Abstract
Background
Studies have examined the interaction of MAOA genotype with childhood maltreatment in relation to depressive symptomatology and alcohol abuse with conflicting findings. Both high and low activity allele combinations have been shown to be protective for maltreated children with direction of findings varying by study methodology and participant’s sex.
Methods
Participants in a prospective cohort design study involving court substantiated cases of child abuse and neglect and a matched comparison group were followed up into adulthood and interviewed (N = 802). Eighty-two percent consented to provide blood, 631 gave permission for DNA extraction and analyses, and 575 were included in the final sample. This sample included male, female, White, and Non-White (primarily Black) participants. Symptoms of dysthymia, major depression and alcohol abuse were assessed using the NIMH Diagnostic Interview Schedule-III-R.
Results
Significant three-way interactions, MAOA genotype by abuse by sex, predicted dysthymic symptoms. Low-activity MAOA genotype buffered against symptoms of dysthymia in physically abused and multiply maltreated women. Significant three-way interactions, MAOA genotype by sexual abuse by race, predicted all outcomes. Low-activity MAOA genotype buffered against symptoms of dysthymia, major depressive disorder and alcohol abuse for sexually abused White participants. The high-activity genotype was protective in the Non-White sexually abused group.
Conclusions
This prospective study provides evidence that MAOA interacts with child maltreatment to predict mental health outcomes. Reasons for sex differences and race findings are discussed.
Keywords: Child abuse and neglect, MAOA genotype, depression, dysthymia, alcohol abuse, prospective longitudinal design
Research has shown that adults who were abused and/or neglected as children are at increased risk for a variety of negative consequences, including depression, alcohol abuse, and violent and criminal behavior [1–19]. At the same time, several scholars have called attention to a group of children who appear resilient despite histories of childhood adversities including maltreatment [20–27].
Rresearchers have begun to examine gene by environment interactions to understand differences in outcomes for children exposed to early adversities. Studies have focused on monoamine oxidase A (MAOA) genotype as a moderator between the environmental stressor of child maltreatment and subsequent outcomes. MAOA is a gene located on the X chromosome (Xp11.23-11.4) that encodes the MAOA enzyme which breaks down neurotransmitters serotonin (5-HT), norepinephrine (NE) and dopamine (DA) [28]. Variability in MAOA genotype is due to a polymorphism of the MAOA gene with a variable number of tandem repeats (VNTR). The variability has been shown to correlate with transcription with shorter, 3-repeat allele, being less active than the longer, 4-repeat allele [29–31]. MAOA has been implicated in affective functioning in knock-out mice suggesting that normal MAOA activity is important for anger expression [32]. Neurotransmitters 5-HT, NE and DA that MAOA metabolizes have been linked to depressive and substance abuse symptomatology [33] which are also outcomes predicted by child maltreatment [2, 3, 10, 34–36].
Caspi et al [37] found that monoamine oxidase A (MAOA) genotype moderated the relationship between child maltreatment and antisocial and violent offences in males, with high-activity MAOA protecting maltreated children. This research has since been replicated [38–40], replicated in part [41–43], or not replicated [44, 45]. A recent meta-analysis showed a significant effect of the interaction of MAOA genotype and child maltreatment on antisocial behaviors [46]. Most these studies have been conducted with White males. However, some research [47, 48] with females reported that low-activity allele was protective, opposite to Caspi et al [37] and one study that examined these relationships separately in Blacks and Whites found different results [41].
Although the MAOA enzyme metabolizes neurotransmitters implicated in depressive symptomatology and alcohol abuse, few studies have examined whether MAOA interacts with child maltreatment to predict these outcomes. Cicchetti et al. [49] reported a significant interaction of MAOA genotype and level of maltreatment in the prediction of depressive symptomatology using a sample of low-income adolescents. Children who were more severely maltreated and had low MAOA activity displayed more symptoms of depression than those with high-activity MAOA and severe levels of maltreatment. A study of White adults [50], using retrospective self-reports of physical and sexual abuse, found that MAOA genotype interacted with abuse to predict Major Depressive Disorder symptoms in men and women, but in the opposite direction to Cicchetti et al [49]. Abuse and high activity MAOA were associated with more depression symptoms, compared to those with low activity MAOA and abuse histories [50].
