Abstract
Purpose
To compare the efficacy of four cycles of paclitaxel–bleomycin, etoposide, and cisplatin (T-BEP) to four cycles of bleomycin, etoposide, and cisplatin (BEP) in previously untreated patients with intermediate-prognosis germ-cell cancer (GCC).
Patients and Methods
Patients were randomly assigned to receive either T-BEP or standard BEP. Patients assigned to the T-BEP group received paclitaxel 175 mg/m2 in a 3-hour infusion. Patients who were administered T-BEP received primary granulocyte colony-stimulating factor (G-CSF) prophylaxis. The study was designed as a randomized open-label phase II/III study. To show a 10% improvement in 3-year progression-free survival (PFS), the study aimed to recruit 498 patients but closed with 337 patients as a result of slow accrual.
Results
Accrual was from November 1998 to April 2009. A total of 169patients were administered BEP, and 168 patients were administered T-BEP. Thirteen patients in both arms were ineligible, mainly as a result of a good prognosis of GCC (eight patients administered BEP; six patients administered T-BEP) or a poor prognosis of GCC (one patient administered BEP; four patients administered T-BEP). PFS at 3 years (intent to treat) was 79.4% in the T-BEP group versus 71.1% in the BEP group (hazard ratio [HR], 0.73; CI, 0.47 to 1.13; P [log-rank test] = 0.153). PFS at 3 years in all eligible patients was 82.7% versus 70.1%, respectively (HR, 0.60; CI: 0.37 to 0.97) and was statistically significant (P = 0.03). Overall survival was not statistically different.
Conclusion
T-BEP administered with G-CSF seems to be a safe and effective treatment regimen for patients with intermediate-prognosis GCC. However, the study recruited a smaller-than-planned number of patients and included 7.7% ineligible patients. The primary analysis of the trial could not demonstrate statistical superiority of T-BEP for PFS. When ineligible patients were excluded, the analysis of all eligible patients demonstrated a 12% superior 3-year PFS with T-BEP, which was statistically significant.
INTRODUCTION
The treatment of metastatic germ-cell cancer (GCC) with cisplatin-etoposide based chemotherapy results in the cure of the majority of patients.1 In the current consensus classification for nonseminoma and seminoma, the following three prognostic categories are defined; a good-prognosis group with a 5-year disease-free survival (DFS) of 90%, an intermediate-prognosis group with a 5-year DFS of 70%, and a poor-prognosis group with a 5-year DFS of 45%.2 For all three prognostic groups, the administration of bleomycin, etoposide, and cisplatin (BEP) is the standard treatment.
Three decades of chemotherapy studies that tested shortened intervals between chemotherapy cycles, the concept of alternating or sequential chemotherapy, and recent studies of high-dose chemotherapy plus autologous peripheral stem-cell support have not proven to be superior to the gold standard of BEP.3–5
An alternative strategy could be the incorporation of a new effective agent in the BEP regimen. Paclitaxel has demonstrated activity in two phase II studies in patients with platinum-refractory cancer.6,7 Therefore, the European Organisation for Research and Treatment of Cancer (EORTC) decided to investigate the addition of paclitaxel to BEP. In a formal paclitaxel (Taxol; Bristol-Myers Squibb International, Brussels, Belgium)–bleomycin, etoposide, and cisplatin (T-BEP) dose-finding study, paclitaxel was administered at dose levels of 75, 125, 175, and 200 mg/m2 in a 3-hour infusion on day 1 before the start of BEP.8 Primary granulocyte colony-stimulating factor (G-CSF) prophylaxis was applied. A dose of paclitaxel 175 mg/m2 was feasible with full-dose BEP, and this regimen was chosen for the intended randomized multicenter study.
The randomized phase II/III study of T-BEP versus BEP in patients with intermediate-prognosis GCC reported in this article was conducted in the framework of EORTC, the German Testicular Cancer Study Group/Association of Urologic Oncology, the Medical Research Council (Testis Cancer study 21)/United Kingdom National Cancer Research Institute Testicular Cancer Clinical Study Group, and the Spanish Germ-Cell Cancer Group.
PATIENTS AND METHODS
Patients
Patients were eligible for the study if they had intermediate-prognosis metastatic GCC according to International Germ Cell Cancer Concensus as follows: for nonseminoma, all of (1) a testis or retroperitoneal primary tumor, (2) α-fetoprotein (AFP) ≥ 1,000 but ≤ 10,000 U/L, human chorionic gonadotropin ≥ 5,000 U/L (1,000 ng/mL) but ≤ 50,000 U/L (10,000 ng/mL), or lactate dehydrogenase ≥ 1.5× but ≤ 10× the upper limit of normal, and (3) no liver, bone, brain, or other nonpulmonary visceral metastases; and for pure seminoma, (1) any primary site, (2) any lactate dehydrogenase and any human chorionic gonadotropin, (3) nonpulmonary visceral metastases present, and (4) AFP within the normal range. Patients were not accepted if they had previously received chemotherapy, had a creatinine clearance less than 40 mL/min, or were less than 16 or greater than 50 years of age. All patients provided ethics board– approved written informed consent.
Treatment
Standard BEP consisted of cisplatin 20 mg/m2 days 1 through 5 and etoposide 100 mg/m2 administered days 1 through 5 for four cycles. Bleomycin was administered at a dose of 30 mg weekly for 12 weeks (total dose of bleomycin, 360 mg). Patients administered T-BEP received paclitaxel 175 mg/m2 given as a 3-hour infusion on day 1, before starting standard BEP, for four cycles. Paclitaxel was supplied by Bristol-Myers Squibb International. G-CSF (filgrastim and later pegfilgrastim) was used for primary prophylaxes in patients allocated to the T-BEP group. In patients who received BEP, G-CSF was used only as secondary prophylaxis.
Chemotherapy dose modifications for hematologic toxicity are presented in detail in Appendix Tables A1 and A2 (online only).
After grade 3 or 4 mucosal toxicity or diarrhea, the following cycle of chemotherapy was delayed until recovery. For patients allocated to the T-BEP group, the dose of paclitaxel was reduced by 25% for all remaining cycles. For clinically significant hypersensitivity reactions during paclitaxel administration, the infusion was discontinued, and patients were treated with antihistamines and epinephrine and were retreated at the discretion of the investigator.
The actual dose-intensity was calculated as follows for all drugs except bleomycin for which the total dose in milligrams was used.
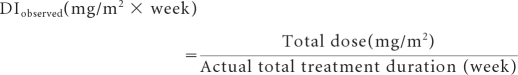 |
The relative dose intensity (RDI) was the ratio of the observed dose intensity to the weekly dose planned per protocol and expressed as a percentage. The average RDI for BEP or T-BEP was obtained by averaging the RDI of the individual components of each regimen.
Response Assessment
After completion of chemotherapy, patients with normal tumor marker levels and no clinical or radiologic evidence of residual masses were classified as complete responders and were monitored without additional therapy. Patients in whom markers normalized but who showed evidence of residual tumor mass underwent debulking surgery unless the initial histologic diagnosis was pure seminoma. The protocol advised complete macroscopic resection of all tumor remnants. These patients were classified as complete responders if the histologic examination showed no viable cancer. If viable malignancy had been resected completely, patients were classified as having been rendered disease free by chemotherapy plus surgery. Patients in whom the surgical resection of residual viable cancer was incomplete, patients who had a continuing increase of tumor markers, or patients who had disease progression while receiving chemotherapy or within 2 months after the completion of chemotherapy were classified as incomplete responders. Rising tumor markers or an increase in tumor volume (unless this was caused by mature teratoma that was completely resectable) was considered progression of disease. Patients with residual masses who did not undergo complete debulking surgery were classified as nonassessable for response and included in progression-free survival (PFS) and survival analyses. Events in the PFS analysis were incomplete response, progression of disease, and death.
