Abstract
Purpose
We evaluated whether patients with human epidermal growth factor receptor 2 (HER2) –positive primary breast tumors had metastatic tumors that were HER2 positive (concordant) or HER2 negative (discordant). We then evaluated whether treatment with trastuzumab or chemotherapy before biopsy of the metastasis had any effect on the rate of HER2 discordance. We also compared the overall survival durations of patients with HER2-concordant and -discordant tumors.
Patients and Methods
We retrospectively identified all patients who initially had been diagnosed with HER2-positive (immunohistochemistry 3+ and/or fluorescent in situ hybridization positive) primary breast cancer between 1997 and 2008 at MD Anderson Cancer Center who also had metastatic tumor biopsy results available for review.
Results
We included 182 patients who met our criteria. Forty-three (24%) of the 182 patients with HER2-positive primary tumors had HER2-negative metastatic tumors. The HER2 discordance rates differed significantly on the basis of whether patients received chemotherapy (P = .022) but not on the basis of whether patients received trastuzumab (P = .296). Patients with discordant HER2 status had shorter overall survival than did patients with concordant HER2 status (hazard ratio [HR], 0.43; P = .003). A survival difference remained among the 67 patients who received trastuzumab (HR, 0.56; P = .083) and 101 patients who did not (HR, 0.53; P = .033) before their metastasis biopsies.
Conclusion
We confirmed that loss of HER2-positive status in metastatic tumors can occur in patients with primary HER2-positive breast cancer. Our data strongly support the need for biopsies of metastatic lesions to accurately determine patient prognosis and appropriate use of targeted therapy.
INTRODUCTION
Molecular markers help clinicians manage metastatic breast cancer systemically by indicating when endocrine therapy, chemotherapy, or human epidermal growth factor receptor 2 (HER2) –targeted therapy (such as trastuzumab or lapatinib) is appropriate. However, confirmatory biopsies of suspected metastases are not always performed in the routine oncologic care setting.
The HER2/neu gene is amplified in 20% to 25% of primary breast cancer cases, and without biopsies of metastases, clinicians may assume that the HER2 status of the metastatic tumor is the same as that of the primary tumor. However, previous studies1–18 have suggested the possibility of discordant HER2 status between primary and metastatic tumors. Furthermore, some studies13,19–22 have suggested that trastuzumab may convert disease status from HER2 positive in a primary tumor to HER2 negative. Most studies to date, however, have included small groups of HER2-positive patients (Table 1); therefore, the extent of HER2 discordance between primary and metastatic tumor sites has not been conclusively established, and it is unclear whether trastuzumab increases this discordance. Furthermore, to the best of our knowledge, there have been no previous studies evaluating whether clinical factors affect these discordance rates. Because previous studies had small sample sizes, more information is also needed about the possible effects of HER2 discordance on overall survival (OS) durations. It has been suggested that patients with HER2 discordance might have poorer survival durations than patients with concordance between primary and residual22 or metastatic tumor sites.13
Table 1.
Previous Studies of HER2 Status Discordance
| Previous Study | Location | HER2-Positive Primary Tumor |
HER2-Negative Primary Tumor |
||||
|---|---|---|---|---|---|---|---|
| No. of Patients: |
Discordance Rate (%) | No. of Patients: |
Discordance Rate (%) | ||||
| With HER2-Positive Primary Tumors | With HER2-Negative Metastatic Tumors or Primaries After PST | With HER2-Negative Primary Tumors | With HER2-Positive Metastatic Tumors | ||||
| Masood et al1 | Metastatic | 12 | 1 | 8 | 38 | 0 | 0 |
| Shimizu et al2 | Metastatic | 8 | 0 | 0 | 13 | 0 | 0 |
| Simon et al3 | Metastatic | 31 | 2 | 6 | 91 | 2 | 2 |
| Tanner et al4 | Metastatic | 13 | 0 | 0 | 33 | 0 | 0 |
| Vincent-Salomon et al23 | Primary | 13 | 2 | 15 | 29 | 0 | 0 |
| Salomon et al5 | Metastatic | 11 | 2 | 18 | — | — | — |
| Xu et al6 | Metastatic | 15 | 0 | 0 | — | — | — |
| Gancberg et al7 | Metastatic | 34 | 2 | 6 | 66 | 3 | 5 |
| Taucher et al8 | Primary | 21 | 2 | 10 | 64 | 2 | 3 |
| Burstein et al19 | Primary | 23 | 6 | 26 | — | — | — |
| Regitnig et al9 | Metastatic | 4 | 0 | 0 | 27 | 4 | 15 |
| Carlsson et al10 | Metastatic | 23 | 0 | 0 | 24 | 0 | 0 |
| Zidan et al11 | Metastatic | 14 | 1 | 7 | 7 | 0 | 0 |
| Gong et al12 | Primary/Metastatic | 18 | 2 | 12 | 40 | 0 | 0 |
| Pectasides et al13 | Metastatic | 16 | 6 | 38 | — | — | — |
| Hurley et al20 | Primary | 23 | 10 | 43 | — | — | — |
| D'Andrea et al14 | Metastatic | 24 | 3 | 13 | — | — | — |
| Harris et al21 | Primary | 18 | 2 | 11 | — | — | — |
| Mittendorf et al22 | Primary | 25 | 8 | 32 | — | — | — |
| Simmons et al15 | Metastatic | 13 | 0 | 0 | 22 | 2 | 9 |
| Lower et al16 | Metastatic | 140 | 90 | 64 | 242 | 37 | 15 |
| Wilking et al17 | Metastatic | 43 | 8 | 19 | 108 | 7 | 6 |
| Thompson et al18 | Metastatic | 14 | 1 | 7 | 123 | 3 | 2 |
Abbreviations: HER2, human epithelial growth factor receptor 2; PST, preoperative systemic treatment.