Research on alcohol abuse to date has also produced conflicting results. In a sample of Native American adult women, Ducci et al [51] found that MAOA genotype interacted with self-reported childhood sexual abuse to predict alcohol abuse: individuals with low levels of MAOA and abuse histories reported a higher number of symptoms, compared to those with high levels of MAOA and abuse histories. Studies with adolescents in Sweden [52], [53] found a clear difference between boys and girls in the interaction of family adversity and genotype predicting alcohol-related problem behaviors and risky alcohol consumption. Boys with the low activity and girls with high activity MAOA genotype were at increased risk, if they were also exposed to family adversity [54].
In summary, several studies have reported interactions of the MAOA genotype and child maltreatment to predict a variety of outcomes. However, the direction of these relationships has varied considerably, with differences in the operationalization of maltreatment, study design (cross-sectional versus longitudinal), sex, race/ethnicity, and outcome assessed. Some studies have used documented cases of childhood abuse and neglect, whereas others have relied on retrospective self-reports. Thus, it is difficult to draw firm conclusions about these relations.
The current research builds upon previous studies by examining the interaction of child maltreatment and MAOA genotype using a prospective cohort design with court documented cases of childhood physical abuse, sexual abuse, and neglect and matched controls who were followed up into adulthood and assessed. This study offers several advantages for testing the gene (MAOA) x environment (child abuse and neglect) interaction. First, this study involves a clear operationalization and lack of ambiguity about the child maltreatment in our sample by using documented cases of child abuse and neglect. Second, there is a comparison group matched on the basis of age, sex, race/ethnicity and approximate social class background [55]. Third, we use a structured diagnostic interview to assess psychiatric disorders. Fourth, we have a diverse sample with males, females, Whites, and non-Whites. Finally, we have followed our participants into adulthood so that we can compare risks beyond adolescence and into adulthood.
This paper examines the interaction of child maltreatment and the MAOA genotype in relation to three adult mental health outcomes -- major depressive disorder (MDD), dysthymia (DD), which has been shown to have a common genetic underpinning to MDD [56], and alcohol abuse (AA). We ask two basic questions: (1) Does child maltreatment interact with MAOA genotype to predict MDD, DD, and AA? (2) Does the interaction between childhood maltreatment and MAOA genotype in predicting outcomes of MDD, DD, and AA differ by race, sex or type of maltreatment experienced?
Method
Participants
This research is based on a specialized cohort design study in which abused and neglected children were matched with non-abused and non-neglected children and followed prospectively into young adulthood [18]. Only court substantiated cases of child abuse and neglect were included. Cases were drawn from the records of county juvenile and adult criminal courts in a metropolitan area in the Midwest during the years 1967 through 1971. To avoid potential problems with ambiguity in the direction of causality, and to ensure that temporal sequence was clear (that is, child neglect or abuse led to subsequent outcomes), abuse and neglect cases were restricted to those in which children were 11 years of age or less at the time of the abuse or neglect incident.
The abuse and neglect group includes three types: physical abuse, sexual abuse, and neglect. Physical abuse cases included injuries such as bruises, welts, burns, abrasions, lacerations, wounds, cuts, bone and skull fractures, and other evidence of physical injury. Sexual abuse charges varied from relatively non-specific charges of “assault and battery with intent to gratify sexual desires” to more specific charges of “fondling or touching in an obscene manner,” sodomy, incest, rape, and so forth. Neglect cases reflected a judgment that the parents’ deficiencies in child care were beyond those found acceptable by community and professional standards at the time. These cases represented extreme failure to provide adequate food, clothing, shelter, and medical attention to children.
