Abstract
Purpose
Persistent androgen signaling is implicated in castrate-resistant prostate cancer (CRPC) progression. This study aimed to evaluate androgen signaling in bone marrow–infiltrating cancer and testosterone in blood and bone marrow and to correlate with clinical observations.
Patients and Methods
This was an open-label, observational study of 57 patients with bone-metastatic CRPC who underwent transiliac bone marrow biopsy between October 2007 and March 2010. Patients received oral abiraterone acetate (1 g) once daily and prednisone (5 mg) twice daily. Androgen receptor (AR) and CYP17 expression were assessed by immunohistochemistry, testosterone concentration by mass spectrometry, AR copy number by polymerase chain reaction, and TMPRSS2-ERG status by fluorescent in situ hybridization in available tissues.
Results
Median overall survival was 555 days (95% CI, 440 to 965+ days). Maximal prostate-specific antigen decline ≥ 50% occurred in 28 (50%) of 56 patients. Homogeneous, intense nuclear expression of AR, combined with ≥ 10% CYP17 tumor expression, was correlated with longer time to treatment discontinuation (> 4 months) in 25 patients with tumor-infiltrated bone marrow samples. Pretreatment CYP17 tumor expression ≥ 10% was correlated with increased bone marrow aspirate testosterone. Blood and bone marrow aspirate testosterone concentrations declined to less than picograms-per-milliliter levels and remained suppressed at progression.
Conclusion
The observed pretreatment androgen-signaling signature is consistent with persistent androgen signaling in CRPC bone metastases. This is the first evidence that abiraterone acetate achieves sustained suppression of testosterone in both blood and bone marrow aspirate to less than picograms-per-milliliter levels. Potential admixture of blood with bone marrow aspirate limits our ability to determine the origin of measured testosterone.
INTRODUCTION
Recent clinical findings link persistent androgen signaling to castrate-resistant prostate cancer (CRPC) progression in some patients, leading to reevaluation of the widely held view that progression after castration is androgen independent.1 Geller2 framed this hypothesis more than 2 decades ago; more recently, Mohler et al3 and Titus et al4 provided findings that support the hypothesis that persistent androgen signaling is implicated in resistance to castration. Clinical findings of significant benefit, with occasional striking regression of CRPC and recently confirmed overall survival (OS) benefit after CYP17 lyase inhibition or more specific androgen receptor (AR) blockade, establish the therapeutic relevance of Geller's hypothesis.5–12 These findings have fostered interest in understanding the potential benefit of targeting AR signaling in the castrate-resistant state.
We performed a translational study treating patients with bone-metastatic CRPC with abiraterone acetate. We demonstrate that testosterone is depleted in blood and bone marrow aspirate to less than picograms-per-milliliter (pg/mL) levels after treatment and propose a candidate predictive signature based on findings in bone marrow–infiltrating prostate cancer cells.
PATIENTS AND METHODS
This open-label, single-center study was designed to evaluate androgen signaling in bone marrow–infiltrating cancer and testosterone in blood and bone marrow aspirate and to correlate findings with clinical observations. Secondary objectives included assessing treatment efficacy and safety and exploring the association between levels of circulating androgens and bone marrow aspirate androgens before and during treatment.
Patients had histologically confirmed adenocarcinoma of the prostate and castrate-resistant bone-metastatic disease progression. Disease progression was defined as documented osseous or soft-tissue metastatic progression or prostate-specific antigen (PSA) progression according to Prostate Cancer Clinical Trials Working Group II criteria (PCWG II).13 Patients were required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤ 2, a serum testosterone level ≤ 50 ng/dL (sustained by medical or surgical castration), normal serum potassium levels, and adequate adrenal, renal, hepatic, and bone marrow function (Appendix, online only). All patients provided written informed consent, and the study protocol was approved by the MD Anderson Cancer Center institutional review board.
Treatment and Evaluation
Patients were treated with abiraterone acetate 1 g daily as four 250-mg tablets at least 1 hour before or 2 hours after a meal and prednisone 5-mg tablets twice daily. Screening and baseline evaluations included complete medical history, physical examination, complete blood count, serum electrolytes and chemistry, PSA levels, testosterone concentration, radionuclide bone scan, and tumor imaging (chest x-ray and pelvic and abdominal computed tomography scans). Safety assessments, using National Cancer Institute Common Terminology Criteria for Adverse Events version 3, were completed every 4 weeks, along with physical examination and select serum chemistry, electrolyte, PSA, and testosterone evaluations. Bone marrow biopsy and aspirate (∼5 mL) were performed before treatment, at week 8, and at end of study. End-of-study biopsy was performed on treatment discontinuation. Matching blood plasma and serum were collected within 2 hours of biopsy.
