Abstract
Purpose
Use of robotics in oncologic surgery is increasing; however, reports of safety and efficacy are from highly experienced surgeons and centers. We performed a population-based analysis to compare laparoscopic hysterectomy and robotic hysterectomy for endometrial cancer.
Patients and Methods
The Perspective database was used to identify women who underwent a minimally invasive hysterectomy for endometrial cancer from 2008 to 2010. Morbidity, mortality, and cost were evaluated using multivariable logistic and linear regression models.
Results
We identified 2,464 women, including 1,027 (41.7%) who underwent laparoscopic hysterectomy and 1,437 (58.3%) who underwent robotic hysterectomy. Women treated at larger hospitals, nonteaching hospitals, and centers outside of the northeast were more likely to undergo a robotic hysterectomy procedure, whereas black women, those without insurance, and women in rural areas were less likely to undergo a robotic hysterectomy procedure (P < .05 for all). The overall complication rate was 9.8% for laparoscopic hysterectomy versus 8.1% for robotic hysterectomy (P = .13). The adjusted odds ratio (OR) for any morbidity for robotic hysterectomy was 0.76 (95% CI, 0.56 to 1.03). After adjusting for patient, surgeon, and hospital characteristics, there were no significant differences in the rates of intraoperative complications (OR, 0.68; 95% CI, 0.42 to 1.08), surgical site complications (OR, 1.49; 95% CI, 0.81 to 2.73), medical complications (OR, 0.64; 95% CI, 0.40 to 1.01), or prolonged hospitalization (OR, 0.85; 95% CI, 0.64 to 1.14) between the procedures. The mean cost for robotic hysterectomy was $10,618 versus $8,996 for laparoscopic hysterectomy (P < .001). In a multivariable model, robotic hysterectomy was significantly more costly ($1,291; 95% CI, $985 to $1,597).
Conclusion
Despite claims of decreased complications with robotic hysterectomy, we found similar morbidity but increased cost compared with laparoscopic hysterectomy. Comparative long-term efficacy data are needed to justify its widespread use.
INTRODUCTION
Hysterectomy is the standard of care for endometrial cancer. The procedure is traditionally performed through a laparotomy and has been associated with substantial perioperative morbidity. In the 1990s, laparoscopic hysterectomy for endometrial cancer was introduced. Compared with open hysterectomy, the laparoscopic procedure is associated with lower morbidity and shorter hospital stays and has become the preferred treatment option for many surgeons.1
In the last decade, robotic surgery has emerged as an alternative minimally invasive surgical strategy for a number of cancers. Although initially used for radical prostatectomy, robotically assisted surgery has now been adopted for a wide range of procedures including hysterectomy.2 Robotic assistance affords many advantages including three-dimensional visualization, increased freedom of instrument movement, and enhanced ergonomics and surgeon comfort.2,3
Despite the potential benefits of robotic hysterectomy, studies comparing it with laparoscopic hysterectomy have been small in size, nonrandomized, and limited to highly experienced surgeons and centers.4–11 In one of the largest studies to date that included 103 robotic hysterectomies, median blood loss and operative times were lower for robotic compared with laparoscopic hysterectomy.5 Although these studies are informative and demonstrate the feasibility of the procedure, its safety and efficacy in the community may be far different.
The use of robotic surgery is increasing.2 Although a variety of factors influence the uptake of new technologies, marketing often plays a significant role.12,13 Previous work has shown that many new surgical technologies are adopted when only minimal data are available.12,14–16 This is problematic not only because these technologies may not improve clinical outcomes, but also because they are frequently associated with increased cost.3,17,18 The goal of our analysis was to compare the perioperative morbidity, resource utilization, and cost of laparoscopic and robotic hysterectomy in a large cohort of women with endometrial cancer treated throughout the United States.
PATIENTS AND METHODS
Data Source
The Perspective database (Premier, Charlotte, NC) was used. Perspective is a voluntary, fee-supported database originally developed to measure resource utilization and quality of care (Appendix, online only). Perspective samples more than 500 acute care hospitals throughout the United States that contribute data on inpatient admissions.19 In addition to demographics, disease characteristics, and procedures, the database collects information on all billed services. The Perspective database is validated and has been used in a number of outcomes studies.20,21 In 2006, Perspective recorded approximately 5.5 million hospital discharges, which represent approximately 15% of nationwide hospitalizations.19,21
Cohort Selection and Surgical Procedures
Our analysis included women who underwent a minimally invasive hysterectomy for endometrial cancer (International Classification of Diseases, Ninth Revision [ICD-9] codes 182.0 to 182.8) between October 2008 and March 2010. Patients were stratified into the following two groups based on the type of hysterectomy performed: laparoscopic (ICD-9 code 68.41 or 68.51) or robotic (ICD-9 code 17.42 or 17.44). Women who underwent either a laparoscopically assisted vaginal hysterectomy (ICD-9 code 68.51) or a total laparoscopic hysterectomy (ICD-9 code 68.51) were included. Patients who had ICD-9 codes for both a robotically assisted and a laparoscopic procedure were included in the robotic hysterectomy cohort. Performance of lymphadenectomy was noted for each patient and defined through identification of any ICD-9 code for nodal sampling.
Clinical and Demographic Characteristics
Demographic data analyzed included age (< v ≥ 60 years of age), race (white, black, or other), marital status (married, single, or unknown), and insurance status (Medicare, Medicaid, commercial, self-pay, or unknown). The hospitals in which patients were treated were characterized based on location (urban or rural), region of the country (northeast, midwest, west, or south), size (< 400, 400 to 600, and > 600 beds), and teaching status (teaching or nonteaching). Risk adjustment for comorbid conditions was performed using the Charlson comorbidity index.22 The ICD-9 coding to define the Charlson index as reported by Deyo et al23 was used.
Procedure Volume
For each surgeon and hospital, we determined the total number of laparoscopic and robotic hysterectomies performed during the study period. Because not all physicians and hospitals contributed data for the entire study period, we calculated annualized procedure volumes. The annualized procedure volume was estimated by dividing the total number of patients who underwent a procedure by the number of years a given surgeon or hospital contributed at least one procedure. The volumes were then divided to create three approximately equal tertiles of surgeon and hospital volume (low, intermediate, and high).24,25 Separate volume estimates were determined for laparoscopic and robotic procedures.