A critical element of the design was the establishment of a comparison group, matched to the maltreatment group as closely as possible on the basis of sex, age, race, and approximate family socio-economic status during the time period under study (1967 through 1971). To accomplish this matching, the sample of abused and neglected cases was first divided into two groups on the basis of their age at the time of the abuse or neglect incident. Children who were under school age at the time of the abuse or neglect were matched with children of the same sex, race, date of birth (+/− 1 week), and hospital of birth through the use of county birth record information. For children of school age, records of more than 100 elementary schools for the same time period were used to find matches with children of the same sex, race, date of birth (+/− 6 months), and same class in same elementary school during the years 1967 through 1971. Overall, there were matches for 74% of the abused and neglected children [55].
This research began in 1986 as an archival study focused on the “cycle of violence” [18]. A second phase located and interviewed 1,196 individuals between 1989 and 1995 and 896 of the study participants were re-interviewed in 2001–2002. Data for the present study were collected during 2003–2005 in the context of a medical status examination (including blood collection through venipuncture) and interview of 806 participants. Of those interviewed, 638 (82%) consented to provide blood and 631 gave permission for DNA extraction and analyses. IRB regulations that define prisoners as a vulnerable population requiring additional protections prevented the collection of blood from 31 incarcerated participants.
Although there was attrition associated with death, refusals, and our inability to locate individuals over the various waves of the study, the composition of the samples at the four time points has remained about the same. The abuse and neglect group represented 56–58% at each time period; Whites were 62–66%; and males were 48–51% of the samples. There were no significant differences across the samples on these variables or in mean age across the four phases of the study.
The average age of participants is 41 years old (SD = 3.85; range = 31–51). Approximately half the sample is female (48.7%) and 60.8% is White, non-Hispanic. Using participant-reported race/ethnicity, the non-white/minority group includes African Americans (35.1%) and Hispanics (4.1%). The average highest grade of school completed for the sample was 11.47 (SD = 2.19) and the median occupational level [57] was semi-skilled workers (only 11.3% were in the professions). Thus, the overall sample is skewed toward the lower end of the socio-economic spectrum. Maltreatment was divided into types: physical abuse (7%), sexual abuse (9%), neglect (72%) and multiple maltreatment (12%).
The interviewers and participants were blind to the purpose of the study, to the inclusion of an abused and/or neglected group, and to the participants’ group membership. Participants were told that they had been selected as part of a large group of individuals who grew up in that area during the late 1960s and early 1970s. Institutional Review Board approval was obtained for the procedures and participants signed a consent form acknowledging that they understood the conditions of their participation and were participating voluntarily.
Assessment of Dysthymia, Major Depressive Disorder, and Alcohol Abuse Symptoms
Assessments of lifetime DSM-III-R symptoms of Dysthymia (DD), Major Depressive Disorder (MDD), and Alcohol Abuse (AA) were based on information from the NIMH Diagnostic Interview Schedule (DIS-III-R) [58]. The DIS-III-R is a highly structured interview designed for use by lay interviewers. Field interviewers received a week of study-specific training and successfully completed practice interviews before beginning the interviews. Field interviewer supervisors recontacted a random 10% of the respondents for quality control. Frequent contacts between field interviewers and supervisors were held to prevent interview drift, to monitor quality, and to provide continuous feedback. Adequate reliability for the DIS has been reported [59]. The DIS has been used in a variety of NIMH sponsored research projects that require psychiatric assessments of large numbers of subjects [60]. Number of symptoms reported by participants were used as outcomes and ranged from 0 to 9 for MDD and AA and 0 to 7 for DD: depression (M = 3.85, SD = 2.76), dysthymia (log transformed to correct high skew, M = 1.12, SD = .66) and alcohol abuse (log transformed to correct high skew, M = 1.00, SD = .81).