In this study, we present the largest data set to date investigating the role of HER2 discordance in breast cancer and evaluating clinical factors that may influence such discordance. We hypothesized that treatment with the HER2-targeted drug trastuzumab or chemotherapy would increase the likelihood that patients with HER2-positive primary tumors would have HER2-negative metastatic tumors. Moreover, we hypothesized that patients with HER2 discordance between their primary and metastatic sites would have shorter OS than that of patients with HER2 concordance. We carried out this study by using records from 182 patients with HER2-positive tumors treated at a single institution.
PATIENTS AND METHODS
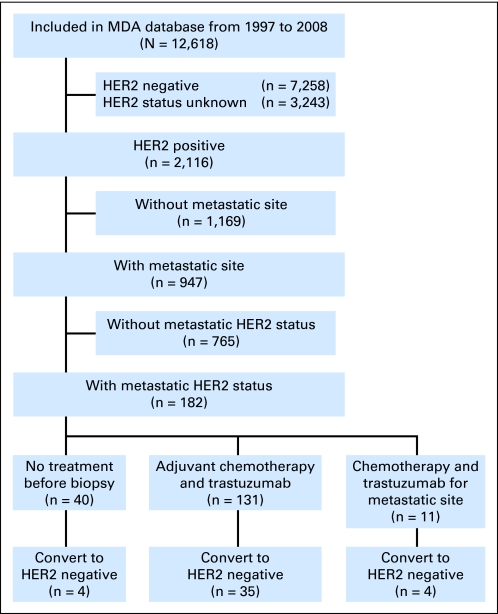
We retrospectively identified all patients from the Breast Medical Oncology database at The University of Texas MD Anderson Cancer Center who, between January 1, 1997, and December 31, 2008, had been diagnosed with primary HER2-positive breast cancer with concurrent or subsequent local or distant metastatic disease and whose primary and metastatic tumors' HER2 expression and/or amplification status was known (Fig 1). We identified 947 patients who were diagnosed with HER2-positive primary breast tumors and had metastases. Of these, 182 patients had known HER2 status in their metastatic tumors. Clinical and histologic characteristics of all patients were entered prospectively into the database on the basis of information obtained from medical records. We distinguished between metastatic tumors found in patients at the time of initial breast cancer diagnosis and metastases found after removal of the primary breast tumor and/or adjuvant systemic treatment. Our study was approved by MD Anderson Cancer Center's institutional review board, which waived the need for written informed consent because of the retrospective nature of the study.
Fig 1.
Study diagram. HER2, human epidermal growth factor receptor 2; MDA, MD Anderson Cancer Center.
Staging and Pathologic Review
The cases of primary breast cancer were staged according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, sixth edition.24 Grading of tumors was done according to the modified Black's nuclear grading system,25 and histologic classification was done according to WHO criteria.26 HER2 status was obtained from patient medical records. We defined HER2 positivity as either the presence of HER2 gene amplification as evident by fluorescent in situ hybridization (FISH) or an immunohistochemical (IHC) analysis score of 3+. Although all new patients with breast cancer at MD Anderson Cancer Center have a central pathologic review by board-certified breast pathologists, no central pathologic re-review was carried out for the purpose of this article. Membranous staining in the IHC assay was scored (0, 1+, 2+, 3+) according to the manufacturer's specifications (Dako, Carpinteria, CA). HER2 status was defined as positive if an IHC assay demonstrated a staining score of 3+ and/or if FISH demonstrated a gene copy ratio of HER2:CEP17 ≥ 2.0. For patients who were originally assessed at other institutions, expression results were obtained as follows: (1) by carrying out de novo staining (if no staining results were available but unstained patient tissue and/or slides were available; three patients), (2) by review of slides stained immunohistochemically by the outside institution at the time of initial presentation by a pathologist as part of clinical routine at MD Anderson Cancer Center (if no unstained slides were available for de novo staining but stained slides were available for review; 46 patients), or (3) by retrieval from the patients' referral documents or communications (if no stained or unstained slides were available; 53 patients). Biopsy sites corresponding to the stained specimens were retrieved from the pathology report. A patient was considered to have estrogen receptor (ER) –positive or progesterone receptor (PR) –positive disease if the tumor had at least 10% nuclear staining. Hormone receptor–positive disease was defined as either ER- or PR-positive disease.