Outcomes
The primary outcome of the study was perioperative morbidity. Secondary outcomes included individual complications, rates of transfusion and reoperation, mortality, and resource utilization. Perioperative morbidity was classified into the following categories: intraoperative complications (bladder injury, ureteral injury, intestinal injury, vascular injury, and other operative injury), surgical site complications (wound complications, abscess, hemorrhage, and bowel obstruction), and medical complications (venous thromboembolism, myocardial infarction, cardiopulmonary arrest, acute renal failure, respiratory failure, cerebrovascular accident, bacteremia/sepsis, shock, and pneumonia). A composite score of overall morbidity was determined based on the occurrence of any one of the complications. For each cohort, we calculated the rates of transfusion and reoperation. Rates of readmission were calculated by determining the number of patients who were readmitted to the same facility within 60 days of the initial surgery for any of the complications previously described.
We also examined a number of process measures and utilization metrics. Patients who required more than 2 days of inpatient care after the procedure were considered to have a prolonged hospitalization. The Perspective database includes an itemized, data-stamped log of all items that are billed to a patient, including drugs, laboratory and radiologic tests, and therapeutic services. Within the Perspectives database, approximately three quarters of hospitals submit direct cost data taken from internal accounting systems. The remaining institutions provide estimates based on Medicare cost to charge ratios.19,21,26 Cost data from the Perspective database have been used in a number of outcomes studies.19,21,26 Cost data from the index admission were examined. The discharge status of each patient was noted. Women who were transferred from an acute care hospital to a skilled nursing facility, nursing home, or an acute or subacute rehabilitation center were considered to have a nonroutine discharge. Readmission for any of the morbidities described earlier was also examined. Perioperative mortality was defined as death during the primary hospitalization.
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests, whereas continuous variables were compared with one-way analysis of variance. The association between the outcomes of interest and the type of procedure performed was assessed using multivariable logistic regression models that included patient, surgeon, and hospital characteristics. Results are reported with odds ratios (ORs) and 95% CIs. Cost estimates more than three standard deviations from the mean were removed, and the remaining cost data were assessed using linear regression models including the variables described earlier. The analysis of the secondary end points was exploratory, and the 95% CIs for these estimates are not adjusted for multiplicity. All analyses were performed with STATA version 11.0 (STATA, College Station, TX) and SAS version 9.2 (SAS Institute, Cary, NC). All statistical tests were two-sided.
RESULTS
A total of 2,464 women who underwent minimally invasive hysterectomy for endometrial cancer were identified. The cohort included 1,027 patients (41.7%) who had a laparoscopic hysterectomy and 1,437 patients (58.3%) who underwent a robotic procedure. The clinical and demographic characteristics of the cohort are listed in Table 1.
Table 1.
Demographics and Clinical Characteristics of the Cohort Stratified by Type of Hysterectomy Performed
| Demographic or Clinical Characteristic | Laparoscopic Hysterectomy |
Robotic Hysterectomy |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| All patients | 1,027 | 41.7 | 1,437 | 58.3 | |
| Age at surgery, years | .12 | ||||
| < 60 | 459 | 44.7 | 597 | 41.6 | |
| ≥ 60 | 568 | 55.3 | 840 | 58.5 | |
| Race | < .001 | ||||
| White | 725 | 70.6 | 1,117 | 77.7 | |
| Black | 77 | 7.5 | 65 | 4.5 | |
| Other | 225 | 21.9 | 255 | 17.8 | |
| Year of diagnosis | .17 | ||||
| 2008 | 152 | 14.8 | 175 | 12.2 | |
| 2009 | 688 | 67.0 | 992 | 69.0 | |
| 2010 | 187 | 18.2 | 270 | 18.8 | |
| Marital status | .60 | ||||
| Married | 548 | 53.4 | 739 | 51.4 | |
| Single | 236 | 23.0 | 351 | 24.4 | |
| Unknown | 243 | 23.7 | 347 | 24.2 | |
| Insurance status | .01 | ||||
| Medicare | 396 | 38.6 | 542 | 37.7 | |
| Commercial | 529 | 51.5 | 765 | 53.2 | |
| Medicaid | 41 | 4.0 | 43 | 3.0 | |
| Self-pay | 38 | 3.7 | 31 | 2.2 | |
| Unknown | 23 | 2.2 | 56 | 3.9 | |
| Hospital location | .003 | ||||
| Urban | 972 | 94.6 | 1,394 | 97.0 | |
| Rural | 55 | 5.4 | 43 | 3.0 | |
| Hospital type | .49 | ||||
| Nonteaching | 403 | 39.2 | 584 | 40.6 | |
| Teaching | 624 | 60.8 | 853 | 59.4 | |
| Hospital size, No. of beds | < .001 | ||||
| < 400 | 297 | 28.9 | 292 | 20.3 | |
| 400-600 | 471 | 45.9 | 548 | 38.2 | |
| > 600 | 259 | 25.2 | 597 | 41.6 | |
| Hospital region | < .001 | ||||
| Midwest | 264 | 25.7 | 377 | 26.2 | |
| Northeast | 251 | 24.4 | 184 | 12.8 | |
| South | 354 | 34.5 | 647 | 45.0 | |
| West | 158 | 15.4 | 229 | 15.9 | |
| Charlson comorbidity index | .37 | ||||
| 1 | 593 | 57.7 | 822 | 57.2 | |
| 2 | 280 | 27.3 | 422 | 29.4 | |
| ≥ 3 | 154 | 15.0 | 193 | 13.4 | |
| Lymphadenectomy | < .001 | ||||
| No | 454 | 44.2 | 330 | 23.0 | |
| Yes | 573 | 55.8 | 1,107 | 77.0 | |
| Surgeon volume* | .57 | ||||
| Low | 373 | 36.3 | 523 | 36.4 | |
| Intermediate | 312 | 30.4 | 461 | 32.1 | |
| High | 342 | 33.3 | 453 | 31.5 | |
| Hospital volume† | .74 | ||||
| Low | 348 | 33.9 | 494 | 34.4 | |
| Intermediate | 353 | 34.4 | 473 | 32.9 | |
| High | 326 | 31.7 | 470 | 32.7 | |
Surgeon volume for laparoscopic hysterectomy is categorized as follows: low, ≤ 3 procedures per year; intermediate, 3.01 to 9.3 procedures per year; and high, > 9.3 procedures per year. Surgeon volume for robotic hysterectomy is categorized as follows: low, < 9 procedures per year; intermediate, 9.01 to 14 procedures per year; and high, > 14 procedures per year.
