Abstract
Purpose
This prospective study examined the factors that predicted sustained adherence to surveillance mammography in women treated for breast cancer.
Methods
Breast cancer survivors (N = 204) who were undergoing surveillance mammography completed questionnaires assessing mammography-related anticipatory anxiety, persistent breast pain, mammography pain, and catastrophic thoughts about mammography pain. Adherence to mammography in the following year was assessed.
Results
In the year after study entry, 84.8% of women (n = 173) returned for a subsequent mammogram. Unadjusted associations showed that younger age, shorter period of time since surgery, and having upper extremity lymphedema were associated with lower mammography adherence. Forty percent of women reported moderate to high levels of mammography pain (score of ≥ 5 on a 0 to 10 scale). Although mammography pain was not associated with adherence, higher levels of mammography-related anxiety and pain catastrophizing were associated with not returning for a mammogram (P < .05). The impact of anxiety on mammography use was mediated by pain catastrophizing (indirect effect, P < .05).
Conclusion
Findings suggest that women who are younger, closer to the time of surgery, or have upper extremity lymphedema may be less likely to undergo repeated mammograms. It may be important for health professionals to remind selected patients directly that some women avoid repeat mammography and to re-emphasize the value of mammography for women with a history of breast cancer. Teaching women behavioral techniques (eg, redirecting attention) or providing medication for reducing anxiety could be considered for women with high levels of anxiety or catastrophic thoughts related to mammography.
INTRODUCTION
There are approximately 2.5 million women living with breast cancer in the United States.1 Evidence-based guidelines regarding cancer surveillance for breast cancer survivors recommend annual mammography.2–4 Early detection by annual mammography may increase survival rates among women with local recurrences and new breast cancers.5–12 Yet, studies suggest that 20% to 55% of breast cancer survivors do not undergo annual surveillance mammograms.13–19
Previous studies of mammography adherence have focused on women without a cancer history. A large number of these studies examined relationships between variables selected from health behavior theories (eg, health belief model) and mammography use.20,21 Findings regarding the relationship between mammography adherence and health behavior theory variables (eg, perceived risk of cancer, perceived mammography benefits) have been mixed.22–32 The factors that contribute to sustained mammography use in breast cancer survivors may differ from the variables identified in women without a cancer history. The current study focused on four variables that may be especially relevant to breast cancer survivors—mammography-related anticipatory anxiety, persistent breast pain, mammography pain, and catastrophic thoughts about mammography pain.
The impact of anxiety on mammography use has been widely studied in women without a cancer history. Two recent reviews33,34 synthesized this literature and found that most studies examined the impact of general anxiety about getting cancer on mammography adherence.26,27,35–38 General anxiety about getting cancer seems to facilitate mammography use, especially when women believe in the efficacy of mammography and have the resources to obtain a mammogram.26,33 Less is known about the impact of anxiety related to undergoing mammography itself (ie, anticipatory anxiety). Studies suggest that mammography-related anticipatory anxiety may contribute to poorer adherence39–41 because women may avoid getting a mammogram to reduce their anxiety. Mammography-related anticipatory anxiety may be an important barrier to sustained mammography use in breast cancer survivors. Mammography is cited as one of the most powerful triggers of psychological distress for breast cancer survivors.42–45
Persistent breast pain and mammography pain may be of high relevance for breast cancer survivors. More than 50% of women treated for breast cancer experience persistent breast pain.46,47 Women with persistent breast pain may be less willing to undergo a mammogram that can cause additional pain. Moreover, data suggest that persistent breast pain contributes to increased pain during mammography.48–50 Although few data regarding mammography pain are available for breast cancer survivors, Kornguth et al51 examined pain during mammography among breast cancer survivors and women with no cancer history. Results showed that breast cancer survivors reported significantly more pain during mammography compared with women with no cancer history.
Catastrophic thoughts about mammography pain (eg, the pain will become overwhelming) may have important implications for mammography use in breast cancer survivors. The cognitive-behavioral model of anxiety highlights the contribution of catastrophic thoughts to increased avoidance of anxiety-provoking situations.52–54 Numerous studies have shown that the tendency to have catastrophic thoughts about pain contributes to more intense pain, increased emotional distress, and avoidance of activities likely to increase pain.55,56 In a study of healthy women, Asghari and Nicholas57 found that catastrophic thoughts about mammography pain were associated with greater pain during the procedure.
In this study, breast cancer survivors who were undergoing routine surveillance mammography completed questionnaires assessing mammography-related anticipatory anxiety, persistent breast pain, mammography pain, and catastrophic thoughts about mammography pain. Mammography use in the following year was assessed via medical record. We hypothesized that women who had higher levels of anticipatory anxiety and persistent breast pain would be less likely to return for a subsequent mammogram. We also expected that women who experienced greater mammography pain and had more catastrophic thoughts about mammography pain would be less likely to return for a subsequent mammogram. Finally, we hypothesized that higher levels of anticipatory anxiety and persistent breast pain before mammography would contribute to more pain and catastrophic thoughts during the mammogram, which would then lead to lower rates of adherence.
METHODS
Participants
Participants were breast cancer survivors (stage I to IIIA) undergoing surveillance mammography at Duke University in 2006 to 2007. Women had completed surgery, radiation, and/or chemotherapy within the past 2 to 10 years. Women who had completed or were receiving adjuvant hormonal therapy were eligible. Women were excluded if they did not speak English or if they were surgically treated with bilateral mastectomy. Of the 247 women approached, 210 (85%) agreed to participate. Six participants were excluded from analyses as a result of cancer recurrence (n = 1) or an abnormal mammogram result (n = 5).
