Abstract
Purpose
In 2002, pegfilgrastim was approved by the US Food and Drug Administration and the benefits of dose-dense breast cancer chemotherapy, especially for hormone receptor (HR) –negative tumors, were reported. We examined first-cycle colony-stimulating factor use (FC-CSF) before and after 2002 and estimated US expenditures for dose-dense chemotherapy.
Methods
We identified patients in Surveillance, Epidemiology, and End Results–Medicare greater than 65 years old with stages I to III breast cancer who had greater than one chemotherapy claim within 6 months of diagnosis(1998 to 2005) and classified patients with an average cycle length less than 21 days as having received dose-dense chemotherapy. The associations of patient, tumor, and physician-related factors with the receipt of any colony-stimulating factor (CSF) and FC-CSF use were analyzed by using generalized estimating equations. CSF costs were estimated for patients who were undergoing dose-dense chemotherapy.
Results
Among the 10,773 patients identified, 5,266 patients (48.9%) had a CSF claim. CSF use was stable between 1998 and 2002 and increased from 36.8% to 73.7% between 2002 and 2005, FC-CSF use increased from 13.2% to 67.9%, and pegfilgrastim use increased from 4.1% to 83.6%. In a multivariable analysis, CSF use was associated with age and chemotherapy type and negatively associated with black/Hispanic race, rural residence, and shorter chemotherapy duration. FC-CSF use was associated with high socioeconomic status but not with age or race/ethnicity. The US annual CSF expenditure for women with HR-positive tumors treated with dose-dense chemotherapy is estimated to be $38.8 million.
Conclusion
A rapid increase in FC-CSF use occurred over a short period of time, which was likely a result of the reported benefits of dose-dense chemotherapy and the ease of pegfilgrastim administration. Because of the increasing evidence that elderly HR-positive patients do not benefit from dose-dense chemotherapy, limiting pegfilgrastim use would combat the increasing costs of cancer care.
INTRODUCTION
In 2002, several events occurred that changed the way in which the adjuvant chemotherapy of breast cancer was administered. The first event was an oral presentation from the Cancer and Leukemia Group B (CALGB; trial 9741) that showed a statistically significant benefit in disease-free (risk ratio, 0.74; 95% CI, 0.59 to 0.93) and overall survival (risk ratio, 0.69; 95% CI, 0.50 to 0.93) for dose-dense chemotherapy with growth-factor (filgrastim) support in women with node-positive early-stage breast cancer, followed by a published article in 2003.1 Although subsequent studies, including a meta-analysis, have confirmed this finding,2–4 the survival benefits appeared to be minimal in patients with hormone receptor (HR) –positive tumors.4 In support of this observation, a reanalysis of several large CALGB adjuvant trials in 2006 found a nonsignificant absolute 2% difference at 5 years between the experimental arm and the control arm in women with HR-positive tumors enrolled onto trial 9741.5 This result is not surprising given that, compared with no chemotherapy, the proportional reduction in breast cancer mortality from any chemotherapy in women greater than 50 year of age is smaller for HR-positive compared with HR-negative patients (11% v 26%).6
The second event was the approval by the US Food and Drug Administration of pegfilgrastim for the prevention of chemotherapy-related infection as manifested by neutropenic fever.7–9 Before the use of pegfilgrastim, dose-dense chemotherapy was given with filgrastim, which required 10 days of subcutaneous injection. In these studies,7–9 one dose of pegfilgrastim (cost, $2,200) per chemotherapy cycle was comparable with the 10 daily injections of filgrastim with regard to the febrile neutropenia (FN) rate, duration of neutropenia, and depth of the absolute neutrophil count. Pegfilgrastim is also safe and logistically easier for widespread use by patients. After the approval of pegfilgrastim, dose-dense therapy could be given with a single injection of growth factor administered the day after treatment. Although guidelines from the American Society of Clinical Oncology and the European Organisation for Research and Treatment of Cancer recommended the use of colony-stimulating factor (CSF) in the first cycle of chemotherapy for treatments in which the risk of FN is greater than 20%,10,11a recent study reported that the uptake of primary prophylaxis in patients with lung and colon cancer treated with high-risk chemotherapy regimens was only 17%.12 In CALGB trial 9741, the FN was 6% without CSF support and 2% with CSF support. Therefore, this regimen did not meet the 20% guidelines for primary prophylaxis.
Although cost-effectiveness analyses have shown a benefit of pegfilgrastim for primary compared with secondary prevention of FN with high-risk regimens,13 less is known about its use for the adjuvant treatment of breast cancer when the risk of FN is lower. Therefore, we performed a population-based study in elderly women with breast cancer to determine patterns of use of CSF in the US before and after 2002. We examined patient and physician characteristics associated with any CSF use and with first-cycle use. We also estimated the costs spent on first-cycle CSF (FC-CSF).4
METHODS
Data Source
We analyzed data from the Surveillance, Epidemiology, and End Results (SEER) –Medicare database.14 SEER provides information on tumor histology, location, stage of disease, treatment, and survival, along with demographic and selected census tract-level information. The Medicare database includes Medicare A (inpatient) and B (outpatient) eligibility status, billed claims, and diagnoses. These two files are linked and provide the ability to define a population-based cohort and determine which patients have been treated with CSF and the dates of service.