Statistical Methods
Means and standard deviations were calculated for age at diagnosis. Clinical characteristics that could be viewed as categorical variables were analyzed by Pearson's χ2 and Fisher's exact tests to determine their association with HER2 status. A two-sample t test was used to determine the differences in mean ages between patients with concordant HER2 status and those with discordant status. OS duration was defined as the time from the day of metastasis biopsy to death or to last follow-up date if patients were alive. Patients who were alive at the last follow-up were censored in the OS analyses. OS was estimated by the Kaplan-Meier product-limit method. Kaplan-Meier curves were used to represent OS over time for the patients in each group. Cox proportional hazard regression models were used to assess the effect of concordant and discordant HER2 status on OS. The analyses were performed by using SAS 9.1 (SAS Institute, Cary, NC), and plots were generated by using S-PLUS 8.0 (Insightful, Seattle, WA).
RESULTS
Patient and Tumor Characteristics
The clinical characteristics of the 182 patients included in our study are listed in Table 2. Nineteen patients had two or more metastatic samples available for HER2 evaluation. HER2 positivity was diagnosed in the primary tumor by both IHC and FISH amplification in 60 patients, by IHC analysis alone in 72 patients (14 patients had no amplification on FISH, and 58 patients had no FISH results available), and by FISH amplification alone in 50 patients (16 patients had tumors that were not positive on IHC, and 34 patients had no IHC results available). Seventy-six patients received trastuzumab with chemotherapy before their metastases were biopsied.
Table 2.
Patient and Tumor Characteristics (all patients)
| Characteristic | No. |
|---|---|
| No. of patients | 182 |
| No. of metastatic sites | |
| 2 | 19 |
| 3 | 2 |
| No. of tumors | 203 |
| Age at primary diagnosis, years | |
| Median | 48.18 |
| Range | 24-85 |
| ER status at primary diagnosis | |
| Positive | 93 |
| Negative | 87 |
| Unknown | 2 |
| PR status at primary diagnosis | |
| Positive | 65 |
| Negative | 113 |
| Unknown | 4 |
| HER2 status of primary tumor | |
| Positive | 182 |
| IHC 3+, FISH-positive | 60 |
| IHC 3+, FISH-negative | 14 |
| IHC 3+, FISH N/A | 58 |
| IHC-negative, FISH-positive | 16 |
| IHC N/A, FISH-positive | 34 |
| Nuclear grade in primary breast tumor | |
| I | 2 |
| II | 35 |
| III | 137 |
| Unknown | 8 |
| Type of biopsy (metastatic site) | |
| Fine-needle aspiration | 130 |
| Core needle biopsy | 27 |
| Excisional biopsy | 46 |
| Metastatic site of biopsy | |
| Lymph node | |
| N1 or N2 (axillary) | 32 |
| N3 | 29 |
| N4 | 11 |
| Chest wall | 21 |
| Skin | 5 |
| Liver | 30 |
| Bone | 21 |
| Brain | 16 |
| Lung | 15 |
| Pleural effusion | 12 |
| Etc* | 11 |
| Trastuzumab | |
| Before biopsy | 76 |
| None | 106 |
| Timing of metastasis diagnosis | |
| At presentation | 34 |
| At recurrence | 148 |
Abbreviations: ER, estrogen receptor; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; N/A, not available; PR, progesterone receptor.
Etc category includes three pelvic mass, two abdominal mass, one adrenal gland, one subpectoral mass, one endometrial mass, one gallbladder mass, one auricular mass, and one pancreas.
HER2 Discordance by FISH and/or IHC
The breakdown of HER2 status, based on the method of measuring HER2, was as follows. Of the 182 patients with HER2-positive primary tumors, 43 patients (24%) had HER2-negative metastatic tumors. Of 132 primary tumors with IHC values of 3+, 30 patients (23%) also had IHC 3+ in their metastatic sites, and 11 patients (8%) had lower IHC status (2+, four patients; 1+, three patients; 0, four patients). Metastases from 91 patients were not evaluated by IHC analysis. For those 91 patients, HER2 status by FISH was available; 66 (73%) tested HER2 positive, and 25 (27%) tested HER2 negative.
Of 110 patients with FISH-positive primary tumors, 80 patients had FISH-positive metastases, 12 had FISH-negative metastases, and metastases for 18 patients were not evaluated by FISH. For those 18 patients, HER2 status by IHC was available; 15 tested HER2 positive, and three tested HER2 negative.
Of 53 patients for whom HER2 status was not reassessed at our institution, 16 (30%) had discordance between primary and metastatic tumor. Conversely, of 80 patients for whom HER2 status was assessed at our institution, 19 (24%) had discordance. Of 19 patients who had two or more metastatic samples available for HER2 analysis, two patients had HER2 discordance between their metastatic samples.