Sample Size and Statistical Analysis
The study was designed as an open-label phase II/III trial. Phase II aimed at the exclusion of a complete-response (CR) rate of 65% (one-sided α = 0.10; power, 95%) with a two-stage optimum Simon design. A maximum of 82 patients taking T-BEP were needed, with the assumption that the true CR rate was 80%. Phase III was planned to show an increase of the 3-year PFS from 75% to 85% with T-BEP (hazard ratio [HR], 0.56) with 80% power and am two-sided α = 0.05. To this aim, 98 events were needed, and it was planned to recruit 498 patients. Randomization was by minimization to ensure the treatment arms were balanced with respect to histology (seminoma v nonseminoma or combined tumors) and the number of patients allocated in each hospital.9,10
The decision to continue the trial into phase III was taken by the EORTC independent data monitoring committee in March 2004. In December 2006, the EORTC independent data monitoring committee requested and reviewed an interim analysis of the phase III trial by using stopping rules for both futility (γ-family with γ = −2) and superiority (γ-family with γ = −3),11 but the boundaries were not crossed, and the study continued. The trial eventually closed to accrual in August 2009 with 337 patients as a result of recruitment problems and difficulties with paclitaxel supply. An independent statistician advised to conduct the final analysis when a minimum of 2 years follow-up was reached for all patients entered before June 2008. With 85 events of PFS observed in the intent-to-treat population, the study had a power of 74% (instead of 80% as planned) to detect the hypothesized difference in PFS if it was present. The CIs of the 3-year PFS rates in both treatment groups were compatible with the hypothesized values (ie, 75% and 85%).
The primary analysis of the response rate was in all eligible patients; patients of PFS and overall survival were in the intent-to-treat population. Sensitivity analyses were conducted in eligible patients and eligible patients who started the allocated treatment (per-protocol population). Survival end points were described by Kaplan-Meier curves, and comparisons were analyzed by using the nonstratified log-rank test. Binary variables were compared by using the χ2 test. The analysis was based on all available data as of January 3, 2011. All tests were two-sided at the 5% significance level with adjustment for the interim analysis (nominal α = 0.0455).
RESULTS
A total of 337 patients from 12 countries entered the trial between November 1998 and April 2009; 169 patients were allocated to the BEP group, and 168 patients were allocated to the T-BEP group.
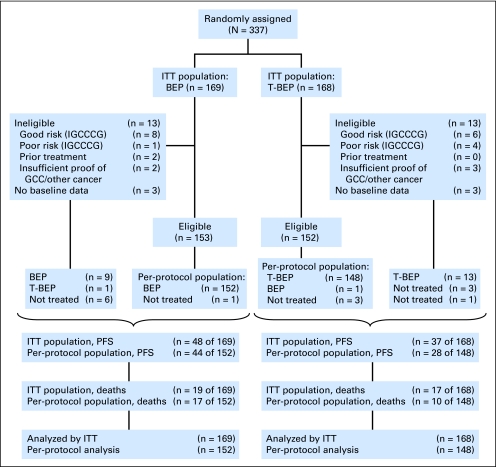
Of these patients, 26 patients were found ineligible (mostly as a result of a different prognostic classification), and for six patients, the eligibility was not completely verifiable (Fig 1). Of importance, distributions of good-risk and poor-risk ineligible patients were uneven because there were numerically more good-risk patients (predestined for a better prognosis) in the standard BEP-treatment group, whereas four of five poor-risk patients (with the associated worse prognosis) had been allocated to the T-BEP group.
Fig 1.
CONSORT diagram. BEP, bleomycin, etoposide, and cisplatin; GCC, germ-cell cancer; IGCCCG, International Germ Cell Cancer Collaborative Group; ITT, intent to treat; PFS, progression-free survival; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Besides the 26 ineligible patients, the following five additional patients were excluded from the per-protocol analysis: one patient in the T-BEP group who received BEP instead and four eligible patients without documented treatment (one patient taking BEP and three patients taking T-BEP).
As of the clinical cutoff date of January 3, 2011, the median follow-up of all patients was 5.3 years. There was no difference in follow-up between the two treatment groups.
The pretreatment characteristics of all patients are listed in Table 1. The two treatment arms were well balanced with respect to age, histology, and disease extent, with the exception of a higher median AFP in the T-BEP arm (499 IU/L in the T-BEP group v 156 IU/L in the BEP group; Kruskal-Wallis P = .0350).
Table 1.
Patient and Disease Characteristics
| Characteristic | Treatment |
|||
|---|---|---|---|---|
| BEP (n = 169) |
T-BEP (n = 168) |
|||
| No. of Patients | % | No. of Patients | % | |
| Patient characteristic | ||||
| Age, years | ||||
| Median | 28 | 28 | ||
| Range | 16-50 | 16-50 | ||
| Interquartile range | 23-35 | 24-35 | ||
| Disease characteristic | ||||
| Histology | ||||
| Seminoma | 14 | 8.3 | 15 | 8.9 |
| Non seminoma | 108 | 63.9 | 103 | 61.3 |
| Combined seminoma and nonseminoma | 44 | 26.0 | 46 | 27.4 |
| Unknown/missing | 3 | 1.8 | 4 | 2.4 |
| Primary site | ||||
| Testis | 156 | 92.3 | 155 | 92.3 |
| Retroperitoneal | 10 | 5.9 | 8 | 4.8 |
| Mediastinal | 1 | 0.6 | 2 | 1.2 |
| Other/missing | 2 | 1.2 | 3 | 1.8 |
| Involved metastatic sites | ||||
| Abdominal lymph nodes | 147 | 86.9 | 144 | 85.8 |
| Mediastinal lymph nodes | 46 | 27.3 | 52 | 30.9 |
| Supraclavicular lymph nodes | 25 | 14.8 | 22 | 13.1 |
| Lung metastases | 81 | 47.9 | 86 | 51.2 |
| Liver metastases | 3 | 1.8 | 4 | 2.4 |
| Bone metastases | 3 | 1.8 | 7 | 4.2 |
| AFP, IU/L* | ||||
| < 1,000 | 117 | 69.2 | 91 | 54.2 |
| 1000 ≤ AFP < 10,000 | 50 | 29.6 | 74 | 44.0 |
| > 10,000 | 0 | 0.0 | 1 | 0.6 |
| Unknown/missing | 2 | 1.2 | 2 | 1.2 |
| Median | 156.0 | 499.0 | ||
| Range | 1.0-8,791.0 | 1.0-1,3448.0 | ||
| Q1-Q3 | 5.0-1,219.0 | 9.0-2,319.0 | ||
| HCG, IU/L | ||||
| < 5,000 | 119 | 70.4 | 116 | 69.0 |
| 5,000 ≤ HCG < 50,000 | 48 | 28.4 | 49 | 29.2 |
| >50,000 | 0 | 0.0 | 1 | 0.6 |
| Unknown/missing | 2 | 1.2 | 2 | 1.2 |
| Median | 423.0 | 267.0 | ||
| Range | 0.0-49,010.0 | 0.0-56,053.0 | ||
| Q1-Q3 | 10.0-5,710.0 | 8.0-7,999.0 | ||
| LDH, ×ULN | ||||
| < 1.5× ULN | 44 | 26.0 | 57 | 33.9 |
| 1.5× ULN ≤ LDH < 10× ULN | 121 | 71.6 | 107 | 63.7 |
| > 10× ULN | 2 | 1.2 | 2 | 1.2 |
| Unknown/missing | 2 | 1.2 | 2 | 1.2 |
| Median | 2.1 | 1.9 | ||
| Range | 0.4-17.8 | 0.0-12.8 | ||
| Q1-Q3 | 1.3-3.9 | 1.2-3.4 | ||
Abbreviations: AFP, α-fetoprotein; BEP, bleomycin, etoposide, and cisplatin; HCG, human chorionic gonadotropin; LDH, lactate dehydrogenase; Q, quartile; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin; ULN, upper limit of normal.