Cohort Selection
We identified all individuals who were age 65 years or older, had a pathologically confirmed primary diagnosis of breast cancer (International Classification of Disease, Ninth Revision [ICD-9] codes 800, 801, 802, 805, 814, 821, 823, 826, 848-854, 856, or 857) from January 1, 1998, through December 31, 2005, and who were treated with chemotherapy within 6-months of diagnosis. We excluded patients who were enrolled onto a non-Medicare health-maintenance organization because the billing claims for these patients are not submitted to Medicare for reimbursement. Patients who were enrolled onto Medicare because of end-stage renal disease and dialysis rather than age and patients with previous primary cancers were also excluded. Age at diagnosis was categorized into 5-year intervals starting at age 65 years. The SEER marital status variable was categorized as married, not married, and unknown.
Socioeconomic Status Score
We generated an aggregate socioeconomic status score from education, poverty level, and income data from the 2000 census tract data as described by Du et al15 Scores of patients were ranked on a scale of 1 to 5 by using a formula that incorporated education, poverty, and income weighted equally, with a score of 1 as the lowest value.
Assessment of Comorbid Disease
To assess the prevalence of comorbid disease in our cohort, we used the Klabunde adaptation of the Charlson comorbidity index (ie, the Klabunde-Charlson index).16,17 Medicare inpatient and outpatient claims were searched for diagnostic codes of the ICD-9 Clinical Modification. Each condition was weighted, and patients were assigned a score on the basis of the Klabunde-Charlson index.17
Physician Characteristics
We matched the treating physician to the CSF claim by using the unique physician identifier number (UPIN) and linking it to the UPIN on file with the American Medical Association. Self-reported primary and secondary specialty codes were defined by oncologist, hematologist, hematologist/oncologist, radiation oncologist, or surgical oncologist. On the basis of the variables in the American Medical Association Masterfile, characteristics of the oncologists that were analyzed included sex, year of graduation, primary employment setting (private v government or academic), location of training (United States v other), and type of degree (Medical Degree v Doctor of Osteopathic Medicine). Practice volumes of physicians (ie, the total number of claims for CSF in the cohort) were analyzed. Physicians with approximately the highest quartile of patients were considered as having high volume, and the cohort was dichotomized at the median as one to nine or 10 or more patients accordingly. We hypothesized that these factors might influence treatment patterns on the basis of previous studies that suggested training and practice environment of the physician influences the use of therapy; however, these were secondary objectives.12,18–20
Treatment Characteristics
We extracted information on chemotherapy from Medicare files by searching the Level II Healthcare Common Procedure Coding System, Current Procedural Terminology codes, ICD-9 Clinical Modification diagnostic codes and procedure codes, the diagnostic-related group code, and the center code from physician claims files, hospital outpatient claims files, or Medicare provider review files. Chemotherapy type was categorized into the following four groups: anthracycline (J9000, J9001, J9178, J9180, and J9211), taxane (J9264, J9265, and J9170), both anthracycline and taxane (A+T), and other (including J9999). We removed patients with chemotherapy codes that were not typical for adjuvant breast cancer treatment. We also classified patients with no lymph node involvement, one to three involved lymph nodes, and greater than three positive lymph nodes for some of the stratified analyses. Chemotherapy duration was categorized as 1 to 13, 14 to 26, and greater than 26 weeks. We searched for Level II Healthcare Common Procedure Coding System codes that corresponded to filgrastim (J1440 and J1441), sargramostim (J2820), and pegfilgrastim (J2505 and Q4053). We excluded patients who received their first CSF before they received chemotherapy. The use of CSF was categorized as a claim at any point in the first year of diagnosis, the use of FC-CSF was categorized as a claim date between the first and second chemotherapy claim dates, and patients who used a combination of FC-CSF, treatment with A+T, and with an average cycle length less than 21 days as having received dose-dense therapy. We calculated the ratio of CSF claims to chemotherapy cycles. Patients with FN were classified with ICD-9 code 780.6 (fever), of which 96% of patients were hospitalized and/or had an additional code for neutropenia (288.0).
Statistical Analysis
Any CSF use was compared with no use of CSF, and FC-CSF use was compared with no FC-CSF use by using χ2 tests and univariate regression with respect to clinical and demographic variables. We used generalized estimating equations to account for correlations of outcome measures among patients who had the same physician. The unit of analysis was the patient. For each patient, the unique UPIN number of the physician was used as the clustering variable. All analyses were conducted with SAS software (version 9.13; SAS Institute, Cary, NC). All statistical tests were two sided.
CSF costs were estimated by using the published 2005 Medicare average sale price of pegfilgrastim. Total annual costs for elderly women with hormone sensitive cancers were calculated by assuming that 4% of HR-positive breast cancer patients received dose-dense therapy. Sensitivity analyses were done by assuming that 6% and 8% of patients received dose-dense chemotherapy (Appendix Table A1, online only).