Procedure
Immediately before undergoing mammography, women completed questionnaires assessing demographic variables, anticipatory anxiety about the mammogram, and persistent breast pain. Women then underwent their mammogram. Immediately after the mammogram (but before results were available), women completed measures of mammography pain and catastrophic thoughts about mammography pain. Medical information and mammography adherence data were collected via medical record. Mammography use in the subsequent year was assessed 15 months after questionnaire completion. For women with no record of a subsequent mammogram, telephone follow-up was conducted to assess whether women had received a mammogram in a clinic outside the Duke University hospital system. Two women reported obtaining a mammogram at an outside clinic, and these women were considered adherent in the analysis.
All participants provided written informed consent. The study protocol was approved by the Duke Institutional Review Board. Women were given $10 for participating in the study.
Measures
Demographic and medical information.
Demographic information was collected as part of the premammogram assessment. Medical information was collected from medical records.
Mammography adherence.
Mammography use in the year after study entry was assessed via medical record. All women in this study received a recommendation to complete a subsequent mammogram in 12 months. Mammography adherence was defined as obtaining a subsequent mammogram within 15 months. A 15-month interval was used to allow for possible scheduling issues, because data suggest that rates of return for annual mammography increase rapidly between 12 and 15 months and then become mostly stable by 15 months.58
Past mammography use was assessed as a potential control variable. Information on mammography use for the period of time after breast cancer surgery until entry onto the study was collected via medical record. The date of each mammogram was recorded. To allow for possible scheduling issues, the following intervals were used to define adherence for each mammogram: an 8-month interval was used for mammograms recommended in 6 months, and a 15-month interval was used for mammograms recommended in 12 months. Past mammography adherence was defined as obtaining consecutive mammograms within recommended intervals before study entry.
Persistent breast pain.
Persistent breast pain was assessed using four items from the Brief Pain Inventory.59 Women rated the worst, least, and average pain intensity during the past week and current pain (eg, “Please rate your worst breast pain in the past week.”). Items were rated on a 0 (no pain) to 10 (pain as bad as you can imagine) scale. A composite pain measure based on the average of these four ratings was computed (α = .91).
Anticipatory anxiety.
An 11-item scale based on the Stanford Acute Stress Reaction Questionnaire60–62 was used to assess mammography-related anticipatory anxiety (eg, restlessness, difficulty sleeping, irritability). This measure assesses acute anxiety reactions to a specific stressor, which was specified as mammography. Women were instructed to rate the extent to which they experienced each symptom of anxiety in the past week including the day of the mammogram. Each item was rated on a 6-point scale (0 = not experienced; 5 = very often experienced). The 11 items included in this scale were based on examination of item variability and exploratory factor analysis.63 Items with adequate variability, factor loadings (> 0.30), and communalities (> 50%) were retained, resulting in an 11-item scale with a single factor solution. Items were summed to create a total score, with higher scores indicating greater anxiety (α = .96).
Mammography pain.
Pain during mammography was assessed using four items from the Brief Pain Inventory.59 Women were asked to rate the worst, least, and average pain intensity during mammography and current breast pain. Each pain item was rated on a 0 (no pain) to 10 (pain as bad as you can imagine) scale. A composite pain measure based on the average of these four ratings was computed (α = .86).
Pain catastrophizing.
The 13-item Pain Catastrophizing Scale64 assessed the degree to which women had catastrophic thoughts about pain during the mammogram (eg, “I became afraid that the pain would get worse.”). Each item was rated on a 5-point scale (0 = not at all; 4 = always), and items were summed to create a total score (α = .93).
Statistical Analyses
Descriptive analyses were conducted. Analyses revealed two variables with non-normal distributions—anticipatory anxiety and pain catastrophizing. On the basis of recommendations for reducing the impact of extreme values on parameters in logistic regression,65 these variables were rank order transformed, and all subsequent analyses were conducted with the transformed variables. To rank order transform each variable, the data were ranked from lowest (one) to highest (n), and tied values were given an average rank. Bivariate analyses (χ2 and t tests) were conducted to examine the relationships between participant characteristics and mammography adherence. Two logistic regressions were conducted, one to test the impact of premammogram variables (persistent breast pain and anticipatory anxiety) on subsequent mammography adherence, and the other to examine the impact of experiences during mammography (mammography pain and pain catastrophizing) on subsequent mammography adherence. Each model controlled for participant characteristics that were associated with mammography adherence in bivariate analyses (P < .10).
Path model analysis was conducted to test whether experiences during mammography mediated the impact of premammogram variables on subsequent mammography adherence. Mediation was tested using the approach66 recommended for analysis with categorical variables. Mplus 5.1 (Muthen & Muthen, Los Angeles, CA)67 was used to calculate estimates for model parameters using robust weighted least squares estimation. The Sobel test68 and a bootstrap approach for obtaining 95% CIs were used to test the significance of indirect effects.
RESULTS
Sample Description
Table 1 lists participant characteristics. On average, women were 5.01 years (standard deviation, 2.28 years; range, 2 to 10 years) from breast cancer diagnosis. More than half of the sample (62.3%) was treated with breast-conserving surgery. The majority of women (96.5%) received adjuvant therapy. Twenty-eight women (13.7%) had upper extremity lymphedema.
Table 1.