Hospital volume for laparoscopic hysterectomy is categorized as follows: low, ≤ 4 procedures per year; intermediate, 4.01 to 11.67 procedures per year; and high, > 11.67 procedures per year. Hospital volume for robotic hysterectomy is categorized as follows: low, ≤ 3.67 procedures per year; intermediate, 3.68 to 10 procedures per year; and high, > 10 procedures per year.
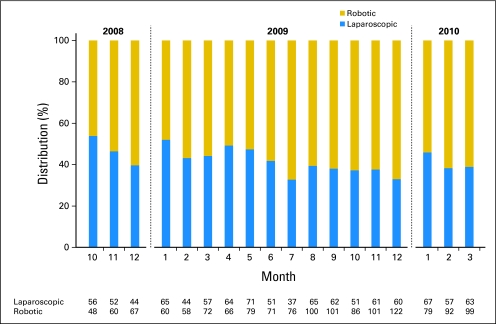
Use of robotic surgery increased with time (Fig 1). In October 2008, 46.2% of the minimally invasive hysterectomies for endometrial cancer were robotic. This increased to 58.2% in June 2009 and to 61.1% in March 2010. In a multivariable model, single women (OR, 1.27; 95% CI, 1.02 to 1.58), women treated at large hospitals (OR, 2.89; 95% CI, 2.21 to 3.78), women treated at nonteaching hospitals (OR, 1.28; 95% CI, 1.04 to 1.59), and women operated on at centers outside the northeast were more likely to undergo a robotic procedure (Table 2). Black women (OR, 0.46; 95% CI, 0.32 to 0.67), women without insurance (OR, 0.56; 95% CI, 0.34 to 0.94), and women residing in rural areas (OR, 0.50; 95% CI, 0.32 to 0.94) were less likely to undergo robotic hysterectomy.
Fig 1.
Distribution of minimally invasive laparoscopic hysterectomies and robotic hysterectomies performed from October 2008 to March 2010.
Table 2.
Multivariable Model of Factors Associated With Performance of Robotic Hysterectomy
| Factor | Robotic Hysterectomy |
|
|---|---|---|
| Odds Ratio | 95% CI | |
| Age at surgery, years | ||
| < 60 | Referent | |
| ≥ 60 | 1.21 | 0.98 to 1.50 |
| Race | ||
| White | Referent | |
| Black | 0.46* | 0.32 to 0.67 |
| Other | 0.61* | 0.49 to 0.76 |
| Year of diagnosis | ||
| 2008 | Referent | |
| 2009 | 1.24 | 0.97 to 1.59 |
| 2010 | 1.34 | 0.99 to 1.80 |
| Marital status | ||
| Married | Referent | |
| Single | 1.27* | 1.02 to 1.58 |
| Unknown | 1.07 | 0.86 to 1.33 |
| Insurance status | ||
| Commercial | Referent | |
| Medicare | 0.83 | 0.66 to 1.04 |
| Medicaid | 0.72 | 0.45 to 1.15 |
| Self-pay | 0.56* | 0.34 to 0.94 |
| Unknown | 1.07 | 0.63 to 1.82 |
| Hospital location | ||
| Urban | Referent | |
| Rural | 0.50* | 0.34 to 0.94 |
| Hospital type | ||
| Teaching | Referent | |
| Nonteaching | 1.28* | 1.04 to 1.59 |
| Hospital size, No. of beds | ||
| < 400 | Referent | |
| 400-600 | 1.40* | 1.10 to 1.79 |
| > 600 | 2.89* | 2.21 to 3.78 |
| Hospital region | ||
| Northeast | Referent | |
| Midwest | 1.92* | 01.49 to 2.49 |
| South | 2.41* | 1.88 to 3.09 |
| West | 2.50* | 1.85 to 3.38 |
| Charlson comorbidity index | ||
| 1 | Referent | |
| 2 | 1.16 | 0.96 to 1.41 |
| ≥ 3 | 0.96 | 0.75 to 1.24 |
P < .05.
Rates of intraoperative complications (4.0% for laparoscopic v 3.0% for robotic; P = .18) and surgical site complications (1.8% for laparoscopic v 2.9% for robotic; P = .08) were similar for the two procedures, whereas medical complications (4.9% for laparoscopic v 2.9% for robotic; P = .01) were more common among women who underwent laparoscopic hysterectomy (Table 3). Prolonged length of stay (> 2 days) was noted in 11.4% of women who underwent laparoscopy compared with 9.9% of women who had a robotic procedure (P = .23). Although reoperation was more common in patients who underwent laparoscopy versus robotic hysterectomy (0.8% v 0.2%, respectively; P = .04), there were no differences in transfusion requirements, readmission, or rates of conversion to open laparotomy (P > .05 for all).
Table 3.
Perioperative Morbidity, Mortality, and Resource Usage of Laparoscopic and Robotic Hysterectomy
| Perioperative Morbidity, Mortality, and Resource Usage | Laparoscopic Hysterectomy |
Robotic Hysterectomy |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| All patients | 1,027 | 41.7 | 1,437 | 58.3 | |
| Any complication | 101 | 9.8 | 116 | 8.1 | .13 |
| Intraoperative complications | 41 | 4.0 | 43 | 3.0 | .18 |
| Bladder injury | 10 | 1.0 | 5 | 0.3 | .05 |
| Ureteral injury | 7 | 0.7 | 6 | 0.4 | .37 |
| Intestinal injury | 5 | 0.5 | 8 | 0.6 | .81 |
| Vascular injury | 1 | 0.1 | 2 | 0.1 | .77 |
| Other operative injury | 32 | 3.1 | 31 | 2.2 | .14 |
| Surgical site complications | 18 | 1.8 | 41 | 2.9 | .08 |
| Wound complication | 15 | 1.5 | 25 | 1.7 | .59 |
| Abscess | 3 | 0.3 | 2 | 0.1 | .41 |
| Hemorrhage | 0 | — | 0 | — | |
| Bowel obstruction | 10 | 1.0 | 29 | 2.0 | .41 |
| Medical complications | 50 | 4.9 | 42 | 2.9 | .01 |
| Venous thromboembolism | 4 | 0.4 | 11 | 0.8 | .24 |
| Myocardial infarction | 0 | — | 0 | — | |
| Cardiopulmonary arrest | 1 | 0.1 | 0 | — | .24 |
| Respiratory failure | 33 | 3.2 | 31 | 2.2 | .10 |
| Renal failure | 12 | 1.2 | 8 | 0.6 | .10 |
| Stroke | 2 | 0.2 | 0 | — | .09 |
| Bacteremia/sepsis | 3 | 0.3 | 2 | 0.1 | .41 |
| Shock | 7 | 0.7 | 5 | 0.3 | .24 |
| Pneumonia | 3 | 0.3 | 2 | 0.1 | .41 |
| Resource usage | |||||
| Subcutaneous emphysema | 2 | 0.2 | 2 | 0.1 | .74 |
| Transfusion | 33 | 3.2 | 31 | 2.2 | .10 |
| Reoperation | 8 | 0.8 | 3 | 0.2 | .04 |
| Length of stay > 2 days | 117 | 11.4 | 142 | 9.9 | .23 |
| Readmission | 0 | — | 2 | 0.1 | |
| Mean hospital cost, $ | 8,996 | 10,618 | < .001 | ||
| Nonroutine discharge | 20 | 1.9 | 22 | 1.5 | .62 |
| Death | 2 | 0.2 | 2 | 0.1 | .74 |
After adjusting for patient, surgeon, and hospital characteristics, there were no statistically significant differences in the rates of intraoperative complications (OR, 0.68; 95% CI, 0.42 to 1.08), surgical site complications (OR, 1.49; 95% CI, 0.81 to 2.73), medical complications (OR, 0.64; 95% CI, 0.40 to 1.01), or prolonged hospitalization (OR, 0.85; 95% CI, 0.64 to 1.14) between the cohorts (Table 4). The overall complication rate was 9.8% in women who underwent laparoscopic hysterectomy compared with 8.1% for women who underwent a robotic procedure (P = .13). The adjusted OR for any morbidity for robotic compared with laparoscopic hysterectomy was 0.76 (95% CI, 0.56 to 1.03). Perioperative mortality was noted in 0.2% of laparoscopic and 0.1% of robotic surgeries (P = .74).