RESULTS
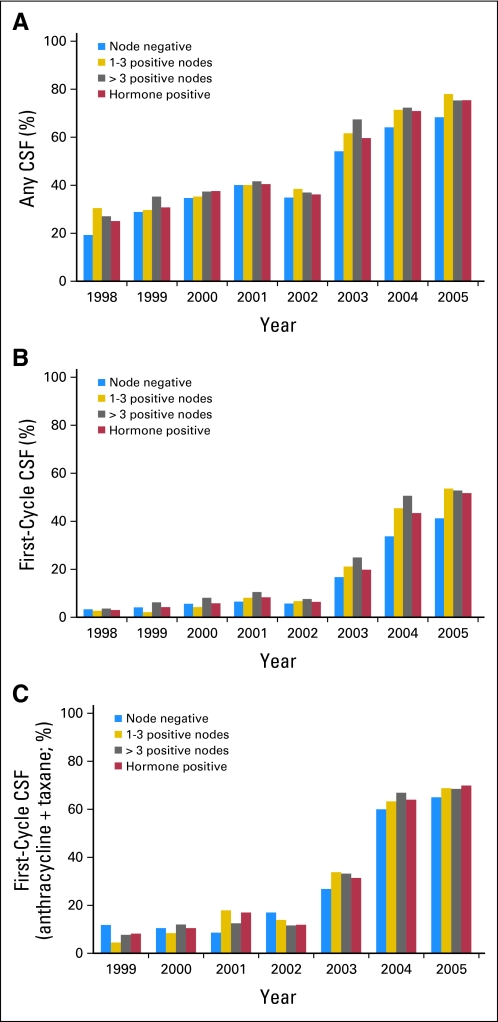
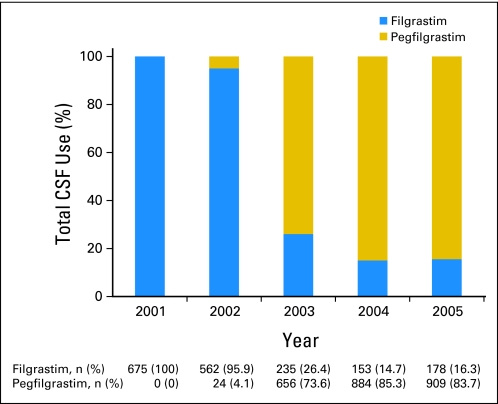
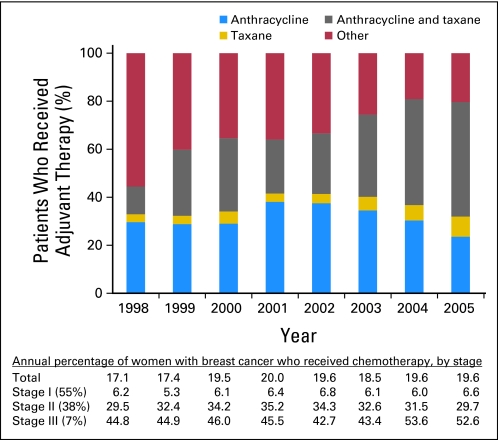
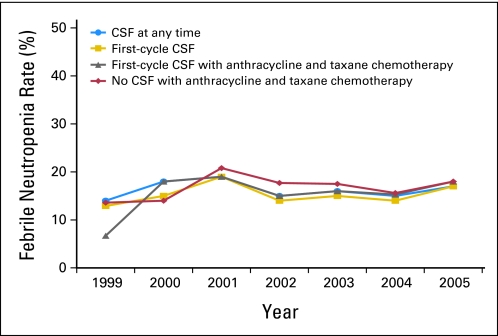
We identified a total of 10,773 women who received adjuvant chemotherapy (20% of the total sample). Of these women, 5,266 patients (48.9%) received CSF, of whom 2,056 patients (19.1%) received CSF with the first cycle of chemotherapy. During the years encompassed by the study (1998 to 2005), CSF use increased from 25.3% to 73.7%, and the most dramatic increase started in 2003 (Fig 1). Similarly, FC-CSF use increased from 6.4% to 46.9% during this time frame, and among women whose adjuvant chemotherapy included A+T, FC-CSF increased from 8.0% to 67.9%. There was little difference in annual rates of CSF use when evaluated by hormone-receptor status and by lymph node involvement (zero, one to three, and > three involved lymph nodes). By 2005, of those who were receiving CSF, 84% were receiving PEG-CSF (Fig 2). Of those who were receiving adjuvant chemotherapy, 48.8% were receiving A+T (Fig 3), 50% of whom had an average cycle length of less than 21 days. The receipt of A+T was similar between hormone receptor–positive and –negative patients (49.3% v 47.8%); however, patients with lymph node–positive cancer had a higher likelihood of receiving A+T (62%) than patients who were lymph node negative (30%). These data were used to inform the cost analysis. The annual rate of FN ranged from 13% to 20% and did not differ over time in patients who did and did not receive CSF (Fig 4). Approximately 50% of patients received CSF with each cycle, and approximately 75% of patients had a ratio of the number of CSF cycles to the number of chemotherapy cycles of 75% or greater.
Fig 1.
Adjuvant colony-stimulating factor (CSF) use over time: (A) CSF at any time during adjuvant chemotherapy; (B) CSF with first cycle of adjuvant chemotherapy; and (C) CSF with first cycle of combination anthracycline and taxane chemotherapy.
Fig 2.
