20:2092–2100. doi: 10.1200/jco.2002.08.035. [DOI] [PubMed] [Google Scholar]
- 87.Brace C, Schmocker S, Huang H, et al. Physicians' awareness and attitudes toward decision aids for patients with cancer. J Clin Oncol. 2010;28:2286–2292. doi: 10.1200/JCO.2009.25.2874. [DOI] [PubMed] [Google Scholar]
- 88.Leighl NB, Shepherd HL, Butow PN, et al. Supporting treatment decision making in advanced cancer: A randomized trial of a decision aid for patients with advanced colorectal cancer considering chemotherapy. J Clin Oncol. 2011;29:2077–2084. doi: 10.1200/JCO.2010.32.0754. [DOI] [PubMed] [Google Scholar]
- 89.O'Brien MA, Whelan TJ, Villasis-Keever M, et al. Are cancer-related decision aids effective? A systematic review and meta-analysis. J Clin Oncol. 2009;27:974–985. doi: 10.1200/JCO.2007.16.0101. [DOI] [PubMed] [Google Scholar]
- 90.Sepucha KR, Belkora JK, Tripathy D, et al. Building bridges between physicians and patients: Results of a pilot study examining new tools for collaborative decision making in breast cancer. J Clin Oncol. 2000;18:1230–1238. doi: 10.1200/JCO.2000.18.6.1230. [DOI] [PubMed] [Google Scholar]
- 91.van Roosmalen MS, Stalmeier PF, Verhoef LC, et al. Randomised trial of a decision aid and its timing for women being tested for a BRCA1/2 mutation. Br J Cancer. 2004;90:333–342. doi: 10.1038/sj.bjc.6601525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Waljee JF, Rogers MA, Alderman AK. Decision aids and breast cancer: Do they influence choice for surgery and knowledge of treatment options? J Clin Oncol. 2007;25:1067–1073. doi: 10.1200/JCO.2006.08.5472. [DOI] [PubMed] [Google Scholar]
- 93.Chao C, Studts JL, Abell T, et al. Adjuvant chemotherapy for breast cancer: How presentation of recurrence risk influences decision-making. J Clin Oncol. 2003;21:4299–4305. doi: 10.1200/JCO.2003.06.025. [DOI] [PubMed] [Google Scholar]
- 94.Plevritis SK, Salzman P, Sigal BM, et al. A natural history model of stage progression applied to breast cancer. Stat Med. 2007;26:581–595. doi: 10.1002/sim.2550. [DOI] [PubMed] [Google Scholar]
- 95.Brekelmans CT, Tilanus-Linthorst MM, Seynaeve C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, BRCA1- and non-BRCA1/2 families as compared to sporadic breast cancer cases. Eur J Cancer. 2007;43:867–876. doi: 10.1016/j.ejca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Lakhani SR, Van De Vijver MJ, Jacquemier J, et al. The pathology of familial breast cancer: Predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and p53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20:2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 97.Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: Differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6:R8–R17. doi: 10.1186/bcr658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Antoniou AC, Sinilnikova OM, Simard J, et al. RAD51 135G–>C modifies breast cancer risk among BRCA2 mutation carriers: Results from a combined analysis of 19 studies. Am J Hum Genet. 2007;81:1186–1200. doi: 10.1086/522611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rebbeck TR, Mitra N, Domchek SM, et al. Modification of BRCA1-associated breast and ovarian cancer risk by BRCA1 interacting genes. Cancer Res. 2011;71:5792–5805. doi: 10.1158/0008-5472.CAN-11-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: A prospective observational study. Lancet. 2007;370:485–492. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 101.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 102.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 103.O'Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 104.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]