Participant Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Participants(N = 204) | % |
|---|---|---|
| Age, years | ||
| Mean | 59.5 | |
| SD | 10.9 | |
| Median | 59.0 | |
| Education, years | ||
| Mean | 14.7 | |
| SD | 2.7 | |
| Median | 14.0 | |
| Ethnicity | ||
| White | 177 | 86.7 |
| Black or African American | 25 | 12.3 |
| Asian or Pacific Islander | 2 | 1.0 |
| Employment status | ||
| Working full or part time | 91 | 44.6 |
| Retired | 82 | 40.2 |
| On sick leave | 3 | 1.5 |
| Unemployed | 25 | 12.3 |
| Unknown/missing | 3 | 1.5 |
| Marital status | ||
| Married/living as married | 144 | 70.6 |
| Not married/living as married | 60 | 29.4 |
| Disease stage | ||
| I | 100 | 49.0 |
| II | 89 | 43.6 |
| IIIA | 15 | 7.4 |
| Surgical treatment | ||
| Breast-conserving surgery | 127 | 62.3 |
| Mastectomy with reconstructive surgery | 40 | 19.6 |
| Mastectomy without reconstructive surgery | 37 | 18.1 |
| Received radiation therapy | 142 | 69.6 |
| Received chemotherapy | 110 | 54.2 |
| Received hormonal therapy | 177 | 87.6 |
| Lymphedema | 28 | 13.7 |
| Years since breast cancer diagnosis | ||
| Mean | 5.01 | |
| SD | 2.28 | |
| Median | 5.04 | |
| How breast cancer was detected | ||
| Mammogram | 105 | 51.5 |
| Clinical examination | 19 | 9.3 |
| Self-examination | 74 | 36.3 |
| Unknown/information unavailable | 6 | 2.9 |
| Past mammography adherence | 170 | 83.3 |
| Subsequent mammography adherence | 173 | 84.8 |
| Anticipatory anxiety score | ||
| Mean | 10.2 | |
| SD | 10.3 | |
| Median | 7.0 | |
| Ongoing breast pain score | ||
| Mean | 1.0 | |
| SD | 1.6 | |
| Median | 0 | |
| Mammography pain score | ||
| Mean | 4.1 | |
| SD | 2.3 | |
| Median | 4.0 | |
| Mammography-related pain catastrophizing score | ||
| Mean | 4.9 | |
| SD | 7.8 | |
| Median | 2.0 | |
Abbreviation: SD, standard deviation.
Persistent breast pain was reported by 43% of women (n = 88), with 38% of women reporting mild pain (rating of 1 to 4) and 5% reporting moderate to severe pain (rating ≥ 5). Mammography pain was reported by 95% of women (n = 193), with 55% reporting mild pain and 40% reporting moderate to severe pain. The majority of women (85%) reported anticipatory anxiety. When using a cutoff of 3 (on a scale of 0 to 5) to indicate the presence of a symptom, 31% of women (n = 63) reported experiencing three or more symptoms of anxiety. Approximately half of women (49%) reported catastrophizing about mammography pain.
Rates of Mammography Adherence
Most women (83.3%) received consecutive on-schedule mammograms from breast cancer surgery to study entry. Among women who were nonadherent, 30 women missed one recommended mammogram, and four women missed two recommended mammograms. Of the 34 women who missed one or more mammograms, 82% were 5 or more years from breast cancer diagnosis.
In the year after study entry, 84.8% of women (n = 173) received a subsequent mammogram. Of the 31 women who did not complete a subsequent mammogram, 61% were within 5 years of breast cancer diagnosis. Past mammography use (from breast cancer surgery to study entry) was not associated with subsequent mammography adherence (P = .93), which may be a result of the concentration of past mammography nonadherence in women who were 5 or more years from breast cancer diagnosis.
Participant Characteristics Associated With Mammography Adherence
Older age and greater time since breast cancer diagnosis were associated with higher rates of subsequent mammography adherence (Table 2). Women who had lymphedema were less likely to undergo a subsequent mammogram compared with women without lymphedema. Subsequent mammography adherence was not associated with other participant characteristics.
Table 2.
Unadjusted Demographic and Medical Correlates of Subsequent Mammography Adherence
| Variable | Adherent (n = 173) |
Nonadherent (n = 31) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .01 | ||||
| Mean | 60.28 | 54.90 | |||
| SD | 10.66 | 11.25 | |||
| Education, years | .99 | ||||
| Mean | 14.73 | 14.73 | |||
| SD | 2.76 | 2.06 | |||
| Ethnicity† | .48 | ||||
| White | 150 | 87.2 | 26 | 83.9 | |
| Asian or Pacific Islander | 2 | 1.2 | 0 | 0 | |
| Black or African American | 20 | 11.6 | 5 | 16.1 | |
| Employment status‡ | .39 | ||||
| Unemployed or sick leave | 25 | 14.6 | 3 | 10.0 | |
| Retired | 72 | 42.1 | 10 | 33.3 | |
| Working full or part time | 74 | 43.3 | 17 | 56.7 | |
| Married | 122 | 70.5 | 22 | 71.0 | .97 |
| Disease stage | .84 | ||||
| I | 86 | 49.7 | 14 | 45.2 | |
| II | 74 | 42.8 | 15 | 48.4 | |
| IIIA | 13 | 7.5 | 2 | 6.5 | |
| Breast-conserving surgery | 105 | 60.7 | 22 | 71.0 | .28 |
| Radiation therapy | 120 | 69.4 | 22 | 71.0 | .89 |
| Chemotherapy | 93 | 53.8 | 17 | 54.8 | .95 |
| Hormonal therapy | 152 | 87.9 | 25 | 80.6 | .20 |
| Lymphedema | 20 | 11.6 | 8 | 25.8 | .04 |
| Years since breast cancer diagnosis | .01 | ||||
| Mean | 5.19 | 4.00 | |||
| SD | 2.22 | 2.40 | |||
| Breast cancer detected by mammogram | 92 | 53.2 | 13 | 41.9 | .34 |
| Adherent to past mammography | 144 | 83.2 | 26 | 83.9 | .93 |
Abbreviation: SD, standard deviation.