Imaging studies were performed at the time of suspected prostate cancer progression or at the treating physician's discretion. Therapy was discontinued at the treating physician's discretion in patients exhibiting clinical progression, which was defined as worsening of preexisting, or development of new, disease-related symptoms.
Assay Methodologies
Tissue and derivatives banking and immunohistochemistry.
The bone marrow specimens were obtained by transiliac biopsy, and samples were processed according to standard MD Anderson Cancer Center decalcification and fixation procedures. After pathologic evaluation, samples were stored in the MD Anderson Cancer Center Prostate Cancer Tissue Bank along with plasma from matching aspirate. Immunohistochemistry (IHC) was performed on 3.5-mm formalin-fixed, paraffin-embedded bone marrow biopsy sections for AR (dilution, 1:50; Dako, Carpinteria, CA) and CYP17 (dilution, 1:450; Novus, Littleton, CO). The AR antibody was a standard diagnostic antibody validated for clinical use, and we used accepted quality control measures. The CYP17 antibody IHC was validated by Western blots combined with IHC and was performed on appropriate positive controls (ie, ovarian lysates) and multitissue controls. A Dako autostainer and standard 3,3-diaminobenzidine were used, as previously described.14 Marker expression was assessed by scoring two fields containing at least 100 tumor cells per specimen and expressed as a percentage. The involvement of cells exhibiting detectable staining was scored as 0 (no staining), 1 (up to 25%), 2 (25% to 75%), or 3 (> 75%). The intensity of staining was scored as zero (no staining), low, or high. Subcellular distribution of biomarker expression was also recorded (membranous, cytosolic, nuclear, or combination).
AR copy numbers.
Details of AR copy number methods are shown in the Appendix (online only).
TMPRSS2-ERG gene rearrangement status.
Details of TMPRSS2-ERG gene rearrangement status assessment are shown in the Appendix (online only).
Mass spectrometry.
Liquid chromatography-tandem mass spectrometry analysis of androgens was performed, as previously described (Appendix, online only).4
Statistical Considerations
The enrollment of approximately 60 patients would provide a data set of 30 paired bone marrow aspirates for evaluation of primary end points, with a 50% anticipated success rate for obtaining adequate bone marrow biopsies and aspirates. A sample size of 30 paired bone marrow aspirates (baseline and week 8) would provide 82% power to detect an effect of at least 0.55 in bone marrow testosterone levels using a two-sided paired t test at a .05 significance level. OS and time to treatment discontinuation from date of treatment initiation were estimated using the Kaplan-Meier method. Correlations between serum and bone marrow testosterone by mass spectrometry were assessed using Pearson's and Spearman's methods. The Wilcoxon signed-rank test was used to assess treatment duration between samples with and without CYP17 expression and AR overexpression and bone marrow testosterone levels between samples with and without CYP17 expression.
RESULTS
Clinical Outcomes
Table 1 summarizes the demographic, clinical, and tumor characteristics of 57 patients enrolled onto the study from October 2007 through March 2010. Of the patients, 57 were evaluable for safety and 56 for clinical response; 27 patients had tumor-infiltrated bone marrow at pretreatment (baseline), and 30 patients had tumor infiltration at any one time point. Median age was 70 years, and eight patients were older than 80 years. All patients had bone-metastatic CRPC. Most had received prior chemotherapy and several lines of hormonal manipulation (Table 1). Twenty patients (35%) had lymph node metastases, and eight patients (14%) had visceral metastases. Most patients had disease-related symptoms at study entry, and the median ECOG PS was 2 (range, 0 to 2).
Table 1.
Patient (N = 57) and Tumor Characteristics
| Characteristic | No. | % |
|---|---|---|
| Patients | ||
| No. of evaluable patients, October 2007 to March 2010 | 56 | |
| Race | ||
| White | 52 | 91 |
| Black | 3 | 5 |
| Hispanic | 2 | 4 |
| Age, years | ||
| Median | 70 | |
| Range | 48-87 | |
| Performance status | ||
| Median | 2 | |
| Range | 0-2 | |
| Prior cytotoxic therapies | ||
| Chemotherapy | 48 | 84 |
| ≥ 2 regimens | 34 | 60 |
| Docetaxel-based regimens | 45 | 79 |
| Radiopharmaceuticals | 7 | 12 |
| Salvage hormonal therapies | ||
| Ketoconazole | 18 | 33 |
| Estrogens and/or ketoconazole | 36 | 65 |
| Prior experimental treatments/novel agents | 21 | 38 |
| Time to CRPC from castration initiation, months | ||
| Median | 26 | |
| Range | 3-132 | |
| Tumors | ||
| PSA, ng/dL | ||
| Median | 95.3 | |
| Range | 3-2,725 | |
| Extent of bone metastases | ||
| < 10 | 10 | 16 |
| ≥ 10 | 47 | 82 |
| > 20 | 39 | 68 |
| Lymph nodes | 20 | 35 |
| Local recurrence | 4 | 7 |
| Visceral metastases | 8 | 14 |
| Liver | 5 | 9 |
| Lung | 2 | 4 |
| Adrenal | 1 | 2 |
| Bone marrow involvement detected by transiliac biopsy | 30 | 53 |
| At baseline | 27 | 47 |
| At week 8 | 18 | 32 |
Abbreviations: CRPC, castrate-resistant prostate cancer; PSA, prostate-specific antigen.
