Introduction
Minimal treatment options exist for advanced, inoperable neurofibromatosis type 2 (NF2), which is a rare tumor-prone disorder. NF2 results from a mutation in the NF2 tumor-suppressor gene on chromosome 22q12. NF2 is genetically and phenotypically distinguished from neurofibromatosis type 1, which results from a mutation in the NF1 tumor-suppressor gene located on chromosome 17q11.1,2 Clinically, NF2 is inherited in an autosomal dominant fashion and manifests through the appearance of bilateral acoustic neuromas (vestibular schwannomas), which lead to progressive hearing loss.3 Vestibular schwannomas and other lesions seen in patients with NF2 that arise progressively from other sites, such as meningiomas, spinal schwannomas, astrocytomas, ependymomas, rarely neurofibromas, and peripheral neuropathies, lead to profound morbidity in this debilitating disease.1,4 Few options are available to these patients outside of surgery, which is the mainstay of treatment for NF2-associated lesions, and, in some instances, radiation therapy.1,2 Despite our understanding of the underlying genetics and molecular pathophysiology of this disorder, patients become debilitated from tumor-related comorbidities. Recently, the anti–vascular endothelial growth factor (VEGF) antibody bevacizumab and erlotinib exhibited promising activity in pilot trials.5–8 Other than these two agents, no medical options are available for patients with NF2 with surgically unresectable disease. Because patients with NF2 harbor an aberration in a single gene, merlin, the protein product of which impacts multiple signals, including PI3-kinase/Akt, Raf/mitogen-activated protein/extracellular signal-regulated kinase, and mammalian target of rapamycin (mTOR), it is conceivable that one of the many agents that target these pathways that have already shown antitumor activity in malignancies will also cause the regression of NF2-related tumors.9–11
Because of its extreme rarity, conducting large clinical trials in NF2 is generally unfeasible.9 Therefore, we enrolled patients with NF2 onto various rationally targeted trials and sought response signals. We reviewed the records of consecutive patients with NF2 who were referred to the Clinical Center for Targeted Therapy (phase I Clinical Trials Program clinic) who were treated in more than one trial starting in January 2007 to December 2010. All trials were approved by the MD Anderson institutional review board, which also granted a waiver of informed consent and a waiver of authorization for this retrospective study. Patients were evaluated every 6 to 8 weeks for response by using RECIST with computed tomography and magnetic resonance imaging.
Case Reports
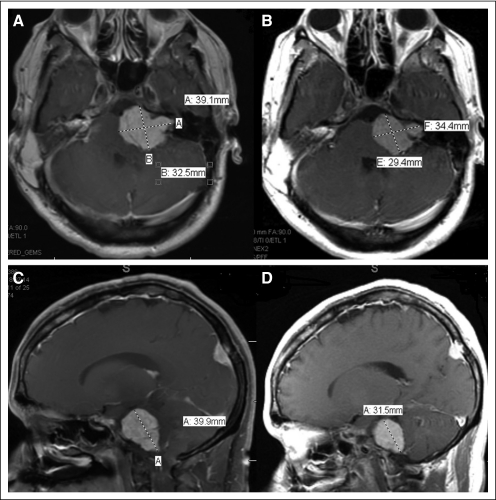
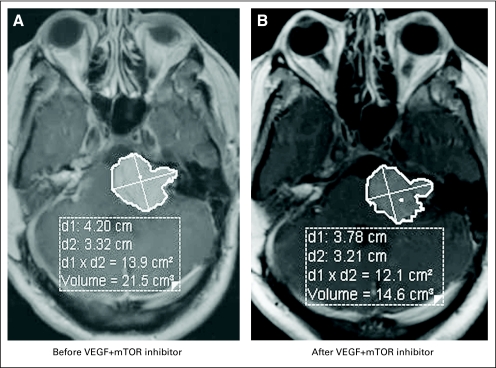
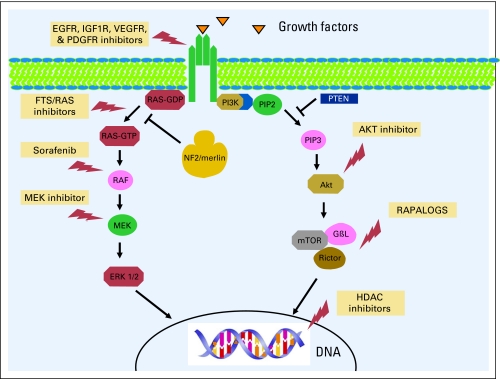
A total of six consecutive patients (men, n = 3 patients) were enrolled onto early clinical trials, the clinical characteristics of which are outlined in Table 1. The median age at diagnosis was 16.5 years (range, 4 to 29 years), and the median age at referral to the phase I Clinical Trials Program was 31.5 years (range, 16 to 41 years). All patients referred for early phase therapy had multiple previous resections of their tumors, including their vestibular schwannomas, and all had hearing deficits. Each patient had more than five tumor sites of histologically proven multiple vestibular schwannomas, hamartomas, meningiomas, and ependymomas. Three patients (patients 3, 5, and 6 as listed in Table 1) had a paternal history of NF2. There was also a significant family history of other malignancies as outlined in Table 1. Mutational analyses for mutations other than NF2 were carried out in two patients who had no other mutations. One patient was tested for c-Met amplification. The other patient was tested for mutations in PIK3CA (codons 532 to 554 in exon 9 or codons 1011 to 1062 in exon 20 of the PIK3CA gene), KRAS, NRAS (codons 12, 13, or 61 of the KRAS or NRAS gene) in the benign nerve-sheath tumor and TP53 (exons 