Age, education, and years since breast cancer diagnosis were examined using independent t tests. The remaining categorical variables were examined using χ2 tests.
In χ2 analysis, ethnicity was coded as white or Asian/Pacific Islander = 0, and African American or black = 1. Ethnicity was missing for one participant.
Employment status was missing for three participants.
Multivariate Logistic Regression Analyses
Each logistic regression included age, years since diagnosis, and presence of lymphedema. Type of surgical treatment was also included as a result of higher levels of mammography pain in women treated with breast-conserving surgery compared with mastectomy (mean score, 4.4 v 3.4, respectively; P = .04). Table 3 lists the adjusted odds of subsequent mammography adherence. Women with higher levels of anticipatory anxiety were less likely to undergo mammography (odds ratio, 0.97 per unit of anticipatory anxiety; P = .03). For example, women with a median rank score on anticipatory anxiety had 32% lower odds of undergoing a subsequent mammogram than women who reported no anticipatory anxiety (rank, 1). Persistent breast pain was not associated with adherence. Women who had more catastrophic thoughts about mammography pain were less likely to undergo a mammogram (odds ratio, 0.93 per unit of pain catastrophizing; P = .02). For example, women with a median rank score on pain catastrophizing had 27% lower odds of undergoing a subsequent mammogram than women who reported no catastrophizing (rank, 1). Pain during mammography was not associated with adherence.
Table 3.
Multivariate Logistic Regression Analyses of Mammography Adherence
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Premammogram variables | |||
| Years since breast cancer diagnosis | 1.21 | 0.99 to 1.46 | .06 |
| Age (in years) | 1.04 | 0.99 to 1.08 | .09 |
| Lymphedema* | 0.34 | 0.12 to 0.94 | .04 |
| Type of surgical treatment† | 0.45 | 0.58 to 3.50 | .45 |
| Ongoing breast pain | 1.09 | 0.84 to 1.41 | .51 |
| Mammography-related anxiety‡ | 0.97 | 0.94 to 0.99 | .04 |
| Variables during mammography | |||
| Years since breast cancer diagnosis | 1.21 | 1.00 to 1.47 | .05 |
| Age (in years) | 1.05 | 1.01 to 1.09 | .02 |
| Lymphedema | 0.38 | 0.14 to 1.05 | .06 |
| Type of surgical treatment | 1.65 | 0.64 to 4.25 | .30 |
| Mammography pain | 1.12 | 0.90 to 1.38 | .28 |
| Mammography-related pain catastrophizing‡ | 0.93 | 0.88 to 0.99 | .02 |
NOTE. Mammography adherence was coded as 0 = nonadherent/did not complete a subsequent mammogram within 15 months, and 1 = adherent/completed subsequent mammogram within 15 months.
Lymphedema was coded as 0 = no lymphedema, and 1 = patient had lymphedema.
Type of surgical treatment was coded as 0 = breast-conserving surgery, and 1 = mastectomy.
Variable was rank order transformed.
Path Model Analysis Testing Mediation
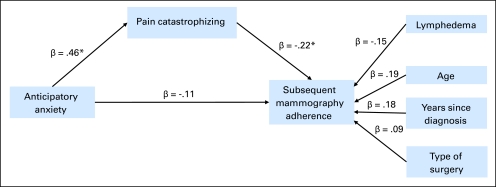
Figure 1 displays the standardized path coefficients for the model. Age, years since diagnosis, type of surgery, and lymphedema were included in the model. Higher anticipatory anxiety (β = 0.46; P < .001) contributed to greater pain catastrophizing during mammography, and anxiety accounted for a significant proportion of the variance in pain catastrophizing (R2 = 24%). Greater pain catastrophizing contributed to lower mammography adherence (β = −0.22; P = .03). The impact of anxiety on mammography adherence was mediated by pain catastrophizing (indirect effect, β = −0.10; 95% CI, −0.20 to −0.004; Sobel test Z = −2.04; P = .04). The indirect relationship between anxiety and mammography adherence accounted for 48% of anxiety's total effect on adherence.
Fig 1.
The impact of anticipatory anxiety on subsequent mammography adherence was mediated by pain catastrophizing. Age, years since breast cancer diagnosis, lymphedema, and type of surgery were included in the model as control variables. Direct paths were estimated between these three variables and subsequent mammography adherence. Lymphedema was coded as follows: 0 = no lymphedema; 1 = patient had lymphedema. Type of surgical treatment was coded as follows: 0 = breast-conserving surgery; 1 = mastectomy. Mammography adherence was coded as follows: 0 = nonadherent/did not complete a subsequent mammogram within 15 months; 1 = adherent/completed subsequent mammogram within 15 months. (*) P < .05.
DISCUSSION
This study extends previous research on mammography adherence by examining breast cancer survivors at the time of surveillance mammography. Several important findings emerged. First, higher levels of anticipatory anxiety and pain catastrophizing were associated with poorer mammography adherence. Second, the impact of anticipatory anxiety on mammography adherence was mediated by pain catastrophizing. Finally, examination of unadjusted correlations showed that younger age, a shorter period of time since diagnosis, and having upper extremity lymphedema were associated with lower mammography adherence.