To date, 49 of 57 patients have discontinued treatment, 30 with evidence of clinical progression, which was paired with imaging progression in 15 of 30 patients and with PSA progression (PCWG II) in six of 30 patients. Thirteen patients did not have clinical progression but discontinued treatment as a result of imaging and/or PSA progression (PCWG II). In addition, six patients discontinued treatment—two as a result of financial hardship, two per treating physician preference, one because of bowel obstruction attributed to radiation enteritis unrelated to study drug or disease, and one because of an ischemic cerebrovascular event unrelated to study drug or disease.
Patients received abiraterone acetate and prednisone treatment for a median of 233 days (7.6 months; range, 28 to 945+ days; 95% CI, 196 to 400 days; Fig 1). Therapy was well tolerated, with most adverse events categorized as grade 1/2 (National Cancer Institute Common Terminology Criteria for Adverse Events) and a safety profile consistent with other reported and anticipated effects of CYP17 inhibition with abiraterone acetate and systemic corticosteroids.5–12 Seven grade 3 events were reported that were possibly related to study drug combination; these included elevated liver function tests (n = 2), hypokalemia (n = 1), hypertension (n = 1), and steroid-related hyperglycemia (n = 3).
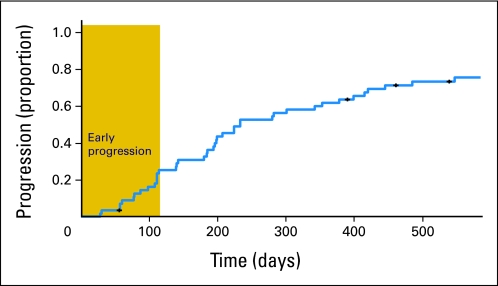
Fig 1.
Kaplan-Meier plot of proportion of patients (y-axis) discontinuing treatment over time (x-axis). Figure illustrates two different types of disease progression occurring either soon after initiation (gold box) or after prolonged treatment.
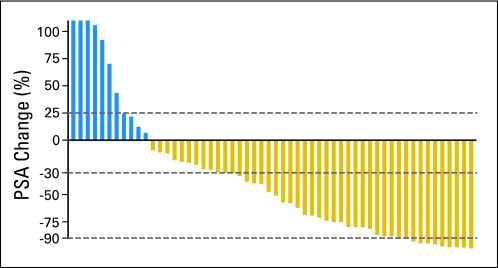
A maximal PSA decline of more than 50% occurred in 28 (50%) of 56 evaluable patients, and nine patients (16%) had a more than 90% decline. PSA decline ≥ 30% occurred in 34 (59%) of 56 patients (Fig 2).
Fig 2.
Waterfall plot representing the percentage changes in serum prostate-specific antigen (PSA) concentrations. Maximal PSA reduction is represented on the right. Dashed lines represent 25%, −30%, and −90% PSA change.
Bone scans were performed in 36 patients after 6 months of treatment. Imaging improvement was recorded in three patients, stable disease in 26 patients, and more than two new lesions in seven patients. Of five patients with hepatic metastases, two were reevaluated by imaging; one was found to have achieved partial response, and one was found to have stable disease. One of three patients with lung metastases had a partial response. Among 10 patients with lymph node involvement reevaluated by imaging, five patients had evidence of a partial response, two had stable disease, and three had progressive disease.
Although the rate of progression seemed to be somewhat steady, with gradual decline over time through 200 days, we observed evidence of two different patterns of clinical outcome with abiraterone acetate: (1) patients who experienced no apparent effect on clinical disease course, and (2) the remainder of patients, who did experience effects (Fig 1). In patients following the first pattern, we observed no symptomatic improvement and no objective evidence of tumor regression; we considered these patients to exhibit primary resistance to treatment (14 [25%] of 56 patients). The remaining patients experienced longer periods of treatment and varied clinical response. ECOG PS improvement was self-reported in 25 (66%) of 38 initially symptomatic patients with delayed progression, whereas there was no patient-reported improvement in 12 symptomatic primary-resistant patients. Of note, the two initially asymptomatic patients in this group became rapidly symptomatic while on treatment. The two groups of patients did not differ significantly with regard to prior treatment or extent of disease at presentation.