Concordance and Discordance by Breast Cancer Clinicopathologic Features
We evaluated the HER2 concordance and discordance rates against various clinical factors (Table 3). There were no statistically significant differences in HER2 discordance rates between patients who received or did not receive trastuzumab (P = .296). Of the 76 patients who received trastuzumab before their metastatic tumor biopsies, 15 (20%) had discordant HER2 status. Similarly, of the 106 patients who did not receive trastuzumab before their metastatic tumor biopsies, 28 (26%) had discordant HER2 status.
Table 3.
Concordance Rates by Clinical Factors (all patients)
| Subgroup | HER2 Status |
P | |||
|---|---|---|---|---|---|
| Concordant(n = 139) |
Discordant(n = 43) |
||||
| No. | % | No. | % | ||
| Trastuzumab | |||||
| None | 78 | 74 | 28 | 26 | .296 |
| Before biopsy | 61 | 80 | 15 | 20 | |
| Timing of metastasis diagnosis | |||||
| At presentation | 30 | 88 | 4 | 12 | .077 |
| At recurrence | 109 | 74 | 39 | 26 | |
| Metastatic location | |||||
| Local | 53 | 72 | 21 | 28 | .212 |
| Distant | 86 | 80 | 22 | 20 | |
| Hormone receptor status | |||||
| Positive | 79 | 77 | 23 | 23 | .865 |
| Negative | 58 | 74 | 20 | 26 | |
| Unknown | 2 | ||||
| Chemotherapy with or without trastuzumab | |||||
| None | 36 | 90 | 4 | 10 | .022 |
| Before biopsy | 103 | 73 | 39 | 27 | |
| Time from diagnosis of breast cancer to biopsy, years | |||||
| ≤ 5 | 95 | 78 | 27 | 22 | .498 |
| > 5 | 44 | 73 | 16 | 27 | |
| Years of breast cancer diagnosis | |||||
| 1997-2004 | 102 | 76 | 32 | 24 | .893 |
| 2005-2008 | 37 | 77 | 11 | 23 | |
| (n = 78) | (n = 28) | ||||
| Chemotherapy without trastuzumab* | |||||
| None | 36 | 90 | 4 | 10 | |
| Before biopsy | 42 | 64 | 24 | 36 | |
Abbreviation: HER2, human epidermal growth factor receptor 2.
Among 106 patients who had chemotherapy without trastuzumab, the total number of patients with HER2 status concordance was 78; total with discordance, 28.
In contrast, there were statistically significant differences in discordance rates between those who did and did not receive chemotherapy, whether or not it was given with trastuzumab (P = .022). Of the 142 patients who received chemotherapy (with or without trastuzumab) before their metastatic tumor biopsies, 39 (27%) had discordant HER2 status. Of the 40 patients who did not receive chemotherapy before their metastatic tumor biopsies, four (10%) had discordant HER2 status. Moreover, among patients who did not receive trastuzumab, there were statistically significant differences in HER2 discordance rates between patients who did and did not receive chemotherapy (P = .003).
Those who had a more than a 1-month interval from primary breast cancer to subsequent relapse tended to have higher rates of HER2 discordance than did those with metastasis at presentation. However, there was no statistically significant difference in HER2 discordance rates between patients whose metastases were diagnosed at presentation and those whose metastases were diagnosed at recurrence (P = .077). Additionally, there was no statistically significant difference in HER2 discordance rates between those whose biopsies were of distant versus local metastases (P = .212) or between patients with hormone receptor–positive versus hormone receptor–negative tumors (P = .865).
OS and HER2 Discordance
We next asked whether patients with HER2 discordance had poorer survival. We tested our hypothesis by using data from the 168 patients with distant metastases. We excluded the 14 patients with only locoregional metastasis because they had different clinical stages and prognoses from those with distant metastasis. Patient and tumor characteristics of those with distant metastasis are summarized by HER2 status in Table 4. Patients with discordant HER2 status were significantly older than those without discordant HER2 status (P = .013). The HER2-concordant group included a large percentage of premenopausal women and patients with grade 3 disease (P = .023 and P < .001, respectively).
Table 4.
Characteristics of Patients With Distant Metastases by HER2 Status
| Characteristic | HER2 Status |
P | |||
|---|---|---|---|---|---|
| Concordant(n = 133) |
Discordant(n = 35) |
||||
| No. | % | No. | % | ||
| Age at primary diagnosis, years | |||||
| Mean | 47.04 | 52.26 | .013 | ||
| SD | 11.27 | 9.42 | |||
| Menopausal status | |||||
| Premenopausal | 76 | 57 | 12 | 34 | .023 |
| Postmenopausal | 57 | 43 | 22 | 63 | |
| Unknown | 0 | 0 | 1 | 3 | |
| ER status | |||||
| Positive | 68 | 51 | 18 | 51 | .993 |
| Negative | 64 | 48 | 17 | 49 | |
| Unknown | 1 | 1 | 0 | 0 | |
| PR status | |||||
| Positive | 45 | 34 | 15 | 43 | .368 |
| Negative | 85 | 64 | 20 | 57 | |
| Unknown | 3 | 2 | 0 | 0 | |
| Trastuzumab | |||||
| None | 77 | 58 | 24 | 69 | .251 |
| Before biopsy | 56 | 42 | 11 | 31 | |
| Tumor grade | |||||
| I | 0 | 0 | 2 | 6 | < .001 |
| II | 20 | 15 | 10 | 28 | |
| III | 107 | 80 | 22 | 63 | |
| Unknown | 6 | 5 | 1 | 3 | |
| Distant metastatic site | |||||
| Without visceral | 46 | 35 | 16 | 45 | .225 |
| With visceral | 87 | 65 | 19 | 55 | |
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor; SD, standard deviation.