Increase in use of pegfilgrastim after US Food and Drug Administration approval. CSF, colony-stimulating factor.
Fig 3.
Change in adjuvant chemotherapy regimens over time in elderly women with breast cancer.
Fig 4.
Febrile neutropenia rates over time with colony-stimulating factor (CSF) use at any time during adjuvant chemotherapy, with the first cycle of any adjuvant chemotherapy, with the first cycle of combination anthracycline and taxane chemotherapy, and without CSF.
Demographic, clinical, and physician characteristics associated with CSF use are listed in Table 1. Clinical factors associated with any CSF use included older age, white race, treatment in later years of the study, metropolitan area of residence, being married, having a higher socioeconomic status, comorbidity, and a lower stage of disease, chemotherapy type, and duration of chemotherapy. Physician factors associated with CSF use were a location of training outside the United States, female sex, and having a Doctor of Osteopathic Medicine as opposed to a Medical Degree (P < .05 for all). In our multivariable model, the only factors that remained associated with decreased CSF use were younger age, black race (odds ratio [OR], 0.80; 95% CI, 0.68 to 0.95), Hispanic ethnicity (OR, 0.68; 95% CI, 0.48 to 0.96), nonmetropolitan residence, chemotherapy type, and shorter duration of chemotherapy (Table 2). Physician characteristics associated with less CSF use included training in the United States, whereas treatment by an oncologist was associated with increased CFS use.
Table 1.
Baseline Demographics and Clinical Characteristics of the Cohort
| Patient Demographics and Clinical Characteristics | No. of Patients (N = 10,773) | Any CSF v No CSF (n = 5,266; 48.9%) |
First-Cycle CSF v No CSF (n = 2,056; 19.1%) |
||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age at diagnosis, years | |||||
| 65-69 | 4,030 | 2,009 | 49.8* | 859 | 21.3* |
| 70-74 | 3,696 | 1,844 | 49.9 | 675 | 18.3 |
| 75-79 | 2,193 | 1,073 | 48.9 | 400 | 18.2 |
| ≥ 80 | 854 | 340 | 39.8 | 122 | 14.3 |
| Race | |||||
| White | 9,322 | 4,594 | 49.3* | 1,789 | 19.2 |
| Black | 828 | 357 | 43.1 | 147 | 17.8 |
| Hispanic | 143 | 57 | 39.9 | 21 | 14.7 |
| Missing or other | 480 | 258 | 53.7 | 102 | 21.2 |
| Residence | |||||
| Urban | 9,802 | 4,864 | 49.6* | 1,904 | 19.4* |
| Rural | 971 | 402 | 41.4 | 152 | 15.6 |
| Marital status | |||||
| Married | 5,731 | 2,864 | 50.0* | 1,130 | 19.7 |
| Unmarried | 4,733 | 2,247 | 47.5 | 862 | 18.2 |
| Unknown | 309 | 155 | 50.2 | 64 | 20.7 |
| Socioeconomic status | |||||
| Lowest (first) quintile | 1,358 | 634 | 46.7* | 237 | 17.4* |
| Second quintile | 2,067 | 961 | 46.5 | 339 | 16.4 |
| Third quintile | 2,371 | 1,169 | 49.3 | 467 | 19.7 |
| Fourth quintile | 2,413 | 1,190 | 49.3 | 467 | 19.4 |
| Highest (fifth) quintile | 2,559 | 1,308 | 51.1 | 545 | 21.3 |
| Comorbidity score | |||||
| 0 | 8,689 | 4,284 | 49.3* | 1,657 | 19.1 |
| 1 | 1,604 | 772 | 48.1 | 306 | 19.1 |
| > 1 | 480 | 210 | 43.7 | 93 | 19.4 |
| Tumor stage | |||||
| I | 1,966 | 864 | 43.9* | 294 | 15.0* |
| II | 6,683 | 3,190 | 47.7 | 1,133 | 17.0 |
| III | 2,124 | 1,212 | 57.1 | 629 | 29.6 |
| Tumor grade | |||||
| High | 4,880 | 2,425 | 49.7 | 923 | 18.9 |
| Low | 5,061 | 2,457 | 48.5 | 993 | 19.6 |
| Unknown | 832 | 384 | 46.1 | 140 | 16.8 |
| Chemotherapy type | |||||
| Taxane + anthracycline | 3,433 | 2,338 | 68.1* | 1,254 | 36.5* |
| Anthracycline | 3,436 | 1,751 | 50.9 | 594 | 17.3 |
| Taxane | 558 | 185 | 33.1 | 75 | 13.4 |
| Other | 3,346 | 992 | 30.0 | 133 | 3.9 |
| Chemotherapy duration, weeks | |||||
| 1-13 | 3,492 | 1,518 | 43.5* | 605 | 17.3* |
| 14-26 | 5,331 | 2,668 | 50.1 | 1,038 | 19.5 |
| > 26 | 1,950 | 1,080 | 55.4 | 413 | 21.2 |
| Hormone-receptor status | |||||
| Negative | 3,016 | 1,474 | 48.9 | 603 | 20.0* |
| Positive | 6,424 | 3,174 | 49.4 | 1,245 | 19.4 |
| Missing | 1,333 | 618 | 46.4 | 208 | 15.6 |
| Oncologist training | |||||
| Non–United States | 2,860 | 1,604 | 56.1* | 647 | 22.6* |
| United States | 7,561 | 3,503 | 46.3 | 1,353 | 17.9 |
| Oncologist degree | |||||
| DO | 444 | 190 | 42.8* | 83 | 18.7 |