Median OS for the entire cohort was 555 days (18.2 months; 95% CI, 440 to 965+ days; range, 49 to 1,037+ days).
Tissue and Aspirate Collection
Pretreatment bone marrow biopsies revealed tumor involvement in 27 (47%) of 56 patients, 25 (44%) of whom had ≥ 5% tumor infiltration evaluable for analysis (Table 1). Eleven of these were patients who discontinued treatment within 4 months of initiation. Bone marrow aspirates were collected pretreatment in 49 (88%) of 56 patients, at week 8 in 44 (84%) of 56 patients, and on treatment discontinuation in 27 (55%) of 49 patients. Six patients did not yield bone marrow aspirates for study at any time point (dry tap). Bone marrow aspirates from two or more time points were collected in 40 (71%) of 56 patients. Blood plasma and serum samples were collected pretreatment in 52 patients (93%), at week 8 in 49 patients (88%), and on treatment discontinuation in 43 (88%) of 49 patients.
Bone Marrow Aspirate and Blood Testosterone and Dihydrotestosterone Concentrations
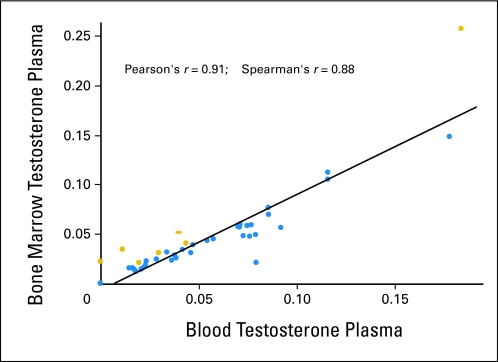
Bone marrow aspirate and blood plasma testosterone and dihydrotestosterone (DHT) measurements by mass spectrometry confirmed metabolite depletion at 8 weeks of treatment and at end of study (on treatment discontinuation) (Table 2; see also Appendix Fig A1, online only). Additional random measurements of circulating testosterone levels at different time points before discontinuation confirmed testosterone depletion (41 samples, 15 patients with prolonged treatment). In the subset of patients with both pretreatment plasma and bone marrow aspirate study specimens available for analysis, a strong correlation (Pearson's r = 0.91) between pretreatment circulating and microenvironment bone marrow aspirate testosterone levels was found by mass spectrometry (Appendix Fig A1). Bone marrow aspirate plasma testosterone concentration was higher than circulating blood plasma testosterone in seven of 42 available cases with matching evaluable samples. Pretreatment bone marrow aspirate DHT was undetectable in the bone marrow aspirate in 46 of 48 measured samples. In 52 samples, mean blood DHT was 0.0116 ng/mL, with a range of 0.0000 to 0.0459 ng/mL. It was undetectable in 27 of 52 plasma samples. No correlation was seen between blood and bone marrow DHT, but this could be a result of the undetectable levels of DHT (< pg/mL). DHT was not detected in any bone marrow aspirate after treatment, whereas it remained detectable in only two of 49 blood 8-week treatment samples; of note, testosterone was undetectable in those samples. Bone marrow aspirate and blood DHT were undetectable on treatment discontinuation (Table 2).
Table 2.
Blood and Bone Marrow Aspirate Plasma Testosterone Concentrations Assessed by Mass Spectrometry
| Androgen | Baseline |
8 Weeks |
End of Study* |
|||
|---|---|---|---|---|---|---|
| Range (ng/mL) | No. | Range (ng/mL) | No. | Range (ng/mL) | No. | |
| Testosterone | ||||||
| Blood plasma | 0.0000-0.214 | 52 | 0.0000†-0.0129 | 49 | 0.0000 | 32 |
| Bone marrow aspirate plasma | 0.0000-0.257 | 49 | 0.0000 | 44 | 0.0000 | 27 |
| Dihydrotestosterone | ||||||
| Blood plasma | 0.0000-0.0459 | 52 | 0.0000-0.0203‡ | 49 | 0.0000 | 32 |
| Bone marrow aspirate plasma | 0.0000-0.257§ | 49 | 0.0000 | 44 | 0.0000 | 27 |
End-of-study samples coinciding with week 8 not included.
Only one patient had detectable testosterone.
Two patients had detectable week 8 dihydrotestosterone.
Two patients had detectable pretreatment bone marrow aspirate dihydrotestosterone.