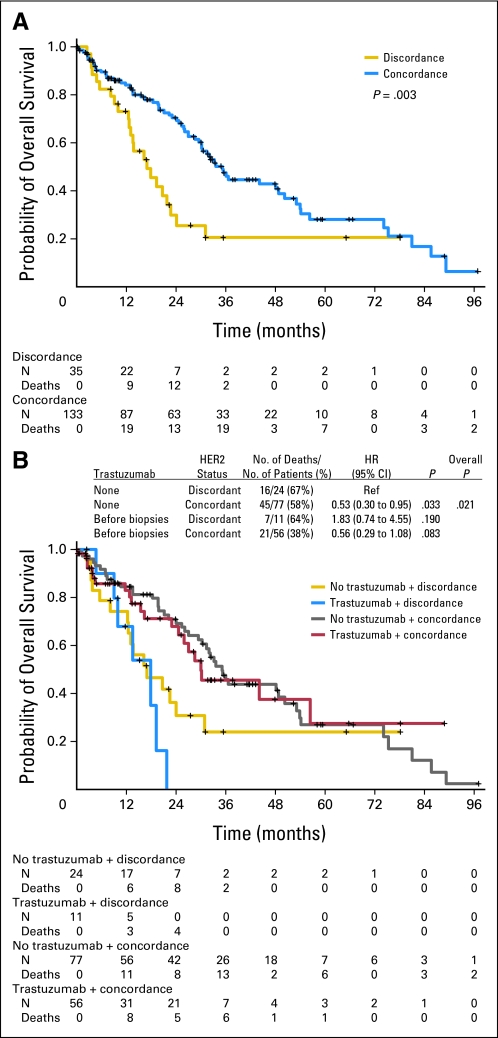
Patients with concordant HER2 status had significantly longer OS than patients with discordant HER2 status (hazard ratio [HR], 0.47; P = .003; Fig 2A), according to univariate analysis. Since age, tumor grade, and menopausal status were significantly associated with HER2 status and might in themselves lead to differences in OS, we controlled for these variables in the multivariate model. The effect of HER2 status remained significant even after adjusting for age, tumor grade (III v II), and menopausal status (post- v premenopausal; HR, 0.36; P < .001; Appendix Table A1, online only). If patients with distant metastases at presentation were excluded (n = 44), patients with concordant HER2 status still had significantly longer OS than patients with discordant HER2 status (HR, 0.39; P < .001).
Fig 2.
(A) Kaplan-Meier overall survival curves by human epidermal growth factor receptor 2 (HER2) status for patients with distant metastases. (B) Kaplan-Meier overall survival curves by combinations of trastuzumab and HER2 status for patients with distant metastases. HR, hazard ratio.
When univariate Cox proportional hazards models were used, concordant HER2 status was significantly associated with longer OS in patients treated with trastuzumab (HR, 0.30; P = .013) and without trastuzumab (HR, 0.53; P = .033) before metastatic tumor biopsy (Fig 2B). This association remained in multivariate Cox proportional hazards models after adjusting for age, tumor grade, and menopausal status (for patients treated with trastuzumab: HR, 0.22; P < .001; for those treated without trastuzumab: HR, 0.40; P = .006; Appendix Table A2, online only). Whether or not the patient received trastuzumab before the metastatic tumor biopsy did not affect OS. In addition, seven of 43 patients who had HER2-negative metastases received HER2-targeted therapy (five with trastuzumab and two with lapatinib) after biopsy, and there was no significant difference in OS on the basis of such therapy (HR, 0.82; P = .719).
DISCUSSION
We found that trastuzumab therapy was not associated with an increase in the loss of HER2 positivity in metastases, whereas chemotherapy was associated with an increase in the loss of such positivity. The overall discordance rate for HER2-positive primary tumors was 24% in our data set. This result indicates that some patients who have not received metastatic tumor biopsies might be receiving unnecessary treatment. Finally, as in our previous study,22 this study supports the finding that patients who have discordance in HER2 status have poor prognosis compared with those who have concordance.
In contrast to our results, several groups3,4,6,8,10 have reported that HER2 status is stable between primary and residual or metastatic tumors, with 82% to 100% concordance when patients did not receive trastuzumab before their residual or metastatic tumor biopsies. However, these studies had small HER2-positive sample sizes.