Many studies have examined the relationship between anxiety and mammography adherence in women without a cancer history.26,27,35–38 Contradictory findings across studies make it difficult to draw conclusions from this literature. Consedine et al33 suggest that the relationship between anxiety and mammography adherence varies across different sources of anxiety. Women could be anxious about cancer in general, particular aspects of undergoing mammography, or receiving an abnormal result. Although anxiety about cancer in general may lead to greater adherence, anxiety about particular aspects of the procedure may contribute to poorer adherence.33 We found that the effects of anticipatory anxiety were mediated by pain catastrophizing. Although anticipatory anxiety might have been related to a variety of aspects of mammography, its effects on adherence were mainly explained by a tendency to have catastrophic thoughts during the mammogram.
It is possible that reports of mammography-related anxiety and catastrophizing reflect women's level of general anxiety. Data suggest that rates of anxiety are elevated among breast cancer survivors, with up to 40% of cancer survivors meeting criteria for depression or anxiety disorders.69–72 General anxiety has been identified as an important barrier to mammography adherence in women without a cancer history.73,74 Future studies are needed to further examine the impact of general anxiety and mammography-related anxiety on adherence.
Studies in women without a cancer history report that 41% to 46% of women implicate pain during their prior mammogram as the reason for not returning.75,76 Instead, our findings underscore the importance of addressing pain catastrophizing and anticipatory anxiety. Pain catastrophizing has been described as the cognitive precursor to pain-related fear and avoidance.77 Evidence suggests that pain catastrophizing leads to avoidance of pain-inducing activities in the context of acute pain.55,78,79 Our findings suggest that women who have more catastrophic thoughts are less likely to return for subsequent mammography.
This study has several limitations. First, the nonexperimental design prevents making causal attributions about the relationships between anxiety, catastrophizing, and adherence. Future research is needed to evaluate these potential cause and effect relationships. Second, data collected from a university medical center may not generalize to community hospitals. In addition, these data were collected in North Carolina and may not generalize to other areas. Third, women in our sample were observed by oncologists. Studies suggest that breast cancer survivors who are observed by oncologists are more likely to have mammograms than women observed by primary care providers.15,80 Finally, it will be important in future studies to examine whether anticipatory anxiety and catastrophic thoughts about pain predict adherence above and beyond health behavior theory variables (eg, perceived risk of cancer).
Past studies indicate that 20% to 55% of breast cancer survivors do not undergo annual mammography.13–19 Studies have reported similar rates of mammography nonadherence across breast cancer survivors in health maintenance organizations,81 Surveillance, Epidemiology, and End Results registries,15,18 controlled trials,82 and community settings.14,17 In this study, mammography adherence rates were high, with only 14% of women missing a mammogram from breast surgery to study entry, 13% missing a mammogram in the year following study entry, and only 2% missing mammograms both before and after study entry. It is noteworthy that 15% of women failed to complete a mammogram in the year after study entry, because this sample was selected from a population of breast cancer survivors who were undergoing surveillance mammograms. Consistent with prior studies,13,15 our data suggest that for many women, mammography adherence varies from year to year.
The results of this study suggest clinicians should be aware that women who are younger, closer to the time of surgery, or have upper extremity lymphedema may be less likely to undergo repeated mammograms. Special attention should be paid to women who have catastrophic thoughts about mammography pain and those who report symptoms of mammography-related anxiety. Teaching women behavioral techniques (eg, redirecting attention) or providing medication for reducing anxiety could be considered for women with high levels of anxiety or catastrophic thoughts related to mammography. Alternatively, it may be sufficient for health professionals to remind selected patients directly that women sometimes avoid repeat mammography because of anxiety or concerns about pain and to re-emphasize the value of mammography for women with a history of breast cancer.
Footnotes
Supported by National Cancer Institute Grant No. F32 CA124068 (R.A.S., primary investigator; F.J.K., mentor; and K.P.W. co-mentor).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Rebecca A. Shelby, Cindy D. Scipio, Mary Scott Soo, Kevin P. Weinfurt, Francis J. Keefe
Collection and assembly of data: Rebecca A. Shelby, Cindy D. Scipio, Mary Scott Soo, Francis J. Keefe
Data analysis and interpretation: Rebecca A. Shelby, Tamara J. Somers, Kevin P. Weinfurt, Francis J. Keefe
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Horner MJ, Ries LAG, Krapcho M, et al. SEER cancer statistics review, 1975-2006. http://seer.cancer.gov/csr/1975_2006/