Molecular Characterization of Bone Marrow Metastases
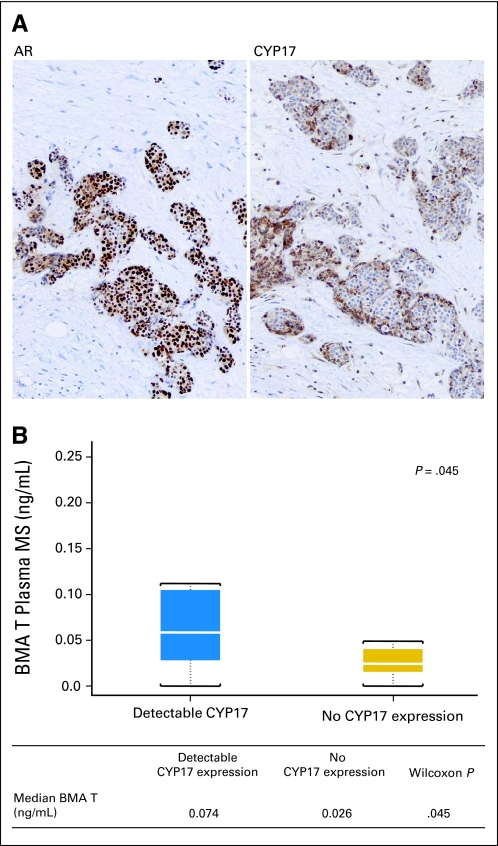
Nuclear AR expression varied in involvement and intensity within and among samples in pretreatment bone marrow biopsies (Table 3). AR expression ranged from 2+ to 3 (50% to 100% involvement) and was of moderate to high intensity. Cytoplasmic CYP17 expression in tumor cells was heterogeneous in involvement and intensity and ranged from 0 to 2+. Homogeneous, intense expression of nuclear AR (involvement ≥ 3, high intensity) in combination with 1+ (≥ 10%) CYP17 tumor expression was correlated with longer time to treatment discontinuation (> 4 months) in the 25 available samples (P < .001, Wilcoxon signed-rank test; Fig 3A, Table 3). Pretreatment CYP17 tumor expression was correlated with increased bone marrow aspirate plasma testosterone concentration (P = .045) in 19 evaluable paired samples (Fig 3B).
Table 3.
Pretreatment Nuclear AR and Cytoplasmic CYP17 Expression and Correlation of Increased Nuclear AR Expression and Cytoplasmic CYP17 Expression With Treatment Duration
| Pretreatment Marker Expression | 0 (No Expression) | 1 (1%-25%) | 2 (26%-75%) | 3 (> 75%) |
|---|---|---|---|---|
| Nuclear AR, No. | 0 | 0 | 8 | 17 |
| Cytoplasmic CYP17, No. | 4 | 5 | 8 | 8 |
| Outcome | Primary Resistance (treatment ≤ 4 months) | Longer Treatment Duration (treatment > 4 months) | P (Wilcoxon signed-rank test) | |
| Pretreatment nuclear AR expression 3+ and CYP17 expression in tumor epithelium | ||||
| No. | 1 | 12 | < .001 | |
| % | 7 | 82 | ||
| Lack of one or both | ||||
| No. | 10 | 2 | ||
| % | 91 | 18 | ||
Abbreviation: AR, androgen receptor.
Fig 3.
(A) Pretreatment intense nuclear androgen receptor (AR) expression in combination with CYP17 expression in the bone marrow–infiltrating tumor of a patient with treatment duration more than 4 months. (B) Pretreatment CYP17 expression in tumor is correlated with increased bone marrow aspirate (BMA) plasma testosterone (T) concentration in 19 cases with paired samples. MS, mass spectrometry.
TMPRSS2-ERG gene rearrangement status and AR copy number were evaluable in 15 of 25 patients with ≥ 20% tumor involvement. TMPRSS2-ERG rearrangement was identified in three of 15 evaluable samples. Pretreatment AR copy numbers were low. Six patients had evaluable serial specimens for analyses. Four patients with longer treatment duration displayed AR copy number increase during treatment, whereas no change was seen in two patients who discontinued therapy because of progression after 8 weeks of treatment.
DISCUSSION
Prostate cancer progression that invariably follows the initial benefit from androgen ablation has, until recently, been considered androgen independent. In addition, the residual concentration of androgens observed after androgen ablation was considered inconsequential, and progression of CRPC was attributed to androgen-independent mechanisms by most investigators. However, recent results support the view that persistent androgen signaling in the castrate-resistant state is functionally significant and a validated therapeutic target in CRPC.2,11,12,15,16 Similarly, the efficacy of this androgen biosynthesis inhibitor recently approved by the US Food and Drug Administration establishes the importance of persistent androgen signaling in CRPC progression.