In our study, 39 (27%) of 142 patients who received chemotherapy (with or without trastuzumab) before their metastatic tumor biopsies had discordant status. This rate was higher than that of patients who did not receive chemotherapy. Another study concluded that HER2 overexpression was unchanged after chemotherapy.8,12,23 Ding et al27 showed that the differential mutation frequencies and structural variation patterns in metastasis and xenograft compared with the primary tumor suggest that secondary tumors may arise from a minority of cells within the primary tumor. However, it is unknown whether chemotherapy can promote clonal selection of HER2/neu-amplified cancers. It is unclear why this effect was found in our study for chemotherapy and not for trastuzumab. It is possible that this increase in the rate of discordance after chemotherapy might be related to the biopsies having sampled HER2-negative subclones.
In the preoperative systemic treatment setting, other investigators13,19–22 have evaluated HER2 expression in paired samples of pretreatment and post-treatment tissues from patients treated with chemotherapy and trastuzumab. On the basis of these previous studies, trastuzumab might be expected to increase the HER2 discordance rate between primary and metastatic tumors, but our data did not support that expectation. In our study, 15 (20%) of 76 patients with HER2-positive primary tumors who received trastuzumab before their metastatic tumor biopsies had HER2-negative metastases compared with 28 (26%) of 106 patients who did not receive trastuzumab. One possible reason for the discrepancy between this and previous studies is that the previous studies compared HER2 status between pretreatment and post-treatment breast tissue, whereas our study compared HER2 status between primary and metastatic tumor tissues. Another possible reason for the discrepancy between studies is that in those studies all patients received both trastuzumab and chemotherapy, but in this study a group of patients did not receive trastuzumab but did receive chemotherapy. The rate of HER2 discordance between primary and metastatic tumor sites might increase after chemotherapy. Whether loss of HER2-positive status in metastasis reflects response to therapy or a mechanism of resistance is unclear. Perceived loss of HER2-positive status in metastasis might reflect heterogeneity of HER2 expression within the tumor.7,28–30
Importantly, our study did show that patients with HER2 discordance had poorer OS than did those with HER2 concordance. Investigators in our group showed in a previous study22 that among patients who had measurable residual disease after they received chemotherapy and trastuzumab in the neoadjuvant setting, those with discordant HER2 status between the initial and residual tumor had a significantly shorter relapse-free survival than patients who had concordant HER2 status. Further, Liedtke et al31 reported that discordance for ER, PR, and HER2 was 18.4%, 40.3%, and 13.6% between primary and metastatic tumor, respectively. In our study, possible reasons for the poor survival outcome of patients with discordance in HER2 status might be that they had not received trastuzumab treatment, that they acquired resistance to trastuzumab and chemotherapy, or that they had altered metabolism of chemotherapy. Whether treatment with trastuzumab should continue if the metastatic lesion tests negatively for HER2 has not been established. In clinical practice, we might withhold a targeted drug if the drug target is not expressed in the metastatic lesion. By discontinuing the drug in such a case, we would avoid unnecessary treatment. However, in the event of a false-negative result for target expression in the metastasis, discontinuing the drug could be eliminating a necessary treatment. A prospective clinical trial to determine the best strategy is needed.
Because this study was retrospective, there is the possibility of selection bias, and this study has a major limitation concerning tumor sampling and testing. Our results are consistent with a lack of reliability in staining methods. HER2 status was taken from pathologic reports of IHC and/or FISH analyses that had been performed by several different pathologists. Discordance results could have been caused by sampling error in focally HER2-positive cancers or by limited accuracy or reproducibility of assays.32The concordance or discordance between HER2 positivity as detected by IHC versus FISH analyses has been shown to be statistically significant.33–35 Previous studies have suggested that variability in IHC testing, including variable types of fixation, different antigen retrieval methods, and subjectivities in observer analysis, may affect results.36,37 In addition, FISH has been shown to be more reproducible than IHC between central and peripheral laboratories.34,38 Therefore, changes in HER2 status may result either from true biologic variation or from inconsistent measurement. It is possible that changes in HER2 expression may occur at different time points in the disease. In this study, among patients who had FISH results available for both the primary and metastatic tumors, the discordance rate was 12% (11 of 91).
In summary, we confirmed that loss of HER2-positive status in metastatic tumors can occur in patients with primary HER2-positive breast cancer who received chemotherapy with or without trastuzumab. Patients with HER2 discordance between their primary and metastatic tumors have shorter OS. However, for our data, inaccurate testing may have caused discordance between primary and metastatic sites. A large prospective study of concordance and/or discordance of HER2 status in metastatic tumors will be needed to confirm our observations by a central reassessment of HER2 status of both the primary and the metastatic lesions by using FISH for all cases, thus minimizing the risk of interassay variability of the results. Our data strongly support the need for biopsies of metastatic lesions in primary HER2-positive breast cancer to accurately determine patient prognosis and appropriate use of targeted therapy.
Appendix
Table A1.