χ2 test for different distribution of AFP (< 1,000 v ≥ 1000 ng/mL), P = .005; Kruskall-Wallis χ2 test for difference in distribution of actual AFP values, P = .035.
Treatment Administered
In the intent-to-treat population, 92.3% of patients allocated to the BEP group completed four cycles of treatment; in patients allocated to the T-BEP group, 87.5% of patients completed four cycles of treatment. In the safety population (all patients who started the allocated treatment) these percentages were 96.3% and 91.3%, respectively. The reasons not to receive the allocated four cycles were mostly due to ineligibility, toxicity (including three patients with allergic reactions to paclitaxel), and refusal.
In the safety population, the median average RDI achieved for all agents was 97.1% in the BEP group and 97.5% in the T-BEP group. Detailed information about the dose intensity and RDI for each component in the two study arms is shown in Appendix Tables A3 and A4 (online only). In patients treated with BEP, 26% of patients had at least one cycle postponed, mainly as a result of hematologic toxicity; in patients who received T-BEP, 16% of patients had at least one cycle postponed, likely as a result of the primary use of G-CSF, as per protocol. In patients who received BEP, G-CSF was applied during at least one cycle in 33% of patients.
Toxicity
Dose reductions were applied at least once in 15% of patients who received BEP and in 12% of patients who received T-BEP. However, 9.3% of patients who received BEP compared with 22% of patients who received T-BEP stopped one drug definitively (mostly bleomycin as a result of pulmonary toxicity [eight patients in each arm] or paclitaxel as a result of allergic reactions, refusal, or reclassification into good-prognosis disease). Grade 3 and 4 neutropenia was encountered more frequently during BEP treatment, which was most likely caused by the restrictive use of G-CSF for secondary prophylaxis only, whereas patients who received T-BEP received G-CSF for primary prophylaxis. However, there were numerically more neutropenic fevers in the T-BEP group than in the BEP group (19% versus 10%, respectively; Table 2). Grades 2 and 3 mucositis/stomatitis and diarrhea occurred more frequently in the T-BEP group. However, grade 4 toxicities were rare in both study arms (Table 2).
Table 2.
Toxicity
| Toxicity | Treatment |
|||
|---|---|---|---|---|
| BEP (N = 161) |
T-BEP (N = 161) |
|||
| No. of Patients | % | No. of Patients | % | |
| Hematologic toxicity | ||||
| Leucocytes < 1.0 × 10 g/L | 17 | 10.6 | 26 | 16.1 |
| Leucocytopenic fever* | 16 | 10 | 31 | 19.2 |
| Platelets < 25 × 10 g/L | 5 | 3.1 | 5 | 3.1 |
| Nonhematologic toxicity | ||||
| Allergic reaction | ||||
| Grades 1-2† | 15 | 9.3 | 30 | 18.6 |
| Grades 3-4 | 0 | 0 | 9 | 5.5 |
| Fatigue | ||||
| Grades 1-2 | 111 | 69 | 103 | 64 |
| Grades 3-4 | 4 | 2.5 | 12 | 7.5 |
| Stomatitis/mucocitis | ||||
| Grades 1-2 | 57 | 35.4 | 72 | 44.7 |
| Grades 3-4 | 4 | 2.5 | 15 | 9.4 |
| Diarrhea | ||||
| Grades 1-2 | 32 | 19.9 | 56 | 34.8 |
| Grades 3-4 | 3 | 1.9 | 16 | 9.9 |
| Sensory neuropathy | ||||
| Grades 1-2 | 46 | 28.5 | 57 | 35.4 |
| Grades 3-4 | 2 | 1.2 | 0 | 0 |
| Other neurotoxicity | ||||
| Grades 1-2 | 23 | 14.3 | 24 | 14.9 |
| Grades 3-4 | 3 | 1.2 | 1 | 0.6 |
| Nausea | ||||
| Grades 1-2 | 117 | 72.7 | 114 | 70.8 |
| Grades 3-4 | 7 | 4.3 | 11 | 6.8 |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Leucocytes < 2.0 × 10 g/L, temperature > 38°C.
Denotes National Cancer Institute Common Toxicity Criteria version 2.0, worst toxicity reported over all cycles.
There were seven fatal adverse events. Of these, four events were possibly related to treatment; two patients (one patient who received T-BEP and one patient who received BEP) died of massive pulmonary embolism between cycles 1 and 2, one patient who received T-BEP died from pulmonary fibrosis that was likely bleomycin related, and one patient developed diarrhea and fever during his first cycle of T-BEP, was admitted 2 days later at a nearby hospital, and died during the diagnostic workup from sudden cardiorespiratory failure. The remaining three fatal events were not treatment related.
Post-Treatment Evaluations and Surgery
After chemotherapy, the majority of patients in both treatment arms had residual lesions (135 patients [80.4%] who received T-BEP versus 134patients (79.3%) who received BEP). Reasons not to resect all lesions, as reported by the investigators on the forms, were mostly due to either multiple lesions or lesions resected at one site that contained no vital cancer, and the decision was made not to resect additional lesions (detailed information is listed in Appendix Tables A5 to A7; online only). Histology was obtained from 119 patients with nonseminoma or mixed seminoma/nonseminoma histologies who received T-BEP and from 116 patients who received BEP. Complete resection of all remnants was possible in 50.8% of the patients who received T-BEP and underwent surgery and in 48.8% of patients who received BEP. Viable cancer was found in nine patients (7%) who received T-BEP and in 15 patients (12.9%) who received BEP.
Response to Treatment
Responses to chemotherapy, with or without surgery, were computed primarily in eligible patients. Because 4.5% of eligible patients did not start the allocated treatment, a computation was also made for eligible patients who started allocated treatment (per-protocol analysis). Analyses of intent to treat, all eligible patients, and per-protocol are listed in Table 3. In the eligible patient population, the rate of CR/no evidence of disease after chemotherapy plus surgery rate was 70.4% in the T-BEP group versus 59.5% in the BEP group (P = .0549). In eligible patients who actually started the allocated treatment (per-protocol population), the response rate (CR/no evidence of disease) was 71.6% in the T-BEP group versus 59.9% in the BEP group (P = .0384).
Table 3.