Table 4.
Multivariable Analysis of Factors Associated With Morbidity for Women Undergoing Minimally Invasive Hysterectomy for Endometrial Cancer
| Factor | Intraoperative Complications |
Surgical Site Complications |
Medical Complications |
Prolonged Hospitalization |
Any Morbidity |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Surgery | ||||||||||
| Laparoscopic | Referent | Referent | Referent | Referent | Referent | |||||
| Robotic | 0.68 | 0.42 to 1.08 | 1.49 | 0.81 to 2.73 | 0.64 | 0.40 to 1.01 | 0.85 | 0.64 to 1.14 | 0.76 | 0.56 to 1.03 |
| Age at surgery, years | ||||||||||
| < 60 | Referent | Referent | Referent | Referent | Referent | |||||
| ≥ 60 | 0.73 | 0.41 to 1.29 | 1.07 | 0.53 to 2.16 | 1.12 | 0.62 to 2.01 | 0.82 | 0.56 to 1.18 | 1.03 | 0.72 to 1.50 |
| Race | ||||||||||
| White | Referent | Referent | Referent | Referent | Referent | |||||
| Black | 0.34 | 0.08 to 1.45 | 2.84* | 1.21 to 6.70 | 2.19* | 1.06 to 4.55 | 1.19 | 0.70 to 2.05 | 1.36 | 0.79 to 2.36 |
| Other | 0.50 | 0.26 to 0.98 | 1.32 | 0.65 to 2.70 | 1.85* | 1.05 to 3.29 | 1.27 | 0.89 to 1.81 | 0.90 | 0.60 to 1.35 |
| Year of diagnosis | ||||||||||
| 2008 | Referent | Referent | Referent | Referent | Referent | |||||
| 2009 | 1.23 | 0.61 to 2.45 | 1.04 | 0.46 to 2.39 | 0.81 | 0.43 to 1.55 | 0.88 | 0.59 to 1.31 | 0.96 | 0.62 to 1.49 |
| 2010 | 1.14 | 0.52 to 2.50 | 0.72 | 0.28 to 1.82 | 0.80 | 0.38 to 1.68 | 0.70 | 0.43 to 1.14 | 0.99 | 0.60 to 1.61 |
| Marital status | ||||||||||
| Married | Referent | Referent | Referent | Referent | Referent | |||||
| Single | 2.02* | 1.21 to 3.39 | 1.26 | 0.64 to 2.48 | 1.34 | 0.77 to 2.33 | 1.25 | 0.88 to 1.76 | 1.54* | 1.09 to 2.19 |
| Unknown | 0.98 | 0.52 to 1.82 | 1.14 | 0.58 to 2.23 | 0.88 | 0.50 to 1.54 | 1.46 | 1.05 to 2.04 | 0.91 | 0.62 to 1.33 |
| Insurance status | ||||||||||
| Commercial | Referent | Referent | Referent | Referent | Referent | |||||
| Medicare | 1.26 | 0.69 to 2.31 | 1.57 | 0.77 to 3.20 | 1.88* | 1.05 to 3.38 | 2.31* | 1.59 to 3.38 | 1.41 | 0.96 to 2.06 |
| Medicaid | 1.55 | 0.52 to 4.60 | 0.54 | 0.07 to 4.22 | 1.17 | 0.38 to 3.62 | 3.21* | 1.76 to 5.83 | 1.64 | 0.81 to 3.32 |
| Self-pay | 0.82 | 0.19 to 3.58 | 2.85 | 0.90 to 8.99 | 0.77 | 0.17 to 3.45 | 1.10 | 0.45 to 2.69 | 1.42 | 0.64 to 3.17 |
| Unknown | 1.15 | 0.26 to 5.17 | 1.77 | 0.37 to 8.42 | 1.57 | 0.43 to 5.74 | 0.86 | 0.29 to 2.51 | 0.94 | 0.35 to 2.60 |
| Hospital location | ||||||||||
| Urban | Referent | Referent | Referent | Referent | Referent | |||||
| Rural | 0.77 | 0.24 to 2.61 | 1.15 | 0.25 to 5.28 | 1.14 | 0.32 to 3.97 | 0.50 | 0.19 to 1.28 | 0.67 | 0.28 to 1.62 |
| Hospital type | ||||||||||
| Teaching | Referent | Referent | Referent | Referent | Referent | |||||
| Nonteaching | 1.69 | 0.94 to 3.04 | 0.52 | 0.23 to 1.18 | 0.85 | 0.45 to 1.61 | 1.05 | 0.74 to 1.49 | 1.02 | 0.69 to 1.52 |
| Hospital size, No. of beds | ||||||||||
| < 400 | Referent | Referent | Referent | Referent | Referent | |||||
| 400-600 | 2.33* | 1.19 to 4.55 | 0.98 | 0.35 to 2.72 | 2.61* | 1.14 to 5.98 | 1.41 | 0.93 to 2.15 | 1.84* | 1.14 to 2.98 |
| > 600 | 1.21 | 0.56 to 2.60 | 1.71 | 0.62 to 4.73 | 2.03 | 0.86 to 4.80 | 1.27 | 0.80 to 2.00 | 1.62 | 0.97 to 2.69 |