Multivariate Analyses of Overall Survival by HER2 Status
| Variable | HR | 95% CI | P |
|---|---|---|---|
| HER2 status | |||
| Discordant | |||
| Concordant | 0.36 | 0.21 to 0.62 | < .001 |
| Age, years | 0.98 | 0.95 to 1.01 | .128 |
| Tumor grade | |||
| I/II | |||
| III | 1.47 | 0.80 to 2.71 | .219 |
| Menopausal status | |||
| Premenopausal | |||
| Postmenopausal | 1.12 | 0.60 to 2.12 | .178 |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hazard ratio.
Table A2.
Multivariate Analyses of Overall Survival by HER2 Status in Subgroups
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Trastuzumab before biopsy | |||
| HER2 status | |||
| Discordant | |||
| Concordant | 0.22 | 0.07 to 0.63 | < .001 |
| Age | 0.97 | 0.91 to 1.03 | .327 |
| Tumor grade | |||
| I or II | |||
| III | 1.66 | 0.54 to 5.18 | .379 |
| Menopausal status | |||
| Premenopausal | |||
| Postmenopausal | 1.06 | 0.34 to 3.35 | .919 |
| No trastuzumab | |||
| HER2 status | |||
| Discordant | |||
| Concordant | 0.40 | 0.21 to 0.77 | .006 |
| Age | 0.98 | 0.94 to 1.01 | .175 |
| Tumor grade | |||
| I or II | |||
| III | 1.42 | 0.69 to 2.90 | .344 |
| Menopausal status | |||
| Premenopausal | |||
| Postmenopausal | 1.18 | 0.55 to 2.56 | .674 |
Abbreviation: HER2, human epidermal growth factor receptor 2.
Footnotes
See accompanying editorial on page 575 and article on page 587; listen to the podcast by Dr Davidson at www.jco.org/podcasts
Supported in part by Grant No. CA016672 from the National Institutes of Health through MD Anderson Cancer Center support, and by the Nellie B. Connally Breast Cancer Research Fund.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Gabriel N. Hortobagyi, Genentech (C), Merck (C), sanofi-aventis (C), Novartis (C) Stock Ownership: None Honoraria: None Research Funding: Gabriel N. Hortobagyi, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Naoki Niikura, Ana M. Gonzalez-Angulo, Naoto T. Ueno
Financial support: Ana M. Gonzalez-Angulo, Naoto T. Ueno
Administrative support: Gabriel N. Hortobagyi, Naoto T. Ueno
Provision of study materials or patients: Naoki Niikura, Ana M. Gonzalez-Angulo, Gabriel N. Hortobagyi, Naoto T. Ueno
Collection and assembly of data: Naoki Niikura, Ana M. Gonzalez-Angulo, Naoto T. Ueno
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Masood S, Bui MM. Assessment of Her-2/neu overexpression in primary breast cancers and their metastatic lesions: An immunohistochemical study. Ann Clin Lab Sci. 2000;30:259–265. [PubMed] [Google Scholar]
- 2.Shimizu C, Fukutomi T, Tsuda H, et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J Surg Oncol. 2000;73:17–20. doi: 10.1002/(sici)1096-9098(200001)73:1<17::aid-jso5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Simon R, Nocito A, Hübscher T, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93:1141–1146. doi: 10.1093/jnci/93.15.1141. [DOI] [PubMed] [Google Scholar]
- 4.Tanner M, Järvinen P, Isola J. Amplification of HER-2/neu and topoisomerase IIalpha in primary and metastatic breast cancer. Cancer Res. 2001;61:5345–5348. [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Xu R, Perle MA, Inghirami G, et al. Amplification of Her-2/neu gene in Her-2/neu-overexpressing and -nonexpressing breast carcinomas and their synchronous benign, premalignant, and metastatic lesions detected by FISH in archival material. Mod Pathol. 2002;15:116–124. doi: 10.1038/modpathol.3880503. [DOI] [PubMed] [Google Scholar]
- 7.Gancberg D, Di Leo A, Cardoso F, et al. Comparison of HER-2 status between primary breast cancer and corresponding distant metastatic sites. Ann Oncol. 2002;13:1036–1043. doi: 10.1093/annonc/mdf252. [DOI] [PubMed] [Google Scholar]
- 8.Taucher S, Rudas M, Mader RM, et al. Influence of neoadjuvant therapy with epirubicin and docetaxel on the expression of HER2/neu in patients with breast cancer. Breast Cancer Res Treat. 2003;82:207–213. doi: 10.1023/B:BREA.0000004378.15859.51. [DOI] [PubMed] [Google Scholar]
- 9.Regitnig P, Schippinger W, Lindbauer M, et al. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J Pathol. 2004;203:918–926. doi: 10.1002/path.1592. [DOI] [PubMed] [Google Scholar]
- 10.Carlsson J, Nordgren H, Sjöström J, et al. HER2 expression in breast cancer primary tumours and corresponding metastases: Original data and literature review. Br J Cancer. 2004;90:2344–2348. doi: 10.1038/sj.bjc.6601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zidan J, Dashkovsky I, Stayerman C, et al. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br J Cancer. 2005;93:552–556. doi: 10.1038/sj.bjc.6602738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Y, Booser DJ, Sneige N. Comparison of HER-2 status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer. 2005;103:1763–1769. doi: 10.1002/cncr.20987. [DOI] [PubMed] [Google Scholar]