Response to Treatment
| Response to Treatment | Treatment |
Total |
||||
|---|---|---|---|---|---|---|
| BEP |
T-BEP |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Intent-to-treat analysis | ||||||
| No. of patients | 169 | 168 | 337 | |||
| Response to treatment | ||||||
| CR to chemotherapy only | 84 | 49.7 | 100 | 59.5 | 184 | 54.6 |
| NED, CR after chemotherapy and surgery | 11 | 6.5 | 8 | 4.8 | 19 | 5.6 |
| Treatment failure | 17 | 10.1 | 7 | 4.2 | 24 | 7.1 |
| Not evaluable | 56 | 33.1 | 47 | 28.0 | 103 | 30.6 |
| Early death as a result of disease | 1 | 0.6 | 2 | 1.2 | 3 | 0.9 |
| Early death as a result of toxicity | 0 | 0.0 | 2 | 1.2 | 2 | 0.6 |
| Early death as a result of other reason | 0 | 0.0 | 2 | 1.2 | 2 | 0.6 |
| Response, CR/NED | 95 | 56.2 | 108 | 64.3 | 203 | 60.2 |
| 95% CI, % | 48.4 to 63.8 | 56.5 to 71.5 | P = .1482 | |||
| All eligible patients | ||||||
| No. of patients | 153 | 152 | 305 | |||
| Response to treatment | ||||||
| CR to chemotherapy only | 80 | 52.3 | 99 | 65.1 | 179 | 58.7 |
| NED, CR after chemotherapy and surgery) | 11 | 7.2 | 8 | 5.3 | 19 | 6.2 |
| Treatment failure | 15 | 9.8 | 5 | 3.3 | 20 | 6.6 |
| Not evaluable | 46 | 30.1 | 36 | 23.7 | 82 | 26.9 |
| Early death as a result of disease | 1 | 0.7 | 1 | 0.7 | 2 | 0.7 |
| Early death as a result of toxicity | 0 | 0.0 | 2 | 1.3 | 2 | 0.7 |
| Early death as a result of other | 0 | 0.0 | 1 | 0.7 | 1 | 0.3 |
| Response, CR/NED | 91 | 59.5 | 107 | 70.4 | 198 | 64.9 |
| 95% CI, % | 51.2 to 67.3 | 62.4 to 77.5 | P = .0549 | |||
| Per-protocol analysis | ||||||
| No. of patients | 152 | 148 | 300 | |||
| Response to treatment | ||||||
| CR to chemotherapy only | 80 | 52.6 | 98 | 66.2 | 178 | 59.3 |
| NED, CR after chemotherapy and surgery | 11 | 7.2 | 8 | 5.4 | 19 | 6.3 |
| Treatment failure | 15 | 9.9 | 5 | 3.4 | 20 | 6.7 |
| Not evaluable | 45 | 29.6 | 34 | 23.0 | 79 | 26.3 |
| Early death as a result of disease | 1 | 0.7 | 1 | 0.7 | 2 | 0.7 |
| Early death as a result of toxicity | 0 | 0.0 | 2 | 1.4 | 2 | 0.7 |
| Response, CR/NED | 91 | 59.9 | 106 | 71.6 | 197 | 65.7 |
| 95% CI, % | 51.6 to 67.7 | 63.6 to 78.7 | P = .0384 | |||
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; CR, complete response; NED, no evidence of disease; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
PFS and Survival
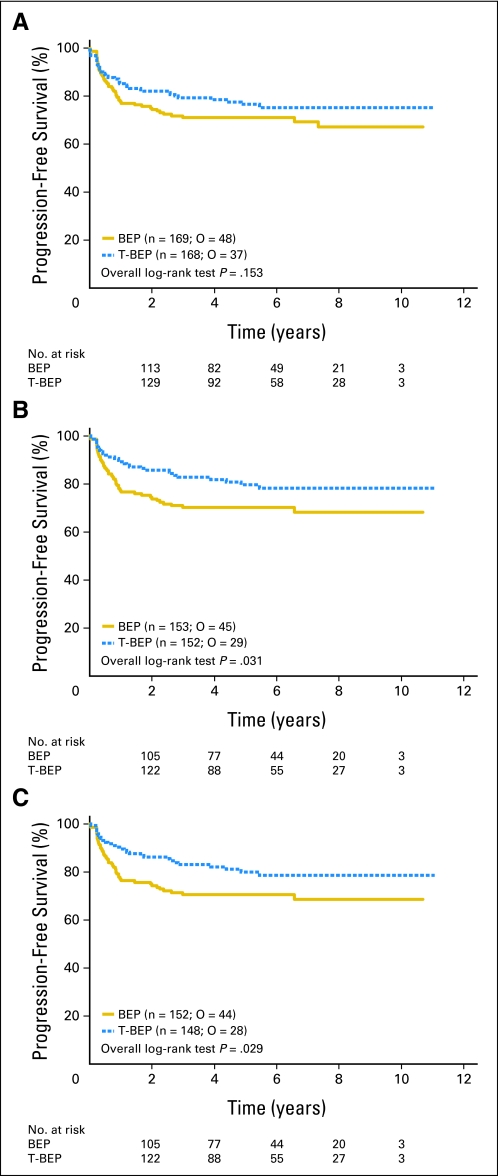
PFS and survival are listed in Table 4. In the intent-to-treat population (including all ineligible patients as well as the patients who did not start the allocated chemotherapy), the 3-year PFS rate was 79.4% in the T-BEP group and 71.1% in the BEP group. PFS curves were not statistically significant (HR, 0.73; CI, 0.47 to 1.13; P = .153; Table 4; Fig 2). With the exclusion of the 26 (7.7%) ineligible patients, 3-year PFS rates were 82.7% versus 70.1%, respectively (HR, 0.60; CI, 0.37 to 0.97, P = .0307). The difference was significant at the adjusted 0.0455 significance level. This may have been due to the fact that ineligible patients were more often ineligible as a result of having poor-risk GCC in the T-BEP arm and, vice versa, having good-risk GCC in the BEP arm. Moreover, most ineligible patients were treated outside of the protocol (good prognosis with BEP). In addition, one patient in each arm received the opposite treatment. With the additional exclusion of the five patients who did not receive the allocated treatment (per-protocol analysis), 3-year PFS rates were 83.2 versus 70.6%, respectively (HR, 0.59; CI, 0.37 to 0.96; P = .0289), similar to the results in eligible patients. Both the PFS analysis in all eligible patients and the per-protocol analysis showed a 12% superior 3-year PFS with T-BEP.
Table 4.