A ≥ 50% maximal PSA decline occurred in 28 (50%) of 56 patients in the study population despite the extensive cancer involvement in patients selected for this study. The favorable safety profile observed was similar to that reported by others and recently confirmed in a phase III study.5–12 Some patients experienced fatigue and weakness, but we could not distinguish these from the adverse effects of progressive prostate cancer.
For the purpose of this study, patients were divided into two categories defined by duration of therapy: those who had undergone ≤ 4 months of therapy and the remainder. The 14 patients (25%) who were treated for ≤ 4 months continued to have worsening of cancer-related symptoms, with no findings to suggest benefit from abiraterone acetate and prednisone treatment, and were considered to have exhibited primary resistance to abiraterone acetate. In contrast, patients who were treated with therapy of longer duration in general experienced improvement in symptoms and had findings consistent with antitumor activity. The present results will not determine the point in progression when resistance to abiraterone acetate emerges. Understanding the relationship between the emergence of resistance and progression will guide further development of therapy.17
This study establishes the feasibility and value of sampling bone marrow biopsies in selected patients to gain insight into the mechanism of resistance to molecularly targeted therapies in human prostate cancer, following the initial bone biopsy experiment reported by Taplin et al.18 Accordingly, uniform and intense tumor nuclear AR expression, coupled with cytoplasmic CYP17 expression, were linked to lack of primary resistance to abiraterone acetate. We also observed a correlation between pretreatment bone marrow aspirate testosterone levels and tumor CYP17 expression.
These associations are consistent with the hypothesis that patients with CRPC demonstrate persistent AR signaling in the tumor microenvironment and are more likely to benefit from therapy with androgen biosynthesis inhibitors. The correlation of pretreatment testosterone levels in blood and bone marrow to CYP17 expression supports the hypothesis that CYP17 expression is functionally meaningful. We have preclinical and clinical tissue-based data in support of increased tumor CYP17 expression after standard androgen ablation strategies as further indirect support of enzyme functionality (data not shown).
As previously reported, abiraterone acetate effectively depletes testosterone in the blood of patients with CRPC.5 We have extended these observations, and to our knowledge, we are the first to demonstrate that abiraterone acetate depletes blood and bone marrow aspirate testosterone and DHT concentrations to less than pg/mL levels and that this depletion is sustained at treatment discontinuation. The potential for blood admixture with bone marrow aspirate limits the ability to determine the site of production of the measured testosterone in bone marrow aspirate. The progression observed in the presence of a depleted testosterone environment leads us to propose that native ligand-independent mechanisms are likely to drive progression during treatment with androgen biosynthesis inhibitors. Our results do not exclude the possibility that altered steroid biosynthesis in the tumor microenvironment accounts for progression in a testosterone-depleted environment. The predominant enzyme that irreversibly converts testosterone to DHT, steroid 5α-reductase type 2, exhibits decreased gene and protein expression in prostate cancer cells during progression of prostate cancer to advanced prostate cancer.4,19,20 Investigators have speculated, though, that a feedback mechanism, such as increased “backdoor” biosynthesis of DHT,21 may act as an alternative ligand-dependent mechanism of resistance to abiraterone acetate; the present DHT results do not support this hypothesis. A shortcoming of our approach was the inability to assess cellular androgen levels given the limited tissue collected from bone. Although the present results establish the feasibility of sampling bone marrow biopsies, critical concerns remain. The potential mixture of blood with bone marrow supernatant does not permit us to conclude that the bone marrow supernatant–detected testosterone is of tumor microenvironment origin. The low level of bone marrow aspirate DHT compared with circulating DHT in 25 of 52 patients, which is consistent with lower levels of metabolite anticipated in metastatic sites, and the correlation of CYP17 expression with bone marrow aspirate testosterone concentration suggests that microenvironment production contributes to measured testosterone in bone marrow aspirate. Although these data are supportive of therapeutically relevant production of steroid hormones, further refinement of techniques to account for blood admixture will be required to provide more definitive conclusions. The study afforded us the opportunity to explore the feasibility of assessing AR copy number and TMPRSS2-ERG in bone marrow biopsy specimens; however, too few specimens were available to assign significance to the observations.
Our findings support the view that persistent androgen signaling is implicated in the progression of CRPC and can be targeted therapeutically. Despite the small sample size, the present data imply a role for AR signaling in disease progression. The observations we report form the basis for the development of a predictive signature that may be used to select patients for treatment with abiraterone acetate.
Acknowledgment
We thank Mark English and Ken Youngren of PAREXEL for editorial assistance.