- 2.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network and American Cancer Society. Breast Cancer: Treatment Guidelines for Patients. Version VIII. Fort Washington, PA: National Comprehensive Cancer Network; 2006. [Google Scholar]
- 4.Smith TJ, Davidson NE, Schapira DV, et al. American Society of Clinical Oncology 1998 update of recommended breast cancer surveillance guidelines. J Clin Oncol. 1999;17:1080–1082. doi: 10.1200/JCO.1999.17.3.1080. [DOI] [PubMed] [Google Scholar]
- 5.Dershaw DD. Mammography in patients with breast cancer treated by breast conservation (lumpectomy with or without radiation) AJR Am J Roentgenol. 1995;164:309–316. doi: 10.2214/ajr.164.2.7839960. [DOI] [PubMed] [Google Scholar]
- 6.Doyle T, Schultz DJ, Peters C, et al. Long-term results of local recurrence after breast conservation treatment for invasive breast cancer. Int J Radiat Oncol Biol Phys. 2001;51:74–80. doi: 10.1016/s0360-3016(01)01625-x. [DOI] [PubMed] [Google Scholar]
- 7.Kaas R, Hart AA, Besnard AP, et al. Impact of mammographic interval on stage and survival after the diagnosis of contralateral breast cancer. Br J Surg. 2001;88:123–127. doi: 10.1046/j.1365-2168.2001.01641.x. [DOI] [PubMed] [Google Scholar]
- 8.Katz ML, Donohue KA, Alfano CM, et al. Cancer surveillance behaviors and psychosocial factors among long-term survivors of breast cancer: Cancer and Leukemia Group B 79804. Cancer. 2009;115:480–488. doi: 10.1002/cncr.24063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollias J, Evans AJ, Wilson AR, et al. Value of contralateral surveillance mammography for primary breast cancer follow-up. World J Surg. 2000;24:983–987. doi: 10.1007/s002680010171. [DOI] [PubMed] [Google Scholar]
- 10.Lash TL, Fox MP, Silliman RA. Reduced mortality rate associated with annual mammograms after breast cancer therapy. Breast J. 2006;12:2–6. doi: 10.1111/j.1075-122X.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- 11.Lash TL, Clough-Gorr K, Silliman RA. Reduced rates of cancer-related worries and mortality associated with guideline surveillance after breast cancer therapy. Breast Cancer Res Treat. 2005;89:61–67. doi: 10.1007/s10549-004-1472-z. [DOI] [PubMed] [Google Scholar]
- 12.Perrone MA, Musolino A, Michiara M, et al. Early detection of recurrences in the follow-up of primary breast cancer in an asymptomatic or symptomatic phase. Tumori. 2004;90:276–279. doi: 10.1177/030089160409000302. [DOI] [PubMed] [Google Scholar]
- 13.Doubeni CA, Field TS, Ulcickas Yood M, et al. Patterns and predictors of mammography utilization among breast cancer survivors. Cancer. 2006;106:2482–2488. doi: 10.1002/cncr.21893. [DOI] [PubMed] [Google Scholar]
- 14.Lash TL, Silliman RA. Medical surveillance after breast cancer diagnosis. Med Care. 2001;39:945–955. doi: 10.1097/00005650-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Keating NL, Landrum MB, Guadagnoli E, et al. Factors related to underuse of surveillance mammography among breast cancer survivors. J Clin Oncol. 2006;24:85–94. doi: 10.1200/JCO.2005.02.4174. [DOI] [PubMed] [Google Scholar]
- 16.Etim AE, Schellhase KG, Sparapani R, et al. Effect of model of care delivery on mammography use among elderly breast cancer survivors. Breast Cancer Res Treat. 2006;96:293–299. doi: 10.1007/s10549-005-9141-4. [DOI] [PubMed] [Google Scholar]
- 17.Andersen MR, Urban N. The use of mammography by survivors of breast cancer. Am J Public Health. 1998;88:1713–1715. doi: 10.2105/ajph.88.11.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schapira MM, McAuliffe TL, Nattinger AB. Underutilization of mammography in older breast cancer survivors. Med Care. 2000;38:281–289. doi: 10.1097/00005650-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Legg JS, Fauber TL, Ozcan YA. The influence of previous breast cancer upon mammography utilization. Womens Health Issues. 2003;13:62–67. doi: 10.1016/s1049-3867(02)00194-9. [DOI] [PubMed] [Google Scholar]
- 20.Yabroff KR, Mandelblatt JS. Interventions targeted toward patients to increase mammography use. Cancer Epidemiol Biomarkers Prev. 1999;8:749–757. [PubMed] [Google Scholar]
- 21.Sohl SJ, Moyer A. Tailored interventions to promote mammography screening: A meta-analytic review. Prev Med. 2007;45:252–261. doi: 10.1016/j.ypmed.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yarbrough SS, Braden CJ. Utility of health belief model as a guide for explaining or predicting breast cancer screening behaviours. J Adv Nurs. 2001;33:677–688. doi: 10.1046/j.1365-2648.2001.01699.x. [DOI] [PubMed] [Google Scholar]
- 23.Lerman C, Kash K, Stefanek M. Younger women at increased risk for breast cancer: Perceived risk, psychological well-being, and surveillance behavior. J Natl Cancer Inst. 1994;16:171–176. [PubMed] [Google Scholar]
- 24.Gross CP, Filardo G, Singh HS, et al. The relation between projected breast cancer risk, perceived cancer risk, and mammography use. J Gen Intern Med. 2006;21:158–164. doi: 10.1111/j.1525-1497.2005.00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvocoressi L, Kasl SV, Lee CH, et al. A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and white women ages 40 to 79 years. Cancer Epidemiol Biomarkers Prev. 2004;13:2096–2105. [PubMed] [Google Scholar]
- 26.Hay JL, McCaul KD, Magnan RE. Does worry about breast cancer predict screening behaviors? A meta-analysis of the prospective evidence. Prev Med. 2006;42:401–408. doi: 10.1016/j.ypmed.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 27.McCaul KD, Schroeder DM, Reid PA. Breast cancer worry and screening: Some prospective data. Health Psychol. 1996;15:430–433. doi: 10.1037//0278-6133.15.6.430. [DOI] [PubMed] [Google Scholar]