4 to 9 of the TP53 gene). The second patient also showed PTEN expression. Treatment and clinical outcomes are outlined in Table 2. Two patients were treated with a RAt sarcoma (RAS) inhibitor (salirasib), and both patients achieved stable disease (SD) for 10 and more than 52 months.12 The patient who achieved SD for more than 4.5 years while treated with the RAS inhibitor had progressive disease in his course before salirasib, which resulted in spinal cord compression with urinary incontinence and lower extremity problems that required surgery. Interestingly, after receiving the RAS inhibitor, the patient had no additional disease progression. One patient had SD after treatment with a mitogen-activated protein kinase 1 inhibitor (mitogen-activated protein/extracellular signal-regulated kinase or MAP/ERK kinase1 inhibitor) for 7 months. The patient was subsequently enrolled onto several target agent-based studies, including one with the multikinase inhibitor sorafenib combined with the histone deacetylase inhibitor valproic acid. On another study, the patient was treated with and did not respond to the combination of valproic acid and the epidermal growth factor receptor inhibitor erlotinib. The patient subsequently had ongoing SD in response to bevacizumab, which is a VEGF antibody, for more than 22 months. The patient, who had some hearing at referral, was treated with bevacizumab and has stable hearing and SD. Two other patients with NF2 treated with bevacizumab and an mTOR inhibitor combination had SD for more than 4 and 9 months. The patient who had SD by RECIST for more than 9 months has, thus far, had a 33% decrease in tumor size by volumetric analysis. Magnetic resonance images of the brain of the patient, with and without contrast, are shown in Figure 1 with both panels demonstrating a response. The volumetric analysis of the response is shown in Figure 2. The neurologic symptoms of the patient also improved with an almost complete flattening of subcutaneous lesions. Adverse-effect profiles of the patients are outlined in Table 2. There were no life-threatening severe adverse events, and the putative mechanism of molecularly targeted therapies used in our patients is shown in Figure 3 (EGFR, epidermal growth factor receptor; IGF1R, insulin-like growth factor 1 receptor; VEGFR, vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; HDAC, histone deacetylase).
Table 1.
Clinical Characteristics of Patients With Neurofibromatosis Type 2 Enrolled Onto Phase I Clinical Trials
| Patient No. | Age at Diagnosis (years) | Age at Referral (years) | Sex | Race | Growth Pattern of NF2 Lesions Before Phase I Therapy* | Family History of NF2 | Other Cancers in the Family |
|---|---|---|---|---|---|---|---|
| 1 | 22 | 26 | M | W | Bilateral vestibular schwannomas, C2-C1 mass, T9-10 mass, growth in size of all lesions | Spontaneous | CLL, large-cell anaplastic lymphoma, breast cancer |
| 2 | 15 | 37 | F | W | Astrocytoma of C2-C3, bilateral vestibular schwannomas, ependymoma C6-C7, meningoma brain, L1 and T12 | Spontaneous | Pancreatic and colon cancer |
| 3 | 16 | 41 | F | W | Bilateral acoustic neuroma, cerebellum meningioma, which affected balance, growth of ependymoma, astrocytoma | Father and half-brother | None |
| 4 | 4 | 16 | F | A | Bilateral vestibular schwannomas, C6 schwannoma/meningioma complex, cauda equina schwannoma, multiple skull-based tumors mostly schwannomas | Spontaneous | None |
| 5 | 29 | 33 | M | A | Multiple schwannomas, vocal paralysis from neurofibroma of the laryngeal nerve, neurofibroma of gluteal branch of the right sciatic nerve, cervical and extradural schwannomas, nerve sheath tumor from left median nerve, left and right ulnar nerve, left acoustic schwannomas | Father | Gastric, pancreatic cancer, lung cancer |
| 6 | 17 | 30 | M | W | Bilateral acoustic neuroma, multiple meningiomas in the brain, cervicomedullary ependymoma, T11, T12, and L1 schwannomas | Father | None |
Abbreviations: A, Asian; CLL, chronic lymphatic leukemia; NF2, neurofibromatosis type 2; W, white.