| MD | 9,977 | 4,917 | 49.3 | 1,917 | 19.2 |
| Specialty | |||||
| Nononcologist | 787 | 357 | 45.4* | 142 | 18.0 |
| Oncologist | 9,634 | 4,750 | 49.3 | 1,858 | 19.3 |
| Oncologist sex | |||||
| Male | 8,250 | 3,971 | 48.1* | 1,536 | 18.6* |
| Female | 2,100 | 1,100 | 52.4 | 451 | 21.5 |
| Oncologist year of graduation | |||||
| 1990s | 1,376 | 774 | 56.2* | 182 | 14.8* |
| 1980s | 3,580 | 1,835 | 51.3 | 731 | 17.5 |
| 1970s | 4,168 | 1,903 | 45.7 | 735 | 20.5 |
| 1960s | 1,226 | 559 | 45.6 | 339 | 24.6 |
| Oncologist practice setting | |||||
| Non–private practice | 2,154 | 1,086 | 50.4 | 430 | 20.0 |
| Private practice | 8,196 | 3,985 | 48.6 | 1,557 | 19.0 |
| Volume | |||||
| 1-9 cases | 5,584 | 2,732 | 48.9 | 1,083 | 19.4 |
| ≥ 10 cases | 4,920 | 2,421 | 49.2 | 934 | 19.0 |
Abbreviations: CSF, colony-stimulating factor; DO, Doctor of Osteopathic Medicine; MD, Doctor of Medicine.
P ≤ .05 (χ2 test).
Table 2.
Multivariable Analysis of Factors Associated With CSF Use Among Women With Breast Cancer Who Received Treatment With Any Adjuvant Chemotherapy
| Any CSF |
First-Cycle CSF |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age at diagnosis, years | ||||
| 65-69 | Referent | Referent | ||
| 70-74 | 1.15 | 1.05 to 1.27* | 0.96 | 0.84 to 1.10 |
| 75-79 | 1.25 | 1.12 to 1.41* | 1.12 | 0.95 to 1.32 |
| ≥ 80 | 1.06 | 0.89 to 1.25 | 1.09 | 0.85 to 1.39 |
| Race | ||||
| White | Referent | Referent | ||
| Black | 0.80 | 0.67 to 0.95* | 0.97 | 0.75 to 1.24 |
| Hispanic | 0.68 | 0.48 to 0.96* | 0.68 | 0.38 to 1.23 |
| Missing or other | 1.03 | 0.82 to 1.29 | 1.04 | 0.78 to 1.38 |
| Residence | ||||
| Metropolitan | Referent | Referent | ||
| Nonmetropolitan | 0.71 | 0.60 to 0.84* | 0.80 | 0.63 to 1.02 |
| Marital status | ||||
| Married | Referent | Referent | ||
| Unmarried | 0.94 | 0.87 to 1.02 | 0.99 | 0.88 to 1.10 |
| Unknown | 1.04 | 0.82 to 1.33 | 1.16 | 0.82 to 1.63 |
| Socioeconomic status | ||||
| Lowest (first) quintile | Referent | Referent | ||
| Second quintile | 0.94 | 0.80 to 1.10 | 0.93 | 0.75 to 1.16 |
| Third quintile | 1.00 | 0.85 to 1.18 | 1.12 | 0.89 to 1.40 |
| Fourth quintile | 0.97 | 0.82 to 1.15 | 1.14 | 0.91 to 1.44 |
| Highest (fifth) quintile | 1.07 | 0.90 to 1.27 | 1.39 | 1.10 to 1.76* |
| Comorbidity score | ||||
| 0 | Referent | Referent | ||
| 1 | 0.92 | 0.82 to 1.03 | 0.98 | 0.83 to 1.16 |
| > 1 | 0.85 | 0.69 to 1.04* | 1.05 | 0.81 to 1.38 |
| Tumor stage | ||||
| I | Referent | Referent | ||
| II/III | 0.98 | 0.88 to 1.10 | 1.03 | 0.87 to 1.22 |
| Tumor grade | ||||
| High | Referent | Referent | ||
| Low | 0.93 | 0.85 to 1.01 | 1.05 | 0.93 to 1.20 |
| Unknown | 0.90 | 0.77 to 1.06 | 0.96 | 0.76 to 1.22 |
| Chemotherapy type | ||||
| Taxane + anthracycline | Referent | Referent | ||
| Anthracycline | 0.73 | 0.64 to 0.83* | 0.41 | 0.36 to 0.48* |
| Taxane | 0.19 | 0.15 to 0.23* | 0.17 | 0.13 to 0.24* |
| Other | 0.25 | 0.22 to 0.29* | 0.09 | 0.07 to 0.11* |
| Chemotherapy duration, weeks | ||||
| 1-13 | 0.69 | 0.61 to 0.77* | 1.19 | 1.00 to 1.42 |
| 14-26 | Referent | Referent | ||
| > 26 | 1.05 | 0.92 to 1.19 | 0.75 | 0.64 to 0.87* |
| Hormone-receptor status | ||||
| Negative | Referent | Referent | ||
| Positive | 1.00 | 0.90 to 1.11 | 1.04 | 0.91 to 1.19 |
| Oncologist training | ||||
| Non–United States | Referent | Referent | ||
| United States | 0.72 | 0.62 to 0.84* | 0.73 | 0.61 to 0.88* |
| Oncologist degree | ||||
| DO | Referent | Referent | ||
| MD | 1.30 | 0.95 to 1.79 | 0.97 | 0.60 to 1.55 |
| Specialty | ||||
| Nononcologist | Referent | Referent | ||
| Oncologist | 1.45 | 1.17 to 1.80* | 1.44 | 1.07 to 1.96* |
| Oncologist sex | ||||
| Male | Referent | Referent | ||
| Female | 1.00 | 0.85 to 1.18 | 0.99 | 0.81 to 1.21 |
| Oncologist year of graduation | ||||
| 1990s | Referent | Referent | ||
| 1980s | 0.92 | 0.75 to 1.13 | 1.16 | 0.92 to 1.47 |
| 1970s | 0.76 | 0.62 to 0.95* | 1.00 | 0.78 to 1.28 |
| 1960s | 0.79 | 0.62 to 1.01 | 0.85 | 0.61 to 1.16 |
| Oncologist practice setting | ||||
| Academic | Referent | Referent | ||
| Private practice | 1.06 | 0.91 to 1.24 | 1.14 | 0.94 to 1.38 |