| Hospital region | ||||||||||
| Northeast | Referent | Referent | Referent | Referent | Referent | |||||
| Midwest | 1.05 | 0.55 to 1.99 | 1.46 | 0.68 to 3.17 | 1.14 | 0.63 to 2.05 | 1.12 | 0.75 to 1.68 | 1.16 | 0.76 to 1.76 |
| South | 0.50 | 0.25 to 1.01 | 0.77 | 0.35 to 1.71 | 0.60 | 0.32 to 1.11 | 0.77 | 0.52 to 1.15 | 0.73 | 0.48 to 1.11 |
| West | 0.74 | 0.34 to 1.61 | 0.74 | 0.24 to 2.25 | 0.23* | 0.08 to 0.66 | 0.73 | 0.44 to 1.22 | 0.60 | 0.35 to 1.06 |
| Charlson comorbidity index | ||||||||||
| 1 | Referent | Referent | Referent | Referent | Referent | |||||
| 2 | 1.05 | 0.63 to 1.77 | 1.27 | 0.68 to 2.36 | 1.68 | 0.97 to 2.90 | 1.43* | 1.04 to 1.97 | 1.23 | 0.87 to 1.73 |
| ≥ 3 | 1.45 | 0.79 to 2.65 | 2.15* | 1.08 to 4.26 | 4.41* | 2.59 to 7.50 | 3.27* | 2.32 to 4.60 | 2.58* | 1.79 to 3.71 |
| Lymphadenectomy | ||||||||||
| No | Referent | Referent | Referent | Referent | Referent | |||||
| Yes | 1.58 | 0.92 to 2.70 | 2.10* | 1.01 to 4.37 | 1.08 | 0.65 to 1.78 | 1.16 | 0.85 to 1.58 | 1.42* | 1.01 to 2.01 |
| Surgeon volume | ||||||||||
| Low | Referent | Referent | Referent | Referent | Referent | |||||
| Intermediate | 0.70 | 0.38 to 1.29 | 0.67 | 0.32 to 1.43 | 0.60 | 0.31 to 1.15 | 0.57* | 0.40 to 0.82 | 0.70 | 0.47 to 1.06 |
| High | 0.88 | 0.45 to 1.71 | 0.85 | 0.39 to 1.84 | 1.06 | 0.65 to 1.78 | 0.42* | 0.28 to 0.65 | 1.06 | 0.70 to 1.62 |
| Hospital volume | ||||||||||
| Low | Referent | Referent | Referent | Referent | Referent | |||||
| Intermediate | 0.57 | 0.31 to 1.05 | 0.83 | 0.40 to 1.70 | 0.90 | 0.48 to 1.70 | 1.25 | 0.87 to 1.79 | 0.69 | 0.46 to 1.03 |
| High | 0.62 | 0.30 to 1.30 | 0.54 | 0.22 to 1.33 | 0.99 | 0.48 to 2.04 | 0.97 | 0.61 to 1.54 | 0.66 | 0.41 to 1.07 |
P < .05.
The mean cost for laparoscopic hysterectomy was $8,996 compared with $10,618 for robotic hysterectomy (P < .001; Table 3). In a multivariable model adjusted for patient characteristics, surgeon factors, and hospital characteristics, the cost for robotic surgery was $1,291 (95% CI, $985 to $1,597) higher than laparoscopic hysterectomy (Table 5). Hospital costs were also greater in single women, patients treated at teaching hospital, patients who underwent lymphadenectomy, and patients with the most comorbidities (P < .05 for all). Hospital costs were lower for patients treated by high-volume surgeons and patients operated on at intermediate-volume centers (P < .05 for both). These results did not change significantly when the cost variable was log-transformed. Separate models of cost for robotic and laparoscopic hysterectomy are also presented in Table 5. To examine whether the cost of robotic hysterectomy decreased with surgical volume, we developed separate models for low- and high-volume surgeons. For low-volume surgeons, the cost of robotic hysterectomy was $1,488 (95% CI, $938 to $2,038) higher than for a laparoscopic procedure; for high-volume surgeons, robotic hysterectomy was $818 (95% CI, $239 to $1,397) more costly than laparoscopy. In our cohort, if all 1,680 minimally invasive hysterectomies performed in 2009 were done robotically, direct hospital costs would have been increased by more than $2,000,000.
Table 5.