PFS and Survival: Primary Table of Results
| PFS and Survival | No. of Patients | Observed Events | T-BEP v BEP |
P (log-rank)* | At 3 Years |
||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | Adjusted 95% CI | % | 95.45% CI | ||||
| PFS | |||||||
| Intent to treat, primary analysis | |||||||
| BEP | 169 | 48 | 0.73 | 0.47 to 1.13 | .1531 | 71.08 | 63.14 to 77.61 |
| T-BEP | 168 | 37 | 0.70† | 0.44 to 1.10† | .1113† | 79.43 | 72.12 to 85.02 |
| All eligible | |||||||
| BEP | 153 | 45 | 0.60 | 0.37 to 0.97 | .0307 | 70.13 | 61.82 to 76.98 |
| T-BEP | 152 | 29 | 0.56† | 0.35 to 0.92† | .0181† | 82.69 | 75.28 to 88.06 |
| Per protocol | |||||||
| BEP | 152 | 44 | 0.59 | 0.37 to 0.96 | .0289 | 70.63 | 62.32 to 77.45 |
| T-BEP | 148 | 28 | 0.55† | 0.34 to 0.91† | .0166† | 83.16 | 75.75 to 88.48 |
| Survival | |||||||
| Intent to treat, primary analysis | |||||||
| BEP | 169 | 19 | 0.89 | 0.46 to 1.74 | .7382 | 89.83 | 83.78 to 93.70 |
| T-BEP | 168 | 17 | 0.88† | 0.44 to 1.75† | .7137† | 91.23 | 85.48 to 94.77 |
| All eligible | |||||||
| BEP | 153 | 17 | 0.64 | 0.30 to 1.39 | .2501 | 89.72 | 83.37 to 93.74 |
| T-BEP | 152 | 11 | 0.64† | 0.29 to 1.41† | .2618† | 94.42 | 88.99 to 97.21 |
| Per protocol | |||||||
| BEP | 152 | 17 | 0.58 | 0.26 to 1.29 | .1700 | 89.65 | 83.26 to 93.69 |
| T-BEP | 148 | 10 | 0.57† | 0.25 to 1.29† | .1698† | 95.04 | 89.71 to 97.64 |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; PFS, progression-free survival; T-BEP, paclitaxel bleomycin, etoposide, and cisplatin.
To be compared with the adjusted .0455 significance level.
Cox models with adjustment for baseline α-fetoprotein.
Fig 2.
Progression-free survival (A) in intent-to-treat population; (B) for all eligible patients; (C) in the per-protocol population. O, observed events.
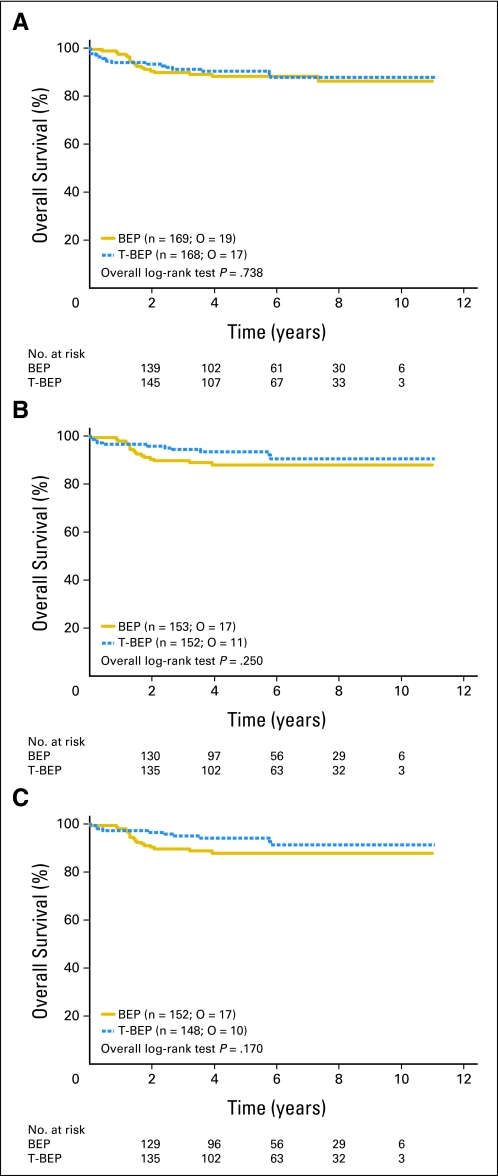
With only 36 events in the intent-to-treat population and 27 events in the per-protocol analysis, differences in overall survival were not statistically significant (HR, 0.89; CI, 0.46 to 1.74; P = .7382 in the intent-to-treat population; HR, 0.58; CI, 0.26 to 1.29; P = .1700 in the per-protocol analysis (Table 4; Fig 3). Adjustment of the comparisons for the baseline AFP values that were imbalanced between groups did not change these results (Table 4).
Fig 3.
Overall survival (A) in the intent-to-treat population; (B) for all eligible patients; and (C) in the per-protocol population. O, observed events.
DISCUSSION
Recent studies of upfront high-dose chemotherapy or dose-intensified etoposide, ifosfamide, and cisplatin chemotherapy in poor-risk GCC have not demonstrated superiority over the standard BEP treatment.4,5 In view of the activity of paclitaxel in the second-line treatment setting in phase 2 studies6,7 the EORTC and other collaborative groups in Europe decided to investigate the addition of paclitaxel to standard BEP in patients with intermediate-risk GCC. The T-BEP dose-finding study showed that the regimen was generally well tolerated, and with the use of primary G-CSF prophylaxis, a dose of paclitaxel 175 mg/m2 was feasible with standard-dose BEP8
This study was designed as a randomized open-label phase II/III study. The study originally aimed to recruit 498 patients. The enrollment onto the study was hampered by logistical problems, including delays in trial initiation in some countries that were anticipated to recruit a large proportion of the total patient group. When paclitaxel finally became generic, and multiple brands became available for use in the European countries, the regulatory burden associated with amending the protocol as well as the cessation of a free-drug supply further reduced the accrual and resulted in the decision by the EORTC to stop the trial with 337 patients randomly assigned.
The primary efficacy analysis (intent to treat) conducted with 85 of 98 events of PFS planned by design had only a 74% power of demonstrating statistical superiority of T-BEP if the hypothesized treatment effect (HR, 0.56) was present. However, extrapolation of the results obtained in the intent-to-treat population (HR, 0.73) to a hypothetical full-sample size of 98 events would not have reached conventional statistical significance with a theoretical 95% CI for the HR that ranged from 0.49 to 1.01.
The intent-to-treat results were confounded by 13 ineligible patients in each arm (8%) with an unfortunate uneven distribution among the two treatment groups with more good-prognosis patients allocated to the BEP group, most of whom were eventually treated outside of the protocol, and more (four of five) poor-prognosis patients allocated to the T-BEP group. This uneven distribution of ineligibles with the associated different prognostic outcome (good prognosis with BEP and poor prognosis with T-BEP) may have biased the results toward the null hypothesis of no difference. Also, for various reasons, several eligible patients (one patient who received BEP and three patients who received T-BEP) did not start the allocated treatment or received the opposite treatment.
The PFS at 3 years (intent to treat) was 79.4% in the T-BEP group versus 71.1% in the BEP group (HR, 0.73; P = .153). However, the PFS for the eligible patient population was 82.7% versus 70.1%, respectively (HR, 0.60; P = .03). This difference reached the adjusted level of statistical significance, as did the analysis in the per-protocol population. Both analyses showed a 12% superior PFS at 3 years. Numerically, these observed differences at 3 years were in the order of magnitude of what was anticipated (10%). As could be expected in the setting of GCC, with salvage regimens available and a low number of events, the overall survival was not statistically different.
This trial failed to demonstrate the superior efficacy of T-BEP in the management of the intended intermediate-prognosis GCC risk population on the basis of its primary analysis. However, the eligible-patient analysis as well as the per-protocol analysis that were thought to alleviate the conservative bias induced by the uneven distribution of ineligible or untreated patients that dragged the results toward the null hypothesis of no difference both reached statistical significance. With a small number of events, survival was not statistically significantly different between the two groups. The T-BEP regimen with the use of primary G-CSF prophylaxis was generally well tolerated with only two toxic deaths (1%) in the framework of this large multicenter study. The study showed a favorable toxicity profile of T-BEP in patients with intermediate-prognosis GCC. Whether or not T-BEP has a potential benefit in the poor-risk GCC patient category remains to be determined because this patient group was not planned to be recruited in the study.