Assessment of MAOA
At mean age 41, DNA was obtained from usable blood samples from 617 study members of 631 who submitted DNA samples using the PureGene (Gentra Systems Inc) system according to the manufacturer’s instructions. The MAOA promoter polymorphism was genotyped by PCR amplification using primers MAOA-F2 5′-(TGCTCCAGAAACATGAGCAC) -3′ and MAOA-R2 5′-(GGACAGGCTGTAGGAGGTGTC) -3′. PCR reactions contained 80 ng of template DNA, 1.0 U AmpliTaq Gold polymerase (Applied Biosystems), 1.0 μM of each primer, 0.2 mM dNTP, 2.0 mM MgCl2, and 2μl of GeneAmp 10x buffer II (Applied Biosystems), in a 20 μl volume. After 12 min at 96°C, 40 cycles were done at 96°C for 15 s, at 67.7°C for 20 s, and at 72°C for 30 s, followed by a final extension step at 72°C for 10 min. Products were resolved by Higher Resolution Microplate Array Diagonal Gel Electrophoresis [61], using an 8% polyacrylamide gel run at 150 V for 1hour and 15 minutes.
Representative genotypes were identified and sequenced using a Beckman-Coulter CEQ8000 semi-automated fluorescent sequencing system to confirm the sizes and number of repeats present in the observed alleles. These samples were included as size standards on subsequent gels. DNA was visualized by staining with ethidium bromide and gel images were captured and analyzed using Kodak 1D image analysis software. Any ambiguous genotypes that could not be resolved by repeat PCR and electrophoresis were determined by direct DNA sequencing. One male was identified as heterozygous for MAOA. Presence of a Y-chromosome was verified by PCR amplification of the marker DYS392. As it is unknown if this person has two copies of the MAOA gene (either through an XXY karyotype or a segmental duplication) or is mosaic for MAOA genotype, he was excluded from further analysis.
Overall, the frequencies of the MAOA alleles were 0.023 for 2 repeats, 0.407 for 3 repeats, 0.014 for 3.5 repeats, 0.550 for 4 repeats, and 0.006 for 5 repeats. Chi-square analysis revealed no evidence for deviation from Hardy-Weinberg Equilibrium (χ2 = 7.9, df = 14, p=.89). There is good agreement about the levels of expression associated with the two most common allelic variants (3 and 4 repeats) of the functional promoter polymorphism in MAOA [29–31]. As these comprised over 95% of the alleles observed, we limited further analyses to these alleles
Individuals with genotypes other than 3- and 4-repeat allele combinations (n=41) were excluded from analyses because levels of expression associated with these are ambiguous [29–31]. Males with one or females with two copies of the 3-repeat allele were designated as low activity, males with one or females with two copies of the 4-repeat allele were designated as high activity[29–31]. Heterozygous females were also included as a separate group. The final sample consisted of 575 participants: 238 (41%) controls and 337 (59%) maltreated.
Data Analysis
To determine whether child maltreatment interacts with MAOA genotype to predict MDD, DD, and AA symptoms, we first conducted Ordinary Least Squares (OLS) regressions. The genotype variables were dummy coded (DC) into two variables [DC1 = low-activity genotype (1), others (0), and DC2 = heterozygous women (1), others (0)] and entered as main effects. Interaction terms were created following criteria set in Cohen et al. [62] by multiplying types of maltreatment by the dummy codes for genotype and entering the interaction terms separately.
To assess whether there were further differences in the child maltreatment by genotype interactions, two regressions assessed three-way interactions (MAOA genotype by type of maltreatment by sex and MAOA genotype by type of maltreatment by race), for each outcome (DD, MDD and AA).
Preliminary Analyses
To assess the representativeness of our sample, we compared the original sample to that analyzed here (n = 575) and found no differences in age, sex, race/ethnicity, and abuse/neglect status. We found a significant difference in the distribution of allele frequencies for Whites compared to Non-Whites (χ2 = 19.66, df = 4, p = .001), although there was no difference between the groups (abuse/neglect versus control) (χ2 = 4.56, df = 4, p = .33). Table S1 in the Supplement presents descriptive statistics. Analyses were run using SPSS, Version 19 [63].