| Volume | ||||
| 1-9 cases | Referent | Referent | ||
| ≥ 10 cases | 1.14 | 0.96 to 1.30 | 1.05 | 0.88 to 1.26 |
Abbreviations: CSF, colony-stimulating factor; DO, Doctor of Osteopathic Medicine; MD, Doctor of Medicine; OR, odds ratio.
P ≤ .05 (corrected for year of diagnosis).
In the adjusted model, characteristics associated with FC-CSF use included chemotherapy type, socioeconomic status, chemotherapy duration, and treatment by an oncologist (Table 2). Age, race, and ethnicity were not associated with FC-CSF use. Notably, FC-CSF was the same in women with hormone receptor–positive and –negative cancers (P = .96).
For HR-positive patients, for whom we assumed there was no survival benefit, the additional annual costs from pegfilgrastim were estimated at $38.8 million if 4% of all women greater than 65 years of age who are diagnosed with hormone-sensitive breast cancer receive dose-dense chemotherapy. We performed a sensitivity analysis by assuming that the use of chemotherapy and treatment with dose-dense therapy has been increasing in the elderly. If the proportion of patients who receive dose-dense therapy increased to 6% or 8%, the CSF costs would increase to $58.1 and $81.4 million, respectively.
DISCUSSION
Our findings show that the use of CSF increased rapidly in 2003 after the approval of pegfilgrastim by the US Food and Drug Administration and the simultaneous reporting of the benefits of dose-dense therapy. The approval of pegfilgrastim was associated with a dramatic increase in FC-CSF use and a shift from filgrastim/sargramostim to pegfilgrastim. Despite the increase in CSF use, the annual rates of FN remained low and relatively stable during this time frame and did not differ between those who did and did not receive CSF. Furthermore, the use of FC-CSF was not different among patients with and without hormone receptor–positive tumors, despite the apparent lack of survival benefit for dose-dense therapy in women with HR-positive tumors. However, for elderly women with hormone-sensitive tumors, for whom the benefits are minimal, the total annual costs were estimated to be greater than $38 million dollars.
The costs to the medical system are directly proportional to the number of women diagnosed with breast cancer who undergo chemotherapy and, of women who undergo chemotherapy, the proportion of women who undergo dose-dense therapy. This number has been rising rapidly. Although women greater than 65 years of age are less likely to be treated with adjuvant chemotherapy than younger women, women greater than 65 years of age represent approximately 44% of all cancer diagnoses. In a scenario in which only 8% of all women greater than 65 years of age with hormone-sensitive stages I to III breast cancer are treated with dose-dense chemotherapy, the annual CSF costs are approximately $81.4 million per year. If these results were extrapolated to women 50 to 64 years of age, for whom survival benefits are also minimal and of whom a higher proportion of women undergo chemotherapy, the annual CSF costs are estimated to be much higher.
The financial burdens associated with cancer-related medical costs have accelerated at a rate beyond those of other medical treatments.21 One major source of this increase can be attributed to spending on new drugs.22 In 2009, CSF expenditures represented the fifth largest individual Part B drug expenditure by the Centers for Medicare and Medicaid Services (approximately $494 million per year).23 The total US annual expenditures for CSFs are estimated to be approximately $3.5 billion.24 For patients with lung and colon cancer, a recent study reported that 96% of CSFs were administered in scenarios in which CSF therapy was not recommended by evidence-based guidelines.12 Furthermore, our findings, which showed similar annual rates of FN in those with and without CSF, are intriguing and recapitulate the minimal reduction of FN seen in CALGB trial 9741 with the addition of CSF. Total annual expenditures for CSF could be significantly reduced if priority were given to individuals who clearly benefit from treatment.