Supplementary Material
Appendix
The following investigators and their institutions participated in this study: N. Dumez, University Hospital, Leuven, Belgium; I. Bodrogi, National Institute of Oncology, Budapest, Hungary; S. Kliesch, Universitäts Klinikum, Muenster, Germany; C. Theodore, Institut Gustave Roussy, Villejuif, France; G. Mead, Southampton General Hospital, Southampton, United Kingdom; G. Osanto, University Medical Center, Leiden, the Netherlands; G. Groenewegen, University Medical Center, Utrecht, the Netherlands; Winter, Klinikum, Schwerin, Germany; A. Horwich, Institute of Cancer Research and Royal Marsden Hospital, Sutton, United Kingdom; T. Gil, Hospital Universitaire Bordet – Erasme, Brussels, Belgium; A. Schütte, Otto Von Guericke Universität, Magdeburg, Germany; M.A. Gerbaek, Aarhus University Hospital, Aarhus, Denmark; A. Heldenreich, Klinikum Philipps, Marburg, Germany; R. Souchon, Allgemeines Krankenhaus, Hagen, Germany; J.B. Vermonken, University Hospital, Antwerpen, Belgium; C. Chevreau, Centre Claudius Regaud, Toulouse, France; J.M. Kerst, National Cancer Institute, Amsterdam, the Netherlands; H.G. Derigs, Johannes Gutenberg Universität, Mainz, Germany; K. Weigang-Köhler, Klinikum, Nurnberg, Germany; R.T.D. Oliver, St. Bartholomews and the London Hospitals, London, United Kingdom; B. Hennemann, Universität, Regensburg, Germany; B. Duclois, Hospital Universitaire de Strasbourg, France; P. Kerbrat, Centre Eugène Marquis, Rennes, France; G. Gravis, Institute Paoli et Calmettes, Merseilles, France; Lehmann, Universitäts Homburg, Germany; J. Sastre, Hosp Universitario San Carlos, Madrid, Spain; H.J. Schmoll, Martin Luther Universität Halle, Germany; D. Hossfeld, Universitäts Krankenhaus Eppendorf, Germany; W.G. Jones, St. James's University Hospital Leeds, United Kingdom; P.M. Wilkinson, Christie Hospital Manchester, United Kingdom; A. Saenz-Cusi, Hospital Clinico Universitario Lozano Blesa, Zaragoza, Spain; C. Dubois, Cliniques Saint Jean Brussels, Belgium; F. Joly, Centre Régional Francois Baclesse, Caen, France; M. Fickers, Atrium Medical Center Heerlen, the Netherlands; J. Aparicio, Hospital Univistario La Fe, Valencia, Spain; C. Peschel, Technische Universität Muenchen, Germany; C. Bokemeyer, Eberhard Karis Universitaet Teubingen, Germany; R.E. Coleman, Weston Park Hospital Sheffield, United Kingdom; R. Persad, Bristol Oncology Center, United Kingdom; M. Sokal (United Kingdom Chief Investigator), Nottingham General/City Hospital, United Kingdom. Participation of the United Kingdom sites was coordinated by the MRC Clinical Trials Unit, London (S. Stenning, P. Pollock).
Table A1.
Protocol Guidelines for Hematologic Toxicity: BEP Dose Modifications (% of starting dose) for Etoposide (VP-16) and CDDP
| WBC × 109/L | Platelets × 109/L |
|||||||
|---|---|---|---|---|---|---|---|---|
| ≥ 100 |
75-99 |
50-74 |
< 50 |
|||||
| VP-16 | DDP | VP-16 | DDP | VP-16 | DDP | VP-16 | DDP | |
| ≥ 2.0 | 100 | 100 | 75 | 100 | 50 | 100 | Delay 4 days | |
| 1.5-1.99 | 75 | 100 | 50 | 100 | 50 | 75 | Delay 4 days | |
| < 1.5 | Delay 4 days | Delay 4 days | Delay 4 days | Delay 4 days | ||||
NOTE. If at the start of a treatment cycle, WBC count has not recovered to ≥ 1.5 × 109/L, and no such recovery was observed in patients treated with secondary prophylaxis with granulocyte colony-stimulating factor, treatment is delayed for 4 days. The same applies if platelets are < 50 × 109/L at the start of a treatment cycle. If WBCs have recovered to between 1.5 × 109/L and 2.0 × 109/L and platelets are between 50 × 109/L and 100 × 109/L, use the scheme shown in Appendix Table A1 for dose modifications in this cycle. Decisions for dose modifications in a given cycle should be made on the values obtained at the retreatment day (ie, dose modifications made in previous cycles should not be maintained). In the treatment of complicated neutropenia and/or to avoid dose delay and/or dose reductions in subsequent cycles, the use of granulocyte colony-stimulating factor is strongly advised. However, in case of complicated neutropenia (ie, grade 3 and 4 infections, despite secondary granulocyte colony-stimulating factor prophylaxis) or neutropenia grade 4 lasting > 7 days despite granulocyte colony-stimulating factor or grade 4 thrombocytopenia that last > 3 days or requiring platelet transfusions, dose modifications should be maintained in all remaining cycles. No dose reduction should be made for myelosuppression during a given treatment cycle. In addition, no dose modification should be made on the basis of the nadir of a previous cycle.
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; CDDP, cisplatin; DDP, diamminedichloroplatinum.
Table A2.
Protocol guidelines for Hematologic Toxicity: T-BEP Dose Modifications (% of starting dose) for Paclitaxel, Etoposide (VP-16), and Cisplatin
| WBC × 109/L | Platelets × 109/L |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥ 100 |
75-99 |
50-74 |
< 50 |
|||||||||
| Paclitaxel | VP-16 | DDP | Paclitaxel | VP-16 | DDP | Paclitaxeli | VP-16 | DDP | Paclitaxel | VP-16 | DDP | |
| ≥ 2.0 | 100 | 100 | 100 | 75 | 75 | 100 | 0 | 50 | 100 | Delay 4 days | ||
| 1.5-1.99 | 50 | 75 | 100 | 50 | 50 | 100 | 0 | 50 | 75 | Delay 4 days | ||
| < 1.5 | Delay 4 days | Delay 4 days | Delay 4 days | Delay 4 days | ||||||||
NOTE. If at the start of a treatment cycle, the WBC count has not recovered to ≥ 1.5 × 109/L and such recovery was not observed at the time of cessation of granulocyte colony-stimulating factor, treatment is delayed for 4 days. The same applies if the start of a treatment cycle platelets are < 50. If WBCs have recovered to between 1.5 × 109/L and 2.0 × 109/L at any time during the past week and platelets are between 50 × 109/L and 100 × 109/L, use the scheme shown in Appendix Table A2 for dose modifications in this cycle. Decisions for dose modifications in a given cycle should be made on values obtained at that occasion (ie, dose modifications made in previous cycles should not be maintained). However, in case of complicated neutropenia (ie, grade 3 and 4 infections) or neutropenia grade 4 that lasts > 7 days or grade 4 thrombocytopenia that lasts > 3 days or requires platelet transfusions, dose modifications should be maintained in all remaining cycles.
Abbreviations: DDP, diamminedichloroplatinum; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Table A3.