All patients with NF2 referred to the phase I Clinical Center for Targeted Therapy had greater than five lesions at the time of referral.
Table 2.
Treatment, Mechanism of Action, Adverse Effects, and Clinical Outcome of Patients With Neurofibromatosis Type 2 Enrolled Onto an Early-Phase Clinical Trial
| Patient No. | Drugs | Mechanism | Adverse Effects | Outcome |
|---|---|---|---|---|
| 1 | FTS | RAS inhibitor | Minimal diarrhea | Stable disease for 4.5 years |
| 2 | FTS | RAS inhibitor | Minimal diarrhea | Stable disease for 10 months |
| 3 | AZD8330 | MEK inhibitor | Stable disease for 7 months | |
| 3 | Sorafinib + valproic acid | VEGFR, PDGFR, and RAF/MEK/ERK pathway inhibitor plus HDAC inhibitor | Rash | Off study early because of a rash |
| 3 | Erlotinib + valproic acid | EGFR inhibitor plus HDAC inhibitor | Drowsiness | Off study because of drowsiness |
| 3 | Erlotinib | EGFR inhibitor | Rash, diarrhea | Off study after 2 months because of a rash |
| 3 | Bevacizumab | VEGF antibody | No significant adverse effects | Stable disease for 9+ months |
| 4 | Bevacizumab | VEGF antibody | No significant adverse effects | Stable disease for 10+ months; ongoing and stable hearing on volumetric analyses |
| 5 | Bevacizumab + temsirolimus | VEGF antibody + mTOR inhibitor | Grade 1 proteinuria, hyperlipidemia | Stable disease for 4+ months |
| 6 | Bevacizumab + temsirolimus | VEGF antibody + mTOR inhibitor | Grade 1 proteinuria, hyperlipidemia | 33% volumetric reduction in size and better gait; symptoms improved; stable disease for> 9+ months |
Abbreviations: EGFR, epidermal growth factor receptor; ERK, extracellular-signal-regulated kinase; FTS-S-trans, trans-farnesylthiosalicylic acid; HDAC, histone deacetylases; MEK, mitogen-activated protein; mTOR, mammalian target of rapamycin; PDGFR, platelet-derived growth factor receptor; RAS, RAt sarcoma; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
Fig 1.
Fig 2.
Fig 3.
Discussion
To our knowledge, this is the first clinical case series that used rational targeted therapies in patients with NF2. Our results showed that patients with NF2 who were referred to a clinical trials center for targeted therapy treatment demonstrated acceptable safety profiles and preliminary evidence of activity, and targeted therapy is a pragmatic option in this rare-disease setting. Consensus statements in a comprehensive NF2 workshop outlined methods for successfully bringing patients with NF2 into clinical trials.9 The subjects discussed included which tumors should be considered for treatment first (vestibular schwannomas v meningiomas), the role of a placebo in NF2 clinical trials, critical end points that should be evaluated when determining therapeutic efficacy and trial outcomes, the type of clinical trial that should be performed (phase 0 and/or II), new candidate drugs, and potential therapeutic approaches to NF2. An updated list of candidate drugs for treating NF2 was also recommended.9 These included antiangiogenic drugs such as the multikinase inhibitors sunitinib and sorafenib and the anti-VEGF antibody bevacizumab. Among these agents, only bevacizumab had been used to treat patients with NF2 who were considered poor candidates for surgery and radiation therapy.5 After bevacizumab treatment, tumors diminished in size in nine of 10 patients, and four of six patients had an imaging response, which was maintained for 11 to 16 months of follow-up. The median best response to treatment was a volumetric reduction of tumor of 26%.5 Of the seven patients who were eligible for a hearing response, four patients had hearing responses, two patients had stable hearing, and one patient had progressive hearing loss. Adverse events were of grade 1 or 2 only, and thus, the drug was well-tolerated.5 After one patient with NF2 had a clinical and radiologic response to erlotinib,8 another 11 patients with NF2 were treated with erlotinib but demonstrated no radiographic or hearing responses.6 Although the improvement in hearing responses in the bevacizumab study were quite promising, the percentage reduction in tumor volume was not as impressive as hoped for. However, single-agent bevacizumab has the potential to broaden the level of response when combined with another targeted agent or agents.