Results
Study Findings
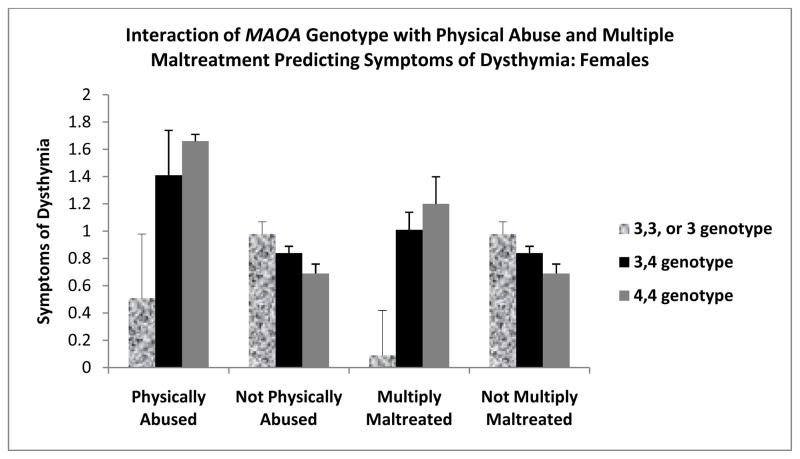
Table 1 shows that none of the maltreatment types interacted with MAOA genotype to predict DD, MDD or AA. Table 2 shows the results of regressions conducted to examine three way interactions between genotype, type of maltreatment, and sex predicting AA, DD, and MDD symptoms. For females only, childhood physical abuse and multiple maltreatment interacted with MAOA genotype to predict dysthymia symptoms, such that the homozygous 3, 3, low-activity allele, but not the homozygous 4, 4, high-activity allele, genpotype was protective for both types of maltreated females (see Figure 1). The heterozygous group tended to fall between the other two groups of females. MAOA genotype did not interact with any of the maltreatment types or in males.1
Table 1.
Two-Way Interactions of MAOA Genotype with Maltreatment Types Predicting Dysthymia, Major Depressive Disorder and Alcohol Abuse Symptoms in Adulthood
| Dysthymiaa (N=571) | Major Depressive Disorder (N=573) | Alcohol Abusea (N=571) | |
|---|---|---|---|
| Predictors | Beta | Beta | Beta |
| Age | .04 | .02 | .03 |
| Sex (Female=1) | .19*** | .19*** | −.30*** |
| Race (White=1) | .02 | .05 | .15*** |
| Genotype DC 1 (3,3=1, others =0) | .11 | .07 | −.07 |
| Genotype DC 2 (heterozygous =1, others=0) | .02 | .02 | −.09 |
| Physical Abuse | .05 | −.001 | −.01 |
| Sexual Abuse | .10 | .05 | .05 |
| Neglect | .23** | .16* | .07 |
| Multiple Maltreatment | .12† | .09 | −.10 |
| Physical Abuse by DC1 | −.05 | −.03 | .02 |
| Physical Abuse by DC2 | .05 | .09† | .04 |
| Sexual Abuse by DC1 | −.04 | −.01 | .04 |
| Sexual Abuse by DC2 | −.01 | .02 | .05 |
| Neglect by DC1 | −.10 | −.04 | −.03 |
| Neglect by DC2 | −.11 | −.08 | .11 |
| Multiple Maltreatment by DC1 | −.04 | −.02 | .06 |
| Multiple Maltreatmetn by DC2 | −.03 | −.04 | .09† |
| Adjusted R2 | .03*** | .06** | .13*** |
Outcomes are log transformed
p < .001;
p < .01;
p < .05;
p < .10
DC1= Dummy code of genotype (3,3=1, others=0); DC2=Dummy code of genotype (heterozygous=1; others =0)
Table 2.