DI and RDI in Safety and Per-Protocol Populations
| DI and RDI | Treatment |
|
|---|---|---|
| BEP | T-BEP | |
| Safety population | ||
| No. of patients | 161 | 161 |
| DI | ||
| CDDP, mg/m2/week | ||
| Median | 32.7 | 32.6 |
| Range | 8.1-35.6 | 6.4-35.6 |
| Q1-Q3 | 31.6-32.9 | 31.4-33.0 |
| VP-16, mg/m2/week | ||
| Median | 162.7 | 163.6 |
| Range | 40.7-186.4 | 31.8-187.7 |
| Q1-Q3 | 154.2-164.7 | 157.4-164.7 |
| BLM, mg/wk | ||
| Median | 29.4 | 29.3 |
| Range | 9.5-30.7 | 9.5-30.7 |
| Q1-Q3 | 27.6-29.6 | 27.2-29.6 |
| Taxol, mg/m2/week | ||
| Median | 57.3 | |
| Range | 13.6-61.0 | |
| Q1-Q3 | 55.0-57.7 | |
| RDI | ||
| CDDP | ||
| Median, % | 98.2 | 97.9 |
| Range, % | 24.4-106.8 | 19.3-106.8 |
| Q1-Q3, % | 94.7-98.8 | 94.3-99.0 |
| No. of patients with ≥ 80% RDI | 154 | 153 |
| % | 95.7 | 95.0 |
| No. of patients with ≥ 90% RDI | 143 | 143 |
| % | 88.8 | 88.8 |
| VP-16 | ||
| Median, % | 97.6 | 98.1 |
| Range, % | 24.4-111.8 | 19.1-112.6 |
| Q1-Q3, % | 92.5-98.8 | 94.4-98.8 |
| No. of patients with ≥ 80% RDI | 152 | 150 |
| % | 94.4 | 93.2 |
| No. of patients with ≥ 90% RDI | 134 | 139 |
| % | 83.2 | 86.3 |
| BLM | ||
| Median, % | 97.9 | 97.7 |
| Range, % | 31.8-102.4 | 31.8-102.4 |
| Q1-Q3, % | 92.0-98.8 | 90.6-98.8 |
| No. of patients with ≥ 80% RDI | 144 | 134 |
| % | 89.4 | 83.2 |
| No. of patients with ≥ 90% RDI | 132 | 122 |
| % | 82.0 | 75.8 |
| Taxol | ||
| Median, % | 98.2 | |
| Range, % | 23.4-104.6 | |
| Q1-Q3, % | 94.3-98.9 | |
| No. of patients with ≥ 80% RDI | 141 | |
| % | 87.6 | |
| No. of patients with ≥ 90% RDI | 133 | |
| % | 82.6 | |
| Per-protocol population | ||
| No. of patients | 152 | 148 |
| DI | ||
| CDDP, mg/m2/week | ||
| Median | 32.7 | 32.7 |
| Range | 8.1-35.6 | 6.4-35.6 |
| Q1-Q3 | 31.6-32.9 | 31.5-33.0 |
| VP-16, mg/m2/week | ||
| Median | 162.8 | 163.8 |
| Range | 40.7-186.4 | 31.8-187.7 |
| Q1-Q3 | 154.2-164.7 | 158.4-164.7 |
| BLM, mg/wk | ||
| Median | 29.6 | 29.3 |
| Range | 9.5-30.7 | 9.8-30.7 |
| Q1-Q3 | 27.5-29.6 | 27.2-29.6 |
| Taxol, mg/m2/week | ||
| Median | 0.0 | 57.4 |
| Range | 0.0-8.2 | 13.6-61.0 |
| Q1-Q3 | 0.0-0.0 | 55.2-57.8 |
| RDI | ||
| CDDP | ||
| Median, % | 98.2 | 98.0 |
| Range, % | 24.4-106.8 | 19.3-106.8 |
| Q1-Q3, % | 94.9-98.8 | 94.6-99.1 |
| No. of patients with ≥ 80% RDI | 146 | 142 |
| % | 96.1 | 95.9 |
| No. of patients with ≥ 90% RDI | 135 | 134 |
| % | 88.8 | 90.5 |
| VP-16 | ||
| Median, % | 97.7 | 98.3 |
| Range, % | 24.4-111.8 | 19.1-112.6 |
| Q1-Q3, % | 92.5-98.8 | 95.0-98.8 |
| No. of patients with ≥ 80% RDI | 144 | 139 |
| % | 94.7 | 93.9 |
| No. of patients with ≥ 90% RDI | 126 | 130 |
| % | 82.9 | 87.8 |
| BLM | ||
| Median, % | 98.8 | 97.7 |
| Range, % | 31.8-102.4 | 32.6-102.4 |
| Q1-Q3, % | 91.7-98.8 | 90.6-98.8 |
| No. of patients with ≥ 80% RDI | 136 | 125 |
| % | 89.5 | 84.5 |
| No. of patients with ≥ 90% RDI | 124 | 115 |
| % | 81.6 | 77.7 |
| Taxol | ||
| Median, % | 98.4 | |
| Range, % | 23.4-104.6 | |
| Q1-Q3, % | 94.7-99.0 | |
| No. of patients with ≥ 80% RDI | 130 | |
| % | 87.9 | |
| No. of patients with ≥ 90% RDI | 124 | |
| % | 83.8 | |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; BLM, bleomycin; CDDP, cisplatin; DI, dose intensity; Q, quartile; RDI, relative dose intensity; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Table A4.
Average RDI in Safety and Per-Protocol Populations
| Average RDI | Treatment |
|
|---|---|---|
| BEP | T-BEP | |
| Safety population | ||
| No. of patients | 161 | 161 |
| Average RDI for BEP component | ||
| Median, % | 97.1 | 97.6 |
| Range, % | 48.8-103.3 | 19.2-103.5 |
| Q1-Q3, % | 92.2-98.8 | 91.9-98.8 |
| No. of patients with RDI ≥ 80% | 154 | 150 |
| % | 95.7 | 93.2 |
| No. of patients with RDI ≥ 90% | 134 | 124 |
| % | 83.2 | 77.0 |
| Average RDI for T-BEP | ||
| Median, % | 97.5 | |
| Range, % | 44.1-102.8 | |
| Q1-Q3, % | 90.3-98.8 | |
| No. of patients with RDI ≥ 80% | 149 | |
| % | 92.5 | |
| No. of patients with RDI ≥ 90% | 121 | |
| % | 75.2 | |
| Per-protocol population | ||
| No. of patients | 152 | 148 |
| Average RDI for BEP component | ||
| Median, % | 97.4 | 97.7 |
| Range, % | 48.8-103.3 | 19.2-103.5 |
| Q1-Q3, % | 92.1-98.8 | 92.3-98.8 |
| No. of patients with RDI ≥ 80% | 146 | 139 |
| % | 96.1 | 93.9 |
| No. of patients with RDI ≥ 90% | 126 | 117 |
| % | 82.9 | 79.1 |
| Average RDI for T-BEP | ||
| Median, % | 97.7 | |
| Range, % | 44.1-102.5 | |
| Q1-Q3, % | 92.1-98.8 | |
| No. of patients with RDI ≥ 80% | 138 | |
| % | 93.2 | |
| No. of patients with RDI ≥ 90% | 114 | |
| % | 77.0 | |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; Q, quartile; RDI, relative dose intensity; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Table A5.