Multivariable Analysis of Factors Associated With Cost for Women Undergoing Minimally Invasive Hysterectomy for Endometrial Cancer
| Factor | All Patients |
Laparoscopic Hysterectomy |
Robotic Hysterectomy |
|||
|---|---|---|---|---|---|---|
| Cost ($) | 95% CI | Cost ($) | 95% CI | Cost ($) | 95% CI | |
| Surgery | ||||||
| Laparoscopic | Referent | — | — | |||
| Robotic | 1,291* | 985 to 1,597 | — | — | ||
| Age at surgery, years | ||||||
| < 60 | Referent | Referent | Referent | |||
| ≥ 60 | 290 | −67 to 648 | 361 | −137 to 856 | 109 | −355 to 573 |
| Race | ||||||
| White | Referent | Referent | Referent | |||
| Black | 676* | 41 to 1,311 | −33 | −822 to 756 | 1,055* | 132 to 1,977 |
| Other | 1,469* | 1,078 to 1,859 | 547* | 6 to 1,088 | 2,167* | 1,642 to 2,691 |
| Year of diagnosis | ||||||
| 2008 | Referent | Referent | Referent | |||
| 2009 | −806* | −1,256 to −356 | −438 | −995 to 119 | −886* | −1,565 to −206 |
| 2010 | −1,043* | −1,554 to −532 | −711* | −1,390 to −32 | −1,179* | −1,867 to −491 |
| Marital status | ||||||
| Married | Referent | Referent | Referent | |||
| Single | 545* | 177 to 913 | 98 | −407 to 602 | 866* | 378 to 1,354 |
| Insurance status | ||||||
| Commercial | Referent | Referent | Referent | |||
| Medicare | 217 | −163 to 597 | 155 | −368 to 677 | 186 | −308 to 680 |
| Medicaid | 929* | 129 to 1,729 | 1,680* | 653 to 2,707 | −80 | −1,201 to 1,040 |
| Self-pay | 4 | −871 to 880 | 303 | −737 to 1,343 | −302 | −1,600 to 996 |
| Unknown | 1,266* | 405 to 2,127 | 327 | −1,020 to 1,673 | 807 | −281 to 1,896 |
| Hospital location | ||||||
| Urban | Referent | Referent | Referent | |||
| Rural | −587 | −1,342 to 166 | −456 | −1,374 to 462 | −412 | −1,564 to 741 |
| Hospital type | ||||||
| Teaching | Referent | Referent | Referent | |||
| Nonteaching | −1,908* | −2,280 to −1,537 | −1,069* | −1,607 to −531 | −1,904* | −2,411 to −1,397 |
| Hospital size, No. of beds | ||||||
| < 400 | Referent | Referent | Referent | |||
| 400-600 | −7 | −434 to 448 | −237 | −836 to 363 | −42 | −705 to 621 |
| > 600 | −417 | −886 to 53 | 213 | −426 to 852 | −774* | −1,463 to 85 |
| Hospital region | ||||||
| Northeast | Referent | Referent | Referent | |||
| Midwest | −138 | −598 to 321 | 303 | −262 to 867 | −937* | −1,626 to −247 |
| South | 232 | −205 to 670 | −486 | −1,039 to 68 | 171 | −483 to 825 |
| West | 893* | 358 to 1,429 | 227 | −507 to 960 | 856* | 97 to 1,616 |
| Charlson comorbidity index | ||||||
| 1 | Referent | Referent | Referent | |||
| 2 | 92 | −234 to 419 | 484* | 34 to 933 | −2 | −426 to 422 |
| ≥ 3 | 1,102* | 668 to 1,536 | 825* | 244 to 1,406 | 1,058 | 477 to 1,639 |
| Lymphadenectomy | ||||||
| No | Referent | Referent | Referent | |||
| Yes | 1,171* | 841 to 1,500 | 1,414* | 986 to 1,842 | 631* | 181 to 1,082 |
| Surgeon volume | ||||||
| Low | Referent | Referent | Referent | |||
| Intermediate | 165 | −234 to 564 | −410 | −1,017 to 197 | 240 | −301 to 781 |
| High | −1,336* | −1,776 to −897 | −1,168* | −1,871 to −465 | −1,441* | −2,043 to −840 |
| Hospital volume | ||||||
| Low | Referent | Referent | Referent | |||
| Intermediate | −416* | −815 to −16 | 690* | 31 to 1,348 | −1,013* | −1,562 to −464 |
| High | 280 | −200 to −762 | 547 | −227 to 1,321 | 365 | −335 to 1,065 |
P < .05.
DISCUSSION
Our findings suggest that robotic hysterectomy offers little short-term benefit over a laparoscopic procedure for women with endometrial cancer. Perioperative morbidity was similar for the two procedures, whereas resource utilization is significantly higher for robotic hysterectomy. Robotic hysterectomy is associated with substantially higher direct hospital costs.
To date, there have been no randomized trials comparing laparoscopic and robotic hysterectomy for endometrial cancer. A recent systematic review of uncontrolled case series of robotic hysterectomy for endometrial cancer that identified 589 procedures found no differences in intraoperative or postoperative complications rates, transfusion requirements, rates of conversion to laparotomy, operative times, or length of stay between women who underwent laparoscopy and women who had a robotically assisted hysterectomy. However, compared with laparoscopy, robotic hysterectomy was associated with clinically insignificant lower blood loss (mean, 182 v 92 mL, respectively). Lymph node yield, a measure of surgical quality, was similar for the two modalities.27 Most of the reports included in this review were from surgeons and centers that possess significant expertise in robotic surgery.4–11 In our population-based analysis, there were no significant differences in the morbidity of robotic and laparoscopic surgery after adjusting for patient, physician, and systems characteristics.
A major concern surrounding the use of robotic surgery is the economic viability of the technology. A single-institution series of 110 patients with endometrial cancer noted that laparoscopic and robotic surgery had similar costs and that both modalities were significantly less costly than open hysterectomy.7 In contrast, a decision model found that laparoscopic hysterectomy was the least expensive treatment from both a hospital and societal perspective.3 In addition to the price of the robot, which ranges from $1 million to $2.25 million, an annual service contract of $140,000 is required, and disposable instruments cost $1,500 to $2,000 per case.13,28 Modeling studies of endometrial cancer have suggested that even if the purchase price of the robot is excluded and the price of disposable instruments is substantially reduced, laparoscopic hysterectomy remains the most economically advantageous.3 A recent analysis of 20 types of robotically assisted procedures noted that the addition of the robot added on average $1,600, or 6% of the total procedure cost.17 In our multivariable model, use of the robot increased direct hospital costs by nearly $1,300, more than 14% of the total hospital cost of a laparoscopic procedure.