In addition to cost, there are toxicities associated with CSF use. In clinical trials, 25% of patients reported musculoskeletal pain when they took pegfilgrastim.8 In addition, pegfilgrastim can cause fever, chills, body aches, and flu symptoms that can be confused with a bacterial infection in patients undergoing chemotherapy. More severe reactions can be shortness of breath and allergic reactions. Finally, there are some reports that suggested there may be a slight increased absolute risk of leukemia (in four of 1,000 patients) after a mean duration of approximately 4 years when pegfilgrastim is given along with chemotherapy.25–28
Black race and Hispanic ethnicity were associated with a significant decrease in the use of any CSF with ORs of 0.80 and 0.68, respectively. However, use was not different by race or ethnicity when CSF was given in the first cycle, in which the majority of patients received pegfilgrastim. Studies have suggested that black women are less likely than white women to receive optimal systemic adjuvant therapy.29,30 It is possible that daily injections were seen as more of a barrier for minorities than a single per-cycle injection or that out-of-pocket costs may impact use, but the exact reasons for this decrease remain unknown and may be important for other therapies that require daily injections.
We acknowledge several important limitations of this study. First, it was possible that patients received CSF by prescription and injected the medication themselves, which resulted in misclassification. However, it has been shown that 95% of patients received CSF in the office of a physician as a result of pharmacy coverage issues, and therefore, we do not feel that this possibility would have substantially affected our findings. Some patients may have received FC-CSFs solely for the prevention of FN rather than with dose-dense intent; however, our analysis required an average cycle of less than 21 days for the treatment to be classified as dose-dense, and thus, the misclassification rate was likely low. We did not have the value of the WBC count, and there is no code specific for FN, and thus, FN may have been misclassified; however 78% of patients had fever severe enough to require hospital admission, and 96% of patients had either a code for neutropenia and/or were hospitalized. Not all patients who received FC-CSF received eight cycles of chemotherapy; therefore, our estimates of the costs associated with the treatment may have been biased slightly upward. Finally, the clinical trials may have been underpowered to detect a survival benefit from dose-dense therapy in women with hormone receptor–positive tumors. However, given the small effect sizes reported (hazard ratio, 0.92; 95% CI, 0.75 to 1.12),4 it is unlikely that an increased number of patients or additional follow-up would have resulted in a clinically meaningful difference between groups.
Our study demonstrated a widespread increase in the use of CSFs and, specifically, of first-cycle pegfilgrastim in elderly women who received adjuvant chemotherapy for breast cancer. This four-fold increase came shortly after published reports of the benefits of dose-dense therapy. Although this approach is effective in women with hormone receptor–negative tumors, the approach has come at a great financial cost in women with hormone receptor–positive tumors who are less likely to have a meaningful benefit. Efforts to improve quality and reduce cost, such as the Quality Oncology Practice Initiative of the American Society of Clinical Oncology are leading the way in changing physician behavior. To reduce the total costs of cancer care, efforts should be made to ensure that these treatments are reserved for only those patients who benefit substantially from their use.
Acknowledgment
This study used the linked Surveillance, Epidemiology, and End Results (SEER) –Medicare database. The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services and the Office of Strategic Planning, Health Care Financing Administration; Information Management Services; and the SEER program tumor registries in the creation of the SEER-Medicare database.
Appendix
Table A1.
Methods for Cost Analysis
| Percentage of All Women > 65 Years of Age Diagnosed With Hormone-Sensitive Breast Cancer Who Received Dose-Dense Therapy | Estimated 2005 Costs of Dose-Dense Therapy for Patients With Hormone Receptor–Positive Breast Cancer (millions of US dollars) |
|---|---|
| 4 | 38.8 |
| 6 | 58.1 |
| 8 | 81.4 |
NOTE. Total annual costs in the Unites States for all patients with breast cancer (N = 180,000) and patients with breast cancer greater than 65 years of age (n = 81,000) were estimated by hormone status (66% of patients were hormone positive). To calculate the proportion of patients who received dose-dense therapy, the proportion of patients with breast cancer within Surveillance, Epidemiology, and End Results who received adjuvant therapy (20%) was multiplied by the estimated proportion of those who received adjuvant therapy with anthracycline and taxane (40%), and the estimated proportion of those who received dose-dense therapy (50%). For this calculation, we assumed that 4% of women with hormone receptor–positive breast cancer who were older than age 65 years received dose-dense therapy. Total costs for dose-dense treatment were calculated by multiplying the 2005 Medicare average pegfilgrastim sales prices per dose by 8 doses ($2,274 per dose) and then multiplying that result by the population of patients receiving dose-dense therapy. Because the proportion of women older than 65 years of age who receive chemotherapy is increasing, and the proportion of women who receive dose-dense therapy is increasing, we performed sensitivity analysis by assuming that 6% and 8% of women received dose-dense therapy.
Footnotes
See accompanying editorial on page 772; listen to the podcast by Drs Smith and Hillner at www.jco.org/podcasts
Supported by Grant No. NCI R01CA134964 from the National Cancer Institute (D.L.H.).