The age at referral did not correlate with clinical outcomes given the small number of patients in our series. Moreover, patients were referred to the Clinical Center for Targeted Therapy only after all other frontline options were exhausted and when patients were in their advanced state of disease. Better responses might occur if targeted agents are offered earlier in the course of the disease trajectory. Most patients had previous resections of their vestibular schwannomas. Because of the nature and design of phase I studies that evaluate only for radiologic responses, hearing responses by audiometry were not measured as an end point in treatment. This is instructive because hearing responses should also be included as an end point in addition to imaging responses in patients with NF2 who are enrolled onto phase I trials.
Preclinical studies have shed light on the molecular pathogenesis of NF2/merlin,10,11 as depicted in Figure 3. Our rational approach of treating patients with advanced, unresectable NF2 lesions with new molecularly targeted therapies on the basis of preclinical evidence demonstrated activity in the form of disease stabilization rather than a true radiologic response. The single patient who had a significant improvement in some symptoms and the disappearance of other symptom received a combination of VEGF and an mTOR inhibitor, although it is not clear if either agent caused the responses. In theory, the combination provided additive or synergistic activity.
In summary, novel targeted agents, alone or in combination with other treatments, are reasonable options for patients with NF2 and may allow rapid proof-of-principle translation of rational therapies (Fig 3). An additional evaluation of several of these combinatorial therapies is warranted.
ACKNOWLEDGMENT
This publication was funded in part by The Denise Terrill Charity Classics. The University of Texas MD Anderson Cancer Center is supported in part by the National Institutes of Health through Cancer Center Support Grant no. CA 016672. This work was supported by National Institutes of Health Grant 5 U01 CA062461 (to R.K.). We thank Ms. Joann Aaron for scientific review and editing of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.Asthagiri AR, Parry DM, Butman JA, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974–1986. doi: 10.1016/S0140-6736(09)60259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans DG. Neurofibromatosis type 2 (NF2): A clinical and molecular review. Orphanet J Rare Dis. 2009;4:16. doi: 10.1186/1750-1172-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu-Emerson C, Plotkin SR. The neurofibromatoses. Part 2: NF2 and schwannomatosis. Rev Neurol Dis. 2009;6:E81–E86. [PubMed] [Google Scholar]
- 4.Baser ME, R Evans DG, Gutmann DH. Neurofibromatosis 2. Curr Opin Neurol. 2003;16:27–33. doi: 10.1097/01.wco.0000053583.70044.ab. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin SR, Stemmer-Rachamimov AO, Barker FG, 2nd, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361:358–367. doi: 10.1056/NEJMoa0902579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plotkin SR, Halpin C, McKenna MJ, et al. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31:1135–1143. doi: 10.1097/MAO.0b013e3181eb328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mautner VF, Nguyen R, Kutta H, et al. Bevacizumab induces regression of vestibular schwannomas in patients with neurofibromatosis type 2. Neuro Oncol. 2010;12:14–18. doi: 10.1093/neuonc/nop010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plotkin SR, Singh MA, O'Donnell CC, et al. Audiologic and radiographic response of NF2-related vestibular schwannoma to erlotinib therapy. Nat Clin Pract Oncol. 2008;5:487–491. doi: 10.1038/ncponc1157. [DOI] [PubMed] [Google Scholar]
- 9.Evans DG, Kalamarides M, Hunter-Schaedle K, et al. Consensus recommendations to accelerate clinical trials for neurofibromatosis type 2. Clin Cancer Res. 2009;15:5032–5039. doi: 10.1158/1078-0432.CCR-08-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norden AD, Drappatz J, Wen PY. Targeted drug therapy for meningiomas. Neurosurg Focus. 2007;23:E12. doi: 10.3171/FOC-07/10/E12. [DOI] [PubMed] [Google Scholar]
- 11.Wen PY, Quant E, Drappatz J, et al. Medical therapies for meningiomas. J Neurooncol. 2010;99:365–378. doi: 10.1007/s11060-010-0349-8. [DOI] [PubMed] [Google Scholar]
- 12.Tsimberidou AM, Rudek MA, Hong D, et al. Phase 1 first-in-human clinical study of S-trans, trans-farnesylthiosalicylic acid (salirasib) in patients with solid tumors. Cancer Chemother Pharmacol. 2010;65:235–241. doi: 10.1007/s00280-009-1027-4. [DOI] [PubMed] [Google Scholar]