Our data are remarkable in that by 2010, more than 60% of all minimally invasive hysterectomies for endometrial cancer were performed robotically despite the limited available data. A number of factors including perceptions, characteristics of early users, and contextual factors have been shown to drive innovation of a new technology.12 For surgical innovations, several studies have demonstrated that the introduction of a new technique often increases aggregate use of a surgical procedure.17,28–30 The introduction of robotic prostatectomy in the United States between 2005 and 2008 was associated with a 60% increase in the number of prostatectomies performed despite a decreasing incidence of prostate cancer.17 Although technologic innovation cannot follow the same developmental process as that of new drugs, there is increasing recognition that more formal regulation is needed.31 The Balliol Collaboration's Innovation, Development, Exploration, Assessment, and Long-Term Study (IDEAL) model proposed that new surgical techniques should evolve from a concept through safety exploration followed by randomized trials before widespread implementation.14,16,31,32 The US Food and Drug Administration is currently revising its regulatory process for medical devices after substantial public criticism.33
We identified a number of disparities in the use of robotic hysterectomy as the technology diffused into practice. Black women were 54% less likely than white women to undergo a robotic procedure, whereas uninsured patients were 44% less likely to have a robotic hysterectomy than patients with commercial insurance. The hospital setting in which patients received care also had a strong impact on the allocation of care. Women treated at large facilities and at nonteaching hospitals were more likely to undergo robotic surgery, whereas women treated at rural hospitals were 50% less likely to undergo a robotic hysterectomy. These disparities mirror those seen with the introduction of laparoscopy for a number of different procedures.34–36
We recognize several important limitations in our study. Because the primary purpose of claims data is for billing, complications are often under-reported. To minimize this bias, we focused our analysis on major perioperative complications that are likely to generate a claim. Any under-reporting of complications would have been equally likely in both cohorts. Although the Perspective database contains a sample of women from throughout the United States, our findings may not be generalizable to the entire US health care system. Perspective lacks data on tumor characteristics such as histology, grade, stage, and depth of invasion that impact treatment. Although we included only patients who underwent minimally invasive surgery, and the indications are the same for both procedures, some degree of procedure selection likely occurred based on tumor characteristics. We cannot exclude the possibility that some patients' procedures were misclassified. However, even during the early months of the study, the relative number of patients who underwent robotic surgery was high, suggesting that ICD-9 coding for robotic surgery was well recognized. With any new procedure, a learning curve exists for physicians, and this is certainly true for robotic surgery.11 We attempted to account for this by including surgical volume as a covariate in our analysis, but we recognize that costs may be lower as surgeons become more familiar with the technology and operative times decrease. Our costing data are based on a nationwide sample of directly reported hospital costs or estimates. Certainly wide variations in cost exist based on whether acquisition and maintenance costs are included and whether costs of disposable instruments are included; however, we feel that, if anything, our costs are likely to underestimate the true costs. A number of factors, including lymphadenectomy, which was more common in the robotic hysterectomy group, also had a strong influence on cost. Finally, it should be recognized that both procedures were associated with low overall morbidity, limiting our power to detect statistically significant differences between groups. Although there were no statistically significant differences in morbidity, there was a trend toward fewer medical complications in the robotic hysterectomy group.
Our findings raise questions as to the role of robotic surgery in the treatment of endometrial cancer. Robotic technology initially gained widespread utilization in urology.2 Because laparoscopic prostatectomy is technically demanding and not routinely performed, robotics introduced a minimally invasive surgical option for prostatectomy.2 In contrast, laparoscopic hysterectomy is well described, technically feasible, and now taught in most training programs.1 However, despite the availability of laparoscopic hysterectomy, a 2008 survey demonstrated that only 8% of gynecologic oncologists used the procedure in more than 50% of their patients.37 Even though laparoscopy is less costly, surgeon preferences for robotics may allow some women to undergo a minimally invasive procedure who may otherwise have undergone laparotomy. Proponents of robotic hysterectomy also argue that robotic capabilities allow surgeons to perform more technically challenging procedures without resorting to laparotomy.3,27 Although difficult to measure, this may be an important advantage of robotics. Finally, further data are needed to compare the oncologic outcomes of robotic and laparoscopic hysterectomy and to compare quality of life after the procedures.
Our study demonstrates that both robotic hysterectomy and laparoscopic hysterectomy are well tolerated and associated with similar morbidity profiles. Despite the rapid uptake of robotic hysterectomy, there seems to be little short-term benefit for the procedure. Compared with laparoscopic hysterectomy, robotic procedures are associated with substantially greater direct hospital costs. Our findings highlight the potential pitfalls of the rapid uptake of new technology before the availability of rigorous data to demonstrate efficacy and cost effectiveness. Defining the comparative effectiveness for new technologies and surgical approaches is necessary before rapid dissemination.
Appendix
The Perspective database (Premier, Charlotte, NC; http://www.premierinc.com) is a voluntary, fee-supported database originally developed to measure resource utilization and quality of care. The Perspective database is the largest inpatient drug utilization database in the United States. Perspective samples more than 500 acute care hospitals throughout the United States that contribute data on inpatient hospital admissions. Perspective contains a representative sample of acute care hospitals throughout the United States. Participating hospitals are drawn from all regions of the United States. Similar to the makeup of hospitals in general, the hospitals sampled by Perspective are predominately midsized, nonteaching facilities more frequently found in metropolitan areas. There is a larger representation of hospitals from the southern United States. All discharges from a given hospital are recorded in the Perspective database. We used data on patients treated from the last quarter of 2008 through the first quarter of 2010.
The Perspective database contains clinical and demographic information and International Classification of Diseases, Ninth Revision, codes for all primary and secondary diagnoses. In addition, Perspective collects data on all billed services. The Perspective database contains an itemized log all of all services charged to a patient including drugs and medications, laboratory, radiologic, and other diagnostic tests, and therapeutic services. Each participating institution submits electronic updates on a quarterly basis. The data are audited regularly to ensure quality and integrity.
All data are entered by trained hospital-level data abstractors. The data are entered into an electronic decision support system and then extracted by Premier via a secure site. On receiving data from participating hospitals, Premier undertakes an extensive data validation and correction process that includes more than 95 quality assurance checks. Data are imported into Premier's engine, where the mapping, error correcting, and quality analysis take place.
Perspectives records hospital costs for each patient visit. Within the Perspectives database, approximately three quarters of hospitals submit direct cost data taken from internal accounting systems. The remaining institutions provide estimates based on Medicare cost to charge ratios. The costing data from Perspectives has been validated in a number of studies that have examined costs and trends in resource utilization.
The Perspective database contains more than 45 million inpatient discharges. To place this into context, in 2006 Perspective recorded approximately 5.5 million hospital discharges, which represent approximately 15% of nationwide hospitalizations. Over the last decade, the Perspective database has been extensively validated and used for a number of studies that have examined resource utilization, quality, and outcomes.