Three-Way Interactions of MAOA Genotype, Type of Child Maltreatment, and Sex Predicting Dysthymia, Major Depressive Disorder, and Alcohol Abuse Symptoms
| Dysthymia Symptomsa (N=573) | Major Depressive Disorder Symptoms (N=573) | Alcohol Abuse Symptomsa (N=571) | |
|---|---|---|---|
| Age | .05 | .03 | .03 |
| Sex (Female=1) | .05 | .14 | −.34** |
| Race (White=1) | .01 | .05 | .15*** |
| Physical Abuse | −.04 | −.08 | −.03 |
| Sexual Abuse | .02 | .03 | −.06 |
| Neglect | .14† | .11 | .02 |
| Multiple Maltreatment | .07 | .06 | −.08 |
| DC1 (3,3=1, others =0) | .08 | .09 | −.05 |
| DC2 (heterozygous =1, others=0) | .10 | .06 | −.07 |
| Physical abuse by DC1 | .05 | .03 | .05 |
| Physical abuse by DC2 | −.05 | .004 | .03 |
| Sexual abuse by DC1 | −.02 | −.04 | .02 |
| Sexual abuse by DC2 | −.05 | .01 | .03 |
| Neglect by DC1 | −.05 | −.03 | −.03 |
| Neglect by DC2 | −.23* | −.15 | .05 |
| Multiple maltreatment by DC1 | .04 | .02 | .07 |
| Multiple maltreatment by DC2 | −.07 | −.06 | .09 |
| Physical abuse by Sex | .21* | .18* | .03 |
| Sexual abuse by Sex | .12 | .13 | .11 |
| Neglect by Sex | .22t | .03 | .13 |
| Multiple Maltreatment by Sex | .09 | .05 | −.02 |
| Sex by DC1 | .07 | −.03 | −.03 |
| Physical Abuse by DC1 by Sex | −.18* | −.12† | −.06 |
| Sexual Abuse by DC1 by Sex | −.02 | .06 | .05 |
| Neglect by DC1 by Sex | −.10 | −.01 | −.001 |
| Multiple by DC1 by Sex | −.14* | −.09 | −.04 |
| Adjusted R2 | .05** | .03* | .11** |
Note:
Outcomes are log transformed
p < .001;
p < .01;
p < .05;
p < .10
DC1= Dummy code of genotype (3,3=1, others=0); DC2=Dummy code of genotype (heterozygous=1; others =0)
Figure 1.
Means of symptoms of dysthymia as a function of MAOA activity and a history of physical abuse and multiple maltreatment in females. MAOA is divided into 3,3 alleles, 4,4 alleles and 3,4 heterozygous. Sexual abuse and multiple maltreatment histories are based on documented court cases. Symptoms of dysthymia were log tranformed to correct for skew. Physically abused or multiply maltreated women with 4,4 high activity genotype were at significantly increased risk (p < .05 for both) for developing dysthymia symptoms than women with 3,3, low-activity genotype. Pattern was absent for non-maltreated females and males.
The results of regressions to test interactions between genotype, type of maltreatment, and race were significant for all three mental health outcomes (AA, DD, and MDD) (see Table 3). Childhood sexual abuse interacted with genotype and race to predict symptoms of DD, MDD and AA. The 3,3 or 3 allele genotype was protective against DD, MDD, and AA symptoms for sexually abused Whites, whereas the 4,4 or 4 allele genotype was protective for non-Whites (see Figure 2A–C).2
Table 3.