- 28.McCaul KD, Branstetter AD, Schroeder DM, et al. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health Psychol. 1996;15:423–429. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 29.Champion V, Maraj M, Hui S, et al. Comparison of tailored interventions to increase mammography screening in nonadherent older women. Prev Med. 2003;36:150–158. doi: 10.1016/s0091-7435(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 30.Russell KM, Champion VL, Skinner CS. Psychosocial factors related to repeat mammography screening over 5 years in African American women. Cancer Nurs. 2006;29:236–243. doi: 10.1097/00002820-200605000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Mickey RM, Durski J, Worden JK, et al. Breast cancer screening and associated factors for low-income African-American women. Prev Med. 1995;24:467–476. doi: 10.1006/pmed.1995.1075. [DOI] [PubMed] [Google Scholar]
- 32.Halabi S, Skinner CS, Samsa GP, et al. Factors associated with repeat mammography screening. J Fam Pract. 2000;49:1104–1112. [PubMed] [Google Scholar]
- 33.Consedine NS, Magai C, Krivoshekova YS, et al. Fear, anxiety, worry, and breast cancer screening behavior: A critical review. Cancer Epidemiol Biomarkers Prev. 2004;13:501–510. [PubMed] [Google Scholar]
- 34.Ackerson K, Preston SD. A decision theory perspective on why women do or do not decide to have cancer screening: Systematic review. J Adv Nurs. 2009;65:1130–1140. doi: 10.1111/j.1365-2648.2009.04981.x. [DOI] [PubMed] [Google Scholar]
- 35.Lagerlund M, Hedin A, Sparén P, et al. Attitudes, beliefs, and knowledge as predictors of nonattendance in a Swedish population-based mammography screening program. Prev Med. 2000;31:417–428. doi: 10.1006/pmed.2000.0723. [DOI] [PubMed] [Google Scholar]
- 36.Consedine NS, Magai C, Neugut AI. The contribution of emotional characteristics to breast cancer screening among women from six ethnic groups. Prev Med. 2004;38:64–77. doi: 10.1016/j.ypmed.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Diefenbach MA, Miller SM, Daly MB. Specific worry about breast cancer predicts mammography use in women at risk for breast and ovarian cancer. Health Psychol. 1999;18:532–536. doi: 10.1037//0278-6133.18.5.532. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz MD, Taylor KL, Willard KS. Prospective association between distress and mammography utilization among women with a family history of breast cancer. J Behav Med. 2003;26:105–117. doi: 10.1023/a:1023078521319. [DOI] [PubMed] [Google Scholar]
- 39.Aro AR, de Koning HJ, Absetz P, et al. Two distinct groups of non-attenders in an organized mammography screening program. Breast Cancer Res Treat. 2001;70:145–153. doi: 10.1023/a:1012939228916. [DOI] [PubMed] [Google Scholar]
- 40.Austin LT, Ahmad F, McNally MJ, et al. Breast and cervical cancer screening in Hispanic women: A literature review using the Health Belief Model. Womens Health Issues. 2002;12:122–128. doi: 10.1016/s1049-3867(02)00132-9. [DOI] [PubMed] [Google Scholar]
- 41.Friedman LC, Webb JA, Weinberg AD, et al. Breast cancer screening: Racial/ethnic differences in behaviors and beliefs. J Cancer Educ. 1995;10:213–216. doi: 10.1080/08858199509528376. [DOI] [PubMed] [Google Scholar]
- 42.Polinsky ML. Functional status of long-term breast cancer survivors: Demonstrating chronicity. Health Soc Work. 1994;19:165–173. doi: 10.1093/hsw/19.3.165. [DOI] [PubMed] [Google Scholar]
- 43.Pelusi J. The lived experience of surviving breast cancer. Oncol Nurs Forum. 1997;24:1343–1353. [PubMed] [Google Scholar]
- 44.Muzzin LJ, Anderson NJ, Figueredo AT, et al. The experience of cancer. Soc Sci Med. 1994;38:1201–1208. doi: 10.1016/0277-9536(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 45.Gurevich M, Devins GM, Wilson C, et al. Stress response syndromes in women undergoing mammography: A comparison of women with and without a history of breast cancer. Psychosom Med. 2004;66:104–112. doi: 10.1097/01.psy.0000109907.78754.5a. [DOI] [PubMed] [Google Scholar]
- 46.Tasmuth T, von Smitten K, Hietanen P, et al. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6:453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 47.Tasmuth T, von Smitten K, Kalso E. Pain and other symptoms during the first year after radical and conservative surgery for breast cancer. Br J Cancer. 1996;74:2024–2031. doi: 10.1038/bjc.1996.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruyninckx E, Mortelmans D, Van Goethem M, et al. Risk factors of pain in mammographic screening. Soc Sci Med . 1999;49:933–941. doi: 10.1016/s0277-9536(99)00181-1. [DOI] [PubMed] [Google Scholar]
- 49.Keemers-Gels ME, Groenendijk RP, van den Heuvel JH, et al. Pain experienced by women attending breast cancer screening. Breast Cancer Res Treat. 2000;60:235–240. doi: 10.1023/a:1006457520996. [DOI] [PubMed] [Google Scholar]
- 50.Sapir R, Patlas M, Strano SD, et al. Does mammography hurt? J Pain Symptom Manage. 2003;25:53–63. doi: 10.1016/s0885-3924(02)00598-5. [DOI] [PubMed] [Google Scholar]
- 51.Kornguth PJ, Keefe FJ, Wright KR, et al. Mammography pain in women treated conservatively for breast cancer. J Pain. 2000;1:268–274. doi: 10.1054/jpai.2000.7884. [DOI] [PubMed] [Google Scholar]
- 52.Beck AT, Emery G, Greenberg RL. Anxiety Disorders and Phobias: A Cognitive Perspective. Cambridge, MA: Basic Books; 2005. [Google Scholar]