- 13.Pectasides D, Gaglia A, Arapantoni-Dadioti P, et al. HER-2/neu status of primary breast cancer and corresponding metastatic sites in patients with advanced breast cancer treated with trastuzumab-based therapy. Anticancer Res. 2006;26:647–653. [PubMed] [Google Scholar]
- 14.D'Andrea MR, Limiti MR, Bari M, et al. Correlation between genetic and biological aspects in primary non-metastatic breast cancers and corresponding synchronous axillary lymph node metastasis. Breast Cancer Res Treat. 2007;101:279–284. doi: 10.1007/s10549-006-9300-2. [DOI] [PubMed] [Google Scholar]
- 15.Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20:1499–1504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lower EE, Glass E, Blau R, et al. HER-2/neu expression in primary and metastatic breast cancer. Breast Cancer Res Treat. 2008;113:301–306. doi: 10.1007/s10549-008-9931-6. [DOI] [PubMed] [Google Scholar]
- 17.Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: Discordances during tumor progression. Breast Cancer Res Treat. 2010;125:553–561. doi: 10.1007/s10549-010-1029-2. [DOI] [PubMed] [Google Scholar]
- 18.Thompson AM, Jordan LB, Quinlan P, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: The Breast Recurrence In Tissues Study (BRITS) Breast Cancer Res. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: A pilot study. J Clin Oncol. 2003;21:46–53. doi: 10.1200/JCO.2003.03.124. [DOI] [PubMed] [Google Scholar]
- 20.Hurley J, Doliny P, Reis I, et al. Docetaxel, cisplatin, and trastuzumab as primary systemic therapy for human epidermal growth factor receptor 2-positive locally advanced breast cancer. J Clin Oncol. 2006;24:1831–1838. doi: 10.1200/JCO.2005.02.8886. [DOI] [PubMed] [Google Scholar]
- 21.Harris LN, You F, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–1207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- 22.Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–7388. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincent-Salomon A, Jouve M, Genin P, et al. HER2 status in patients with breast carcinoma is not modified selectively by preoperative chemotherapy and is stable during the metastatic process. Cancer. 2002;94:2169–2173. doi: 10.1002/cncr.10456. [DOI] [PubMed] [Google Scholar]
- 24.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: Revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 25.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105:97–102. [PubMed] [Google Scholar]
- 26.The World Health Organization: Histological typing of breast tumors. Neoplasma. 1983;30:113–123. [No authors listed] [PubMed] [Google Scholar]
- 27.Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuukasjärvi T, Karhu R, Tanner M, et al. Genetic heterogeneity and clonal evolution underlying development of asynchronous metastasis in human breast cancer. Cancer Res. 1997;57:1597–1604. [PubMed] [Google Scholar]
- 29.Teixeira MR, Pandis N, Bardi G, et al. Clonal heterogeneity in breast cancer: Karyotypic comparisons of multiple intra- and extra-tumorous samples from 3 patients. Int J Cancer. 1995;63:63–68. doi: 10.1002/ijc.2910630113. [DOI] [PubMed] [Google Scholar]
- 30.Campbell LL, Polyak K. Breast tumor heterogeneity: Cancer stem cells or clonal evolution? Cell Cycle. 2007;6:2332–2338. doi: 10.4161/cc.6.19.4914. [DOI] [PubMed] [Google Scholar]
- 31.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pusztai L, Viale G, Kelly CM, et al. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist. 2010;15:1164–1168. doi: 10.1634/theoncologist.2010-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dybdal N, Leiberman G, Anderson S, et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res Treat. 2005;93:3–11. doi: 10.1007/s10549-004-6275-8. [DOI] [PubMed] [Google Scholar]
- 34.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 35.Press MF, Sauter G, Bernstein L, et al. Diagnostic evaluation of HER-2 as a molecular target: An assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res. 2005;11:6598–6607. doi: 10.1158/1078-0432.CCR-05-0636. [DOI] [PubMed] [Google Scholar]
- 36.Sauter G, Lee J, Bartlett JM, et al. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27:1323–1333. doi: 10.1200/JCO.2007.14.8197. [DOI] [PubMed] [Google Scholar]
- 37.Hanley KZ, Birdsong GG, Cohen C, et al. Immunohistochemical detection of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression in breast carcinomas: Comparison on cell block, needle-core, and tissue block preparations. Cancer. 2009;117:279–288. doi: 10.1002/cncy.20034. [DOI] [PubMed] [Google Scholar]
- 38.Paik S, Bryant J, Tan-Chiu E, et al. Real-world performance of HER2 testing: National Surgical Adjuvant Breast and Bowel Project experience. J Natl Cancer Inst. 2002;94:852–854. doi: 10.1093/jnci/94.11.852. [DOI] [PubMed] [Google Scholar]