Footnotes
See accompanying editorial on page 767
Supported by Grant No. R01 CA134964 from the National Cancer Institute, Bethesda, MD (D.L.H.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: William M. Burke, Intuitive Surgical Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jason D. Wright, William M. Burke,Dawn L. Hershman
Financial support: Jason D. Wright
Administrative support: Jason D. Wright
Collection and assembly of data: Jason D. Wright, Abigail S. Charles, Dawn L. Hershman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–5336. doi: 10.1200/JCO.2009.22.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson EB. The evolution of robotic general surgery. Scand J Surg. 2009;98:125–129. doi: 10.1177/145749690909800208. [DOI] [PubMed] [Google Scholar]
- 3.Barnett JC, Judd JP, Wu JM, et al. Cost comparison among robotic, laparoscopic, and open hysterectomy for endometrial cancer. Obstet Gynecol. 2010;116:685–693. doi: 10.1097/AOG.0b013e3181ee6e4d. [DOI] [PubMed] [Google Scholar]
- 4.Veljovich DS, Paley PJ, Drescher CW, et al. Robotic surgery in gynecologic oncology: Program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol. 2008;198:679.e1–9. doi: 10.1016/j.ajog.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Boggess JF, Gehrig PA, Cantrell L, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: Robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199:360.e1–9. doi: 10.1016/j.ajog.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Gehrig PA, Cantrell LA, Shafer A, et al. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol. 2008;111:41–45. doi: 10.1016/j.ygyno.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Bell MC, Torgerson J, Seshadri-Kreaden U, et al. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol. 2008;111:407–411. doi: 10.1016/j.ygyno.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Holloway RW, Ahmad S, DeNardis SA, et al. Robotic-assisted laparoscopic hysterectomy and lymphadenectomy for endometrial cancer: Analysis of surgical performance. Gynecol Oncol. 2009;115:447–452. doi: 10.1016/j.ygyno.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Seamon LG, Bryant SA, Rheaume PS, et al. Comprehensive surgical staging for endometrial cancer in obese patients: Comparing robotics and laparotomy. Obstet Gynecol. 2009;114:16–21. doi: 10.1097/AOG.0b013e3181aa96c7. [DOI] [PubMed] [Google Scholar]
- 10.Seamon LG, Cohn DE, Henretta MS, et al. Minimally invasive comprehensive surgical staging for endometrial cancer: Robotics or laparoscopy? Gynecol Oncol. 2009;113:36–41. doi: 10.1016/j.ygyno.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Lim PC, Kang E, Park do H. A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: A case-matched controlled study of the first one hundred twenty two patients. Gynecol Oncol. 2011;120:413–418. doi: 10.1016/j.ygyno.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–1975. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 13.Kolata G. Results unproven, robotic surgery wins converts. The New York Times. http://www.nytimes.com/2010/02/14/health/14robot.html?pagewanted=all.
- 14.Barkun JS, Aronson JK, Feldman LS, et al. Evaluation and stages of surgical innovations. Lancet. 2009;374:1089–1096. doi: 10.1016/S0140-6736(09)61083-7. [DOI] [PubMed] [Google Scholar]
- 15.Karamlou T, Diggs BS, Ungerleider RM, et al. The rush to atrial septal defect closure: Is the introduction of percutaneous closure driving utilization? Ann Thorac Surg. 2008;86:1584–1590. doi: 10.1016/j.athoracsur.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 16.McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: The IDEAL recommendations. Lancet. 2009;374:1105–1112. doi: 10.1016/S0140-6736(09)61116-8. [DOI] [PubMed] [Google Scholar]
- 17.Barbash GI, Glied SA. New technology and health care costs: The case of robot-assisted surgery. N Engl J Med. 2010;363:701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 18.Breitenstein S, Nocito A, Puhan M, et al. Robotic-assisted versus laparoscopic cholecystectomy: Outcome and cost analyses of a case-matched control study. Ann Surg. 2008;247:987–993. doi: 10.1097/SLA.0b013e318172501f. [DOI] [PubMed] [Google Scholar]
- 19.Lagu T, Rothberg MB, Nathanson BH, et al. The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171:292–299. doi: 10.1001/archinternmed.2011.12. [DOI] [PubMed] [Google Scholar]
- 20.Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 21.Lindenauer PK, Pekow PS, Lahti MC, et al. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303:2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69:871–875. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothberg MB, Pekow PS, Lahti M, et al. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 27.Gaia G, Holloway RW, Santoro L, et al. Robotic-assisted hysterectomy for endometrial cancer compared with traditional laparoscopic and laparotomy approaches: A systematic review. Obstet Gynecol. 2010;116:1422–1431. doi: 10.1097/AOG.0b013e3181f74153. [DOI] [PubMed] [Google Scholar]
- 28.Makarov DV, Yu JB, Desai RA, et al. The association between diffusion of the surgical robot and radical prostatectomy rates. Med Care. 2011;49:333–339. doi: 10.1097/MLR.0b013e318202adb9. [DOI] [PubMed] [Google Scholar]
- 29.Steiner CA, Bass EB, Talamini MA, et al. Surgical rates and operative mortality for open and laparoscopic cholecystectomy in Maryland. N Engl J Med. 1994;330:403–408. doi: 10.1056/NEJM199402103300607. [DOI] [PubMed] [Google Scholar]
- 30.Tunis SR, Bass EB, Steinberg EP. The use of angioplasty, bypass surgery, and amputation in the management of peripheral vascular disease. N Engl J Med. 1991;325:556–562. doi: 10.1056/NEJM199108223250806. [DOI] [PubMed] [Google Scholar]
- 31.McCulloch P, Schuller F. Innovation or regulation: IDEAL opportunity for consensus. Lancet. 2010;376:1034–1036. doi: 10.1016/S0140-6736(10)61386-4. [DOI] [PubMed] [Google Scholar]
- 32.Johnson J, Rogers W, Lotz M, et al. Ethical challenges of innovative surgery: A response to the IDEAL recommendations. Lancet. 2010;376:1113–1115. doi: 10.1016/S0140-6736(10)61116-6. [DOI] [PubMed] [Google Scholar]
- 33.Walker EP. FDA's fast-track medical device approval process under fire. ABC News. http://abcnews.go.com/Health/Wellness/medical-devices-government-office-accountability-finds-holes-fdas/story?id=13368464#.TvnMXtQ7XNU.
- 34.Abenhaim HA, Azziz R, Hu J, et al. Socioeconomic and racial predictors of undergoing laparoscopic hysterectomy for selected benign diseases: Analysis of 341487 hysterectomies. J Minim Invasive Gynecol. 2008;15:11–15. doi: 10.1016/j.jmig.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Guller U, Jain N, Curtis LH, et al. Insurance status and race represent independent predictors of undergoing laparoscopic surgery for appendicitis: Secondary data analysis of 145,546 patients. J Am Coll Surg. 2004;199:567–575. doi: 10.1016/j.jamcollsurg.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Csikesz NG, Tseng JF, Shah SA. Trends in surgical management for acute cholecystitis. Surgery. 2008;144:283–289. doi: 10.1016/j.surg.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 37.Naumann RW, Coleman RL. The use of adjuvant radiation therapy in early endometrial cancer by members of the Society of Gynecologic Oncologists in 2005. Gynecol Oncol. 2007;105:7–12. doi: 10.1016/j.ygyno.2006.11.003. [DOI] [PubMed] [Google Scholar]