- 53.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behav Res Ther. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 54.Wells A. A cognitive model of generalized anxiety disorder. Behav Modif. 1999;23:526–555. doi: 10.1177/0145445599234002. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan MJ, Rodgers WM, Wilson PM, et al. An experimental investigation of the relation between catastrophizing and activity intolerance. Pain. 2002;100:47–53. doi: 10.1016/s0304-3959(02)00206-3. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Asghari A, Nicholas MK. Pain during mammography: The role of coping strategies. Pain. 2004;108:170–179. doi: 10.1016/j.pain.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 58.Partin MR, Slater JS, Caplan L. Randomized controlled trial of a repeat mammography intervention: Effect of adherence definitions on results. Prev Med. 2005;41:734–740. doi: 10.1016/j.ypmed.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Cleeland CS, Ryan KM. Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 60.Cardena E, Holen A, McFarlane A, et al. A multisite study of acute stress reactions to a disaster. In: Widiger TA, Pincus HA, Ross R, et al., editors. DSM-IV Sourcebook, 4. Washington, DC: American Psychiatric Association; 1998. pp. 377–391. [Google Scholar]
- 61.Cardena E, Koopman C, Classen C, et al. Review of the Stanford Acute Stress Reaction Questionnaire. In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran Press; 1996. pp. 293–295. [Google Scholar]
- 62.Cardeña E, Koopman C, Classen C, et al. Psychometric properties of the Stanford Acute Stress Reaction Questionnaire (SASRQ): A valid and reliable measure of acute stress. J Trauma Stress. 2000;13:719–734. doi: 10.1023/A:1007822603186. [DOI] [PubMed] [Google Scholar]
- 63.Jennrich RI, Sampson PF. Rotation for simple loadings. Psychometrika. 1968;31:313–323. doi: 10.1007/BF02289465. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan MJ, Bishop S, Pivik J. The pain catastrophizing scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 65.O'Gorman TW, Woolson RF. On the efficacy of the rank transformation in stepwise logistic and discriminant analysis. Stat Med. 1993;12:143–151. doi: 10.1002/sim.4780120206. [DOI] [PubMed] [Google Scholar]
- 66.MacKinnon DP, Lockwood CM, Brown CH, et al. The intermediate endpoint effect in logistic and probit regression. Clin Trials. 2007;4:499–513. doi: 10.1177/1740774507083434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muthen LK, Muthen BO. Mplus Statistical Analysis With Latent Variables: User's Guide (ed 5) Los Angeles, CA: Muthen & Muthen; 2007. [Google Scholar]
- 68.Sobel ME. Asymptotic intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 69.Grassi L, Rosti G. Psychosocial morbidity and adjustment to illness among long-term cancer survivors: A six-year follow-up study. Psychosomatics. 1996;37:523–532. doi: 10.1016/S0033-3182(96)71516-5. [DOI] [PubMed] [Google Scholar]
- 70.Hegel MT, Moore CP, Collins ED, et al. Distress, psychiatric syndromes, and impairment of function in women with newly diagnosed breast cancer. Cancer. 2006;107:2924–2931. doi: 10.1002/cncr.22335. [DOI] [PubMed] [Google Scholar]
- 71.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;32:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 72.Mehnert A, Koch U. Prevalence of acute and post-traumatic stress disorder and comorbid mental disorders in breast cancer patients during primary cancer care: A prospective study. Psychooncology. 2007;16:181–188. doi: 10.1002/pon.1057. [DOI] [PubMed] [Google Scholar]
- 73.Rakowski W, Meissner H, Vernon SW, et al. Correlates of repeat and recent mammography for women ages 45 to 75 in the 2002 to 2003 Health Information National Trends Survey (HINTS 2003) Cancer Epidemiol Biomarkers Prev. 2006;15:2093–2101. doi: 10.1158/1055-9965.EPI-06-0301. [DOI] [PubMed] [Google Scholar]
- 74.Boyer BA, Cantor RK. Posttraumatic stress among women with breast cancer and their daughters: Relationship with daughters' breast cancer screening. Am J Fam Ther. 2005;33:443–460. [Google Scholar]
- 75.Elwood M, McNoe B, Smith T, et al. Once is enough: Why some women do not continue to participate in a breast cancer screening programme. N Z Med J. 1998;111:180–183. [PubMed] [Google Scholar]
- 76.Marshall G. A comparative study of re-attenders and non-re-attenders for second triennial National Breast Screening Programme appointments. J Public Health Med. 1994;16:79–86. doi: 10.1093/oxfordjournals.pubmed.a042939. [DOI] [PubMed] [Google Scholar]
- 77.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 78.Buer N, Linton SJ. Fear-avoidance beliefs and catastrophizing: Occurrence and risk factor in back pain and ADL in the general population. Pain. 2002;99:485–491. doi: 10.1016/S0304-3959(02)00265-8. [DOI] [PubMed] [Google Scholar]
- 79.Flink IK, Mroczek MZ, Sullivan MJ, et al. Pain in childbirth and postpartum recovery: The role of catastrophizing. Eur J Pain. 2009;13:312–316. doi: 10.1016/j.ejpain.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 80.Earle CC, Burstein HJ, Winer EP, et al. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 81.Elston Lafata, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43:592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 82.Mandelblatt JS, Lawrence WF, Cullen J, et al. Patterns of care in early-stage breast cancer survivors in the first year after cessation of active treatment. J Clin Oncol. 2006;24:77–84. doi: 10.1200/JCO.2005.02.2681. [DOI] [PubMed] [Google Scholar]