Three-Way Interactions of MAOA Genotype, Type of Child Maltreatment, and Race Predicting Major Depressive Disorder, Dysthymia, and Alcohol Abuse Symptoms
| Dysthymia Symptomsa (N=573) | Major Depressive Disorder Symptoms (N=573) | Alcohol Abuse Symptomsa (N=571) | |
|---|---|---|---|
| Age | .02 | .01 | .00 |
| Sex (Female=1) | .18** | .18** | −.30*** |
| Race (White=1) | −.14 | −.12 | .14 |
| Physical Abuse | .03 | −.13 | .20 |
| Sexual Abuse | −.03 | −.05 | −.05 |
| Neglect | .05 | −.03 | .16 |
| Multiple Maltreatment | .13* | .11† | −.10 |
| DC 1 (3,3=1, others =0) | .13 | .04 | −.09 |
| DC 2 (heterozygous =1, others=0) | −.08 | −.08 | −.16 |
| Physical abuse by DC1 | −.03 | −.04 | .05 |
| Physical abuse by DC2 | .09 | .18* | −.03 |
| Sexual abuse by DC1 | .11 | .17* | .25** |
| Sexual abuse by DC2 | .11 | .13 | .13 |
| Neglect by DC1 | −.09 | .04 | −.04 |
| Neglect by DC2 | −.07 | −.06 | .01 |
| Multiple maltreatment by DC1 | −.10 | −.06 | .001 |
| Multiple maltreatment by DC2 | −.07 | −.06 | .20* |
| Physical abuse by race | .04 | .16 | −.24† |
| Sexual abuse by race | .16t | .13 | .14 |
| Neglect by race | .25* | .27* | −.14 |
| Multiple Maltreatment by race | .03 | .02 | −.14† |
| Race by DC1 | −.04 | .02 | .03 |
| Race by DC2 | .12 | .13 | .11 |
| Physical Abuse by DC1 by Race | N/A | N/A | N/A |
| Physical Abuse by DC2 by Race | −.06 | −.11 | .06 |
| Sexual Abuse by DC1 by Race | −.19* | −.22* | −.27** |
| Sexual Abuse by DC2 by Race | −.15† | −.15† | −.11 |
| Neglect by DC1 by Race | −.01 | −.09 | .01 |
| Neglect by DC2 by Race | −.04 | −.02 | .13 |
| Multiple by DC1 by Race | .06 | .04 | .09 |
| Multiple by DC2 by Race | N/A | N/A | N/A |
| Adjusted R2 | .05** | .05** | .13** |
Notes:
Outcomes are log transformed
DC1= Dummy code of genotype (3,3 or 3 =1, others=0); DC2=Dummy code of genotype (3,4 =1; others =0)
p < .001;
p < .01;
p < .05;
p < .10
Figure 2.
Means of symptoms of dysthymia, major depressive disorder and alcohol abuse as a function of MAOA activity and a history of sexual abuse in Whites and Non Whites. MAOA is divided into 3,3or 3 alleles, 4,4 or 4 alleles and 3,4 heterozygous females. Sexual abuse histories are based on documented court cases. Symptoms of dysthymia and alcohol abuse were log tranformed to correct for skew. Sexually abused Whites with 4,4 or 4, high activity, genotype were at significantly increased risk (p < .05 for all) for developing dysthymia, major depressive and alcohol symptoms than sexually abused Whites with 3,3 or 3, low-activity, genotype. Sexually abused Non Whites with 3,3 or 3, low activity, genotype were at significantly increased risk (p < .05 for all) for developing dysthymia, major depressive and alcohol symptoms than sexually abused Non-Whites with 4,4 or 4, high-activity genotype.
Discussion
We did not find that childhood abuse and neglect interacted with MAOA genotype to predict symptoms of Dythymia, Major Depressive Disorder or Alcohol Abuse. However, participant sex and race moderated these relationships and we observed significant interactions between types of maltreatment and genotype for females, but not males, and reverse patterns of vulnerability for Whites and non-Whites.
For women, there were significant interactions in the prediction of Dysthymia, where the high-activity MAOA allele acted as a risk or vulnerability factor for physically abused and multiply maltreated females, consistent with other findings [50]. Thus, high levels of MAOA were associated with increased vulnerability for certain types of maltreatment (physical abuse and multiple) for dysthymia symptomatology as opposed to low levels that have been suggested to act as vulnerability for aggression [41]. It is interesting that depressive disorders have been treated by MAO inhibitors for decades, suggesting that reducing MAOA activity reduces depressive symptomatology [64]. Thus, one might expect that high levels of MAOA, not low levels, should be a risk factor for symptoms of depressive disorders. Research on MAOA activity outside the field of childhood maltreatment has shown some evidence suggestng that the high activity MAOA allele is a vulnerability factor. For example, depressed bereaved participants had more complicated grief symptoms with more MAOA activity than with less [65]. Meyer et al. [66] demonstrated increased MAOA density among depressed individuals compared to healthy controls, suggesting that higher MAOA activity is related to depressive symptomatology among clinically depressed individuals.