Appendix
Patient Exclusion and Inclusion Criteria
A minimum of 4 weeks was required after discontinuation of any prostate cancer hormonal therapy, with the exception of luteinizing hormone–releasing hormone agonists, systemic corticosteroids, chemotherapy, or radiation therapy. Up to two different prior chemotherapy regimens were allowed, with prior taxane-based combinations tabulated as one. Continuation of a bisphosphonate regimen during study was allowed if initiated more than 4 weeks before treatment initiation. Patients with known brain metastases or any serious intercurrent medical condition potentially compromising study participation were ineligible.
Assessment of Androgen Receptor Copy Number
Paraffin-embedded bone marrow biopsy slides were deparaffinized and subjected to ultraviolet/laser capture microdissection (ArcturusXT, Applied Biosystems, Carlsbad, CA) to isolate pure populations of prostate cancer cells. Captured cells were put in 10 μL of formalin-fixed, paraffin-embedded (FFPE) lysis solution and digested with proteinase K (REPLI-g FFPE kit, QIAGEN, Valencia, CA). Extracted DNA was subjected to whole-genome amplification (REPLI-g FFPE kit). DNA products were introduced to real-time polymerase chain reactions (RT-PCR) to detect androgen receptor (AR) gene copy levels using the TaqMan copy number assay (Applied Biosystems). Samples with normal copy number served as controls. Each sample was represented by four technical replicates. Each PCR included a set of primers and a FAM dye–labeled probe specific for AR and a set of primers and a VIC dye–labeled probe for the reference gene RNaseP. The reactions were carried out in a 7500 RT-PCR thermal cycler (Applied Biosystems) that recorded reaction progression and the cycle when plateau was reached (Ct) for each gene and sample. AR copy number was estimated using the 2*2(-ΔΔCt) method as previously described, where ΔCt = [Ct of AR] − [Ct of RNaseP] and ΔΔCt = [ΔCt of the tumor sample] − [ΔCt of the control] (Nguyen DL, et al: Clin Chim Acta 403:207-211, 2009).
TMPRSS2-ERG Gene Rearrangement Status
Prostate tumors containing the TMPRSS2-ERG gene fusion represent an aggressive subtype of prostate cancer with a high susceptibility to evolve into androgen-independent metastatic disease (Yu J, et al: Cancer Cell 17:443-454, 2010; Mehra R, et al: Cancer Res 68:3584-3590, 2008). As a result, the TMPRSS2-ERG gene fusion was evaluated by fluorescence in situ hybridization using break-apart probes of the ERG gene. The probes, consisting of a rhodamine-labeled 5′-ERG probe (BAC RP11-95I21) and a fluorescein isothiocyanate-labeled 3′-ERG probe (BAC RP11-476D17), were obtained from the Children's Hospital of Oakland Research Institute (Oakland, CA). Tissue pretreatment, hybridization, and washing were performed using the Paraffin Pretreatment Reagent Kit I and hybridization reagents (Vysis, Des Plaines, IL), per manufacturer protocols. In cells with no ERG rearrangement, two pairs of colocalized green and red signals were present. In cells with ERG rearrangement, only one pair of colocalized green and red signals was maintained; the other pair either broke into one green signal and one red signal or lost the red signal. A mean of 100 cells was evaluated per tumor focus. A cutoff level of 10% was set for the fluorescence in situ hybridization analysis (ie, a positive signal required at least 10% of the evaluated nuclei showing rearrangement of the ERG gene).
Mass Spectrometry Assessment of Androgens
Blood or bone marrow aspirate plasma specimens were spiked with deuterated 4-androsten-17β-ol-3-one-16,16,17-d3 (T-d3) (CDN Isotopes, Quebec, Canada), extracted, evaporated under vacuum, concentrated using solid-phase columns, and reconstituted in 60% methanol. Testosterone was quantified using a Shimadzu HPLC system (Columbia, MD) coupled to an Applied Biosystems–AB SCIEX QTRAP 5500 mass spectrometer (Foster City, CA). The positive androgen parent-product ion pairs monitored (mass to charge ratio, m/z) were 289.4 to 97.0 for testosterone and 292.4 to 97.0 for the T-d3 internal standard. Pooled human female plasma (Bioreclamation, Hicksville, NY) was used for all our calibration and quality control samples. These plasma samples were spiked with known amounts of normal and/or deuterated testosterone internal standard and then processed exactly as the clinical samples. They were then placed randomly throughout the clinical sample set. The percentages of relative standard deviation (RSD) and analytic recovery in the quality control and calibration curves gives the statistical data for the clinical samples. In this study, a %RSD less than 12% (lowest, 1.67%; highest, 11.8%) was agreed as being acceptable. The US Food and Drug Administration requires that all mass spectrometry assays have %RSD less than 15% for each analyte, or the assay must be discontinued and redesigned. Overall, for the low testosterone concentrations, the variability observed in our quality control and calibration samples demonstrates that our assay is robust, precise, and reproducible. Serum testosterone concentration was performed using standard diagnostic radioimmunoassay in the MD Anderson Special Chemistry Laboratory and was not reported as a result of its low sensitivity.