The interpretation and reporting of data were the sole responsibility of the authors.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jennifer L. Malin, Amgen (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Dawn L. Hershman, Elizabeth T. Wilde,Alfred I. Neugut
Financial support: Dawn L. Hershman
Administrative support: Dawn L. Hershman, Alfred I. Neugut
Collection and assembly of data: Dawn L. Hershman, Donna L. Buono
Data analysis and interpretation: Dawn L. Hershman, Elizabeth T. Wilde, Jason D. Wright, Donna L. Buono, Kevin Kalinsky, Jennifer L. Malin, Alfred I. Neugut
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 2.Venturini M, Del Mastro L, Aitini E, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: Results from a randomized trial. J Natl Cancer Inst. 2005;97:1724–1733. doi: 10.1093/jnci/dji398. [DOI] [PubMed] [Google Scholar]
- 3.Baldini E, Gardin G, Giannessi PG, et al. Accelerated versus standard cyclophosphamide, epirubicin and 5-fluorouracil or cyclophosphamide, methotrexate and 5-fluorouracil: A randomized phase III trial in locally advanced breast cancer. Ann Oncol. 2003;14:227–232. doi: 10.1093/annonc/mdg069. [DOI] [PubMed] [Google Scholar]
- 4.Bonilla L, Ben-Aharon I, Vidal L, et al. Dose-dense chemotherapy in nonmetastatic breast cancer: A systematic review and meta-analysis of randomized controlled trials. J Natl Cancer Inst. 2010;102:1845–1854. doi: 10.1093/jnci/djq409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA . 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Vose JM, Crump M, Lazarus H, et al. Randomized, multicenter, open-label study of pegfilgrastim compared with daily filgrastim after chemotherapy for lymphoma. J Clin Oncol. 2003;21:514–519. doi: 10.1200/JCO.2003.03.040. [DOI] [PubMed] [Google Scholar]
- 8.Holmes FA, O'Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20:727–731. doi: 10.1200/JCO.2002.20.3.727. [DOI] [PubMed] [Google Scholar]
- 9.Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14:29–35. doi: 10.1093/annonc/mdg019. [DOI] [PubMed] [Google Scholar]
- 10.Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 12.Potosky AL, Malin JL, Kim B, et al. Use of colony-stimulating factors with chemotherapy: Opportunities for cost savings and improved outcomes. J Natl Cancer Inst. 2011;103:979–982. doi: 10.1093/jnci/djr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Doan QV, Malin J, et al. The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy. 2009;7:193–205. doi: 10.1007/BF03256152. [DOI] [PubMed] [Google Scholar]
- 14.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 15.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Sax FL, MacKenzie CR, et al. Assessing illness severity: Does clinical judgment work? J Chronic Dis. 1986;39:439–452. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 18.Hershman DL, Buono D, Jacobson JS, et al. Surgeon characteristics and use of breast conservation surgery in women with early stage breast cancer. Ann Surg. 2009;249:828–833. doi: 10.1097/SLA.0b013e3181a38f6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershman DL, Buono D, McBride RB, et al. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer. 2009;115:3848–3857. doi: 10.1002/cncr.24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hershman DL, Buono D, McBride RB, et al. Surgeon characteristics and receipt of adjuvant radiotherapy in women with breast cancer. J Natl Cancer Inst. 2008;100:199–206. doi: 10.1093/jnci/djm320. [DOI] [PubMed] [Google Scholar]
- 21.Vanchieri C. When will the U.S. flinch at cancer drug prices? J Natl Cancer Inst. 2005;97:624–626. doi: 10.1093/jnci/97.9.624. [DOI] [PubMed] [Google Scholar]
- 22.Benson R, Wilson C, Williams MV. Comments on Costs of treating advanced colorectal cancer, Ross et al., Eur J Cancer 1996, 32A, S13–S17. Eur J Cancer. 1998;34:593–594. doi: 10.1016/s0959-8049(97)10025-9. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Department of Health and Human Services. Office of Inspector General Report. 2011. http://oig.hhs.gov/oei/reports/oei-03-09-00510.pdf.
- 24.Doloresco F, Fominaya C, Schumock GT, et al. Projecting future drug expenditures: 2011. Am J Health Syst Pharm . 2011;68:921–932. doi: 10.2146/ajhp100712. [DOI] [PubMed] [Google Scholar]
- 25.Smith RE. Risk for the development of treatment-related acute myelocytic leukemia and myelodysplastic syndrome among patients with breast cancer: Review of the literature and the National Surgical Adjuvant Breast and Bowel Project experience. Clin Breast Cancer. 2003;4:273–279. doi: 10.3816/cbc.2003.n.032. [DOI] [PubMed] [Google Scholar]
- 26.Relling MV, Boyett JM, Blanco JG, et al. Granulocyte colony-stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood. 2003;101:3862–3867. doi: 10.1182/blood-2002-08-2405. [DOI] [PubMed] [Google Scholar]
- 27.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 28.Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: A systematic review. J Clin Oncol. 2010;28:2914–2924. doi: 10.1200/JCO.2009.25.8723. [DOI] [PubMed] [Google Scholar]
- 29.Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25:2522–2527. doi: 10.1200/JCO.2006.10.2749. [DOI] [PubMed] [Google Scholar]
- 30.Hershman DL, Wang X, McBride R, et al. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]