Our finding that MAOA moderates the relationship between childhood maltreatment and dysthymia only in females requires further comment. There are a number of possible reasons for the sex differences in the current study, including hormonal and gene interactions. The MAOA promoter interacts with both corticosteroids and androgens [67, 68] which show sex differences during perinatal development [69]. Additionally, there is evidence that during adolescence, MAOA may interact with testosterone which is differentially active for males and females [47]. Lastly, it is not clear whether females use both alleles of the MAOA genotype, located on the X-chromosome [70], and activation of both alleles may lead to sex differences in MAOA dosage. In the current study, heterozygous females’ symptoms tended to fall between the people with low-activity and high-activity MAOA genotype.
Thus, there are at least three possible ways to interpret the findings that the high activity allele combination may act as a vulnerability only for maltreated females and not males: (1) the effects of MAOA genotype on antisocial behavior and mood are different [50], (2) the direction of expression of MAOA genotype is different for males and females, and (3) these are chance findings. These explanations have different implications regarding the transcription of MAOA, suggesting either sex-based and/or disorder based variation or no variation at all. Based on the findings from this study, none of these explanations can be disqualified. Sex effects on the direction of MAOA genotype vulnerability for maltreated participants have been observed in relation to other mental health outcomes [48, 52, 71, 72]. Thus, it is likely that both sex-based and disorder-based differences exist.
We did not find sex differences in the way genotype moderates the relationship between child maltreatment and alcohol symptoms. Other studies have reported different sex effects in the prediction of alcoholism. Studies by Nillsson and colleagues [52–54] found that the interaction of MAOA and self-reported family functioning predicted adolescent alcohol related problem behaviors and excessive alcohol consumption. The direction of vulnerabilites differed for girls and boys and was stronger for girls. Nillsson and colleagues used retrospective self-reports of poor family functioning as a proxy for abuse and self-report of sexual abuse, clearly different from the operationalizations of abuse and neglect used here which involved official records of physical and sexual abuse and neglect. Thus, it is possible that the discrepancy between the current findings and past research is due to differences in design, participant age, criteria for maltreatment and analyses. It is also possible that these relationships may pertain only to certain types of alcoholism [73]. The Nillsson et al. study focused on the more antisocial type of alcoholism, whereas here we include both types.
We also found an interaction between participant’s race, childhood sexual abuse, and MAOA genotype in the prediction of dysthymia, major depressive disorder and alcohol abuse symptoms. The low-activity genotype acted as a protective factor for Whites, whereas the high-activity genotype acted as a protective factor for non-Whites. Interestingly, Widom and Brzustowicz (2006) examined race differences in the MAOA genotype by maltreatment interaction in the prediction of antisocial behavior using the same sample. They also observed race differences, but found a different pattern – significant interactions for Whites, but not for non-Whites. To our knowledge, no other studies have examined race differences.
Despite a number of strengths, certain limitations of this study should be noted. These findings are based on genetically-inferred (not verified) ethnicities, and this might have led to mistakes in ethnicity. The cases for the current study were drawn from court records in the Midwest of the United States and likely represent the more extreme cases of abuse and neglect. Results may not generalize to cases from other regions and maltreatment based on self-reports. Although the patterns of these results was consistent, because multiple statistical analyses were performed, the findings need to be replicated. In the context of the debate about the validity of findings of studies of gene by environment interactions, the quality of our sample, based on court-substantiated cases of abuse and neglect and matched controls, and the prospective follow-up, makes the possibility of spurious findings less likely. It is also possible that our failure to find significant interactions for males may have been due to the small sample size of certain types, i.e. sexual abuse, and limited power. Finally, gene-environment correlation, the notion that children with particular types of genotypes are more likely to be maltreated, cannot be ruled out here as a potential confound.
Nonetheless, these new findings provide evidence for the role of MAOA genotype as a moderator of the relationship between childhood maltreatment and mental health outcomes. Future research would benefit from replication and examination of other genotypes to further understand how genes and adverse environments play a role in these relationships.
Supplementary Material
Footnotes
The