Fig A1.
Correlation between circulating and bone marrow plasma testosterone by mass spectrometry. A strong correlation was found between circulating and bone marrow testosterone measured by mass spectrometry. Gold dots represent seven (17%) of 42 cases with higher bone marrow than circulating testosterone.
Footnotes
See accompanying article on page 644; listen to the podcast by Dr Taplin at www.jco.org/podcasts
Supported in part by Ortho Biotech Oncology Research & Development (Unit of Cougar Biotechnology), Los Angeles, CA; Prostate Cancer Foundation Young Investigator Award (E.E.), US Department of Defense Prostate Cancer Clinical Trials Consortium Grant No. W81-XWH-09-1-0148 (E.E.) and US Department of Defense Grant No. PC094304 (M.T.); and National Cancer Institute Cancer Center Support Grant No. 5P30 CA16672-35 (E.E.). Funding of editorial assistance was provided by Janssen Global Services.
Presented in part at the American Society of Clinical Oncology 2009 Genitourinary Cancers Symposium, February 26-28, 2009, Orlando, FL; the 44th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2008, Chicago, IL; and the 46th Annual Meeting of the American Society of Clinical Oncology, June 4-8, 2010, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00544440.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Arturo Molina, Cougar Biotechnology/Ortho Biotech (C); Nicole Chieffo, Cougar Biotechnology/Ortho Biotech (C) Consultant or Advisory Role: Eleni Efstathiou, Janssen-Cilag/Johnson & Johnson (C); Christopher J. Logothetis, Johnson & Johnson (U) Stock Ownership: Arturo Molina, Cougar Biotechnology; Nicole Chieffo, Cougar Biotechnology/Ortho Biotech Honoraria: Eleni Efstathiou, Janssen-Cilag/Johnson & Johnson Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eleni Efstathiou, Christopher J. Logothetis
Administrative support: Arturo Molina, Nicole Chieffo, Lisa A. Smith
Collection and assembly of data: Eleni Efstathiou, Mark Titus, Dimitra Tsavachidou, Vassiliki Tzelepi, Anh Hoang, Nicole Chieffo, Lisa A. Smith, Maria Karlou, Patricia Troncoso, Christopher J. Logothetis
Data analysis and interpretation: Eleni Efstathiou, Sijin Wen, Arturo Molina, Christopher J. Logothetis
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–179. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]
- 2.Geller J. Prolonging survival in metastatic prostate cancer: The case for adrenal androgens—Overview and summary of therapeutic controversies in prostatic cancer. J Clin Endocrinol Metab. 1995;80:1074–1078. doi: 10.1210/jcem.80.4.7714070. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Gregory CW, Ford OH, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 4.Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 5.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 6.Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attard G, Reid AH, Olmos D, et al. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69:4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efstathiou E, Troncoso P, Wen S, et al. Initial modulation of the tumor microenvironment accounts for thalidomide activity in prostate cancer. Clin Cancer Res. 2007;13:1224–1231. doi: 10.1158/1078-0432.CCR-06-1938. [DOI] [PubMed] [Google Scholar]
- 15.Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 16.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 17.Efstathiou E, Logothetis CJ. A new therapy paradigm founded on clinical observation. Clin Cancer Res. 2010;16:1100–1107. doi: 10.1158/1078-0432.CCR-09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 19.Lou YR, Nazarova N, Talonpoika R, et al. 5alpha-dihydrotestosterone inhibits 1alpha,25-dihydroxyvitamin D3-induced expression of CYP24 in human prostate cancer cells. Prostate. 2005;63:222–230. doi: 10.1002/pros.20189. [DOI] [PubMed] [Google Scholar]
- 20.Thomas LN, Douglas RC, Lazier CB, et al. Type 1 and type 2 5alpha-reductase expression in the development and progression of prostate cancer. Eur Urol. 2008;53:244–252. doi: 10.1016/j.eururo.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 21.Penning TM. New frontiers in biosynthesis and metabolism. Curr Opin Endocrinol Diabetes Obes. 2010;17:233–239. doi: 10.1097/MED.0b013e3283381a31. [DOI] [PMC free article] [PubMed] [Google Scholar]