Residual Masses at the End of Treatment
| Residual Masses at the End of Chemotherapy | Treatment |
Total (N = 337) |
||||
|---|---|---|---|---|---|---|
| BEP (n = 169) |
T-BEP (n = 168) |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| No residual masses | 22 | 13.0 | 14 | 8.3 | 36 | 10.7 |
| Residual masses | 134 | 79.3 | 135 | 80.4 | 269 | 79.8 |
| Residual masses in nonseminoma or mixed seminoma/nonseminoma patients | 123 | 126 | 249 | |||
| No response form | 13 | 7.7 | 19 | 11.3 | 32 | 9.5 |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Table A6.
Surgery for Residual Masses if Residual Masses in Patient With Nonseminoma
| Surgery for Residual Masses | Treatment |
Total (N = 249) |
||||
|---|---|---|---|---|---|---|
| BEP (n = 123) |
T-BEP (n = 126) |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| No residual masses resected | 22 | 17.9 | 21 | 16.7 | 43 | 17.3 |
| Resection of lesions but at least one remaining | 28 | 22.8 | 31 | 24.6 | 59 | 23.7 |
| At least one lesion not completely resected | 6 | 4.9 | 3 | 2.4 | 9 | 3.6 |
| Complete resection of all masses | 60 | 48.8 | 64 | 50.8 | 124 | 49.8 |
| Unknown | 7 | 5.7 | 7 | 5.6) | 14 | 5.6 |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Table A7.
Histology if Surgery for Residual Masses in Patients With Nonseminoma
| Worst Histology of Resected Specimens | Treatment |
Total (N = 235) |
||||
|---|---|---|---|---|---|---|
| BEP (n = 116) |
T-BEP (n = 119) |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Normal | 6 | 5.2 | 3 | 2.5 | 9 | 3.8 |
| Necrosis/fibrosis | 39 | 33.6 | 41 | 34.5 | 80 | 34.0 |
| Diff.teratoma | 26 | 22.4 | 39 | 32.8 | 65 | 27.7 |
| Viable mal.teratoma | 15 | 12.9 | 9 | 7.6 | 24 | 10.2 |
| Non–germ cell cancer | 2 | 1.7 | 0 | 0.0 | 2 | 0.9 |
| Other | 6 | 5.2 | 7 | 5.9 | 13 | 5.5 |
| Missing | 22 | 19.0 | 20 | 16.8 | 42 | 17.9 |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; Diff, differentiated; Mal, malignant; T-BEP, paclitaxel–bleomycin, etoposide, and cisplatin.
Footnotes
See accompanying editorial on page 769
Supported by Grant No. 2U10 CA011488-28 through 2U10 CA011488-41 from the National Cancer Institute and a donation from the “Kankerbestrijding/Koningin Wilhelmina Foundation,” the Netherlands, through the European Organisation for Research and Treatment of Cancer (EORTC) Charitable Trust; the United Kingdom National Institute for Health Research through the National Cancer Research Network (for United Kingdom sites); the Medical Research Council (MRC) through the MRC Clinical Trials Unit (for coordination of United Kingdom sites); an unrestricted educational grant and free supply of the experimental drug by Bristol-Myers Squibb; and an unrestricted educational grant from Amgen (for activities of the EORTC Genitourinary Group).
The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003643.
Affiliations
Ronald de Wit, Erasmus University Medical Center and Daniel den Hoed Cancer Center, Rotterdam; Alfred J. Witjes, Radboud University Hospital, Nijmegen, the Netherlands; Iwona Skoneczna, Marie Sklodowska–Curie Memorial Cancer Center, Warsaw, Poland; Gedske Daugaard, Rigshospital, Copenhagen, Denmark; Maria De Santis, Ludwig Boltzmann–Institute for Applied Cancer Research Vienna and Applied Cancer Research–Institution for Translational Research Vienna/Kaiser Franz Josef-Spital, Vienna, Austria; August Garin, Cancer Research Center, Moscow, Russia; Nina Aass, Oslo University Hospital and University of Oslo, Oslo, Norway; Peter Albers, Heinrich-Heine-University, Dusseldorf, Germany; Jeffery D. White, Glasgow–Beatson West of Scotland Cancer Centre, Glasgow, United Kingdom; José R. Germa-Lluch, Bellvitge Institute for Biomedical Research, Institut Catala d'Oncologia, Barcelona, Spain; and Sandrine Marreaud and Laurence Collette, European Organisation for Research and Treatment of Cancer Headquarters, Brussels, Belgium.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Ronald de Wit, Gedske Daugaard, Nina Aass, José R. Germa-Lluch, Laurence Collette
Financial support: Ronald de Wit
Provision of study materials or patients: Ronald de Wit, Iwona Skoneczna, Gedske Daugaard, Maria De Santis, August Garin, Nina Aass, Alfred J. Witjes, Peter Albers, Jeffery D. White, José R. Germa-Lluch, Sandrine Marreaud
Collection and assembly of data: Ronald de Wit, Alfred J. Witjes, Peter Albers
Data analysis and interpretation: Ronald de Wit, Iwona Skoneczna, Gedske Daugaard, Maria De Santis, August Garin, Nina Aass, Peter Albers, Jeffery D. White, José R. Germa-Lluch, Sandrine Marreaud, Laurence Collette
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Einhorn LH. Treatment of testicular cancer: A new and improved model. J Clin Oncol. 1990;8:1777–1781. doi: 10.1200/JCO.1990.8.11.1777. [DOI] [PubMed] [Google Scholar]
- 2.International Germ Cell Cancer Collaborative Group (IGCCCG) International Germ Cell Consensus Classification: A prognostic-factor based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 3.Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684. doi: 10.1001/jama.299.6.672. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Nichols CJ, Margolin KA, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumor. J Clin Oncol. 2007;25:247–256. doi: 10.1200/JCO.2005.05.4528. [DOI] [PubMed] [Google Scholar]
- 5.Daugaard G, Skoneczna I, Aass N, et al. A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974) Ann Oncol. 2011;22:1054–1061. doi: 10.1093/annonc/mdq575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Bajorin DF, Schwartz LH, et al. Phase II trial of paclitaxel shows antitumor activity in patients with previously treated germ cell tumor. J Clin Oncol. 1994;12:2277–2283. doi: 10.1200/JCO.1994.12.11.2277. [DOI] [PubMed] [Google Scholar]
- 7.Bokemeyer C, Beyer J, Metzner B, et al. Phase II study of paclitaxel in patients with relapsed or cisplatin-refractory testicular cancer. Ann Oncol. 1996;7:31–44. doi: 10.1093/oxfordjournals.annonc.a010473. [DOI] [PubMed] [Google Scholar]
- 8.de Wit R, Louwerens M, de Mulder PH, et al. Management of intermediate-prognosis germ-cell cancer: Results of a phase I/II study of Taxol-BEP. Int J Cancer. 1999;10:831–833. doi: 10.1002/(sici)1097-0215(19991210)83:6<831::aid-ijc24>3.0.co;2-o. 83. [DOI] [PubMed] [Google Scholar]
- 9.Freedman LS, White SJ. On the use of pocock and simon's method for balancing treatment numbers over prognostic factors in the controlled clinical trial. Biometrics. 1976;32:691–694. [PubMed] [Google Scholar]
- 10.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 11.Hwang IK, Shih WJ, De Cani JS. Group sequential designs using a family of type i error probability spending functions. Stat Med. 1990;9:1439–1445. doi: 10.1002/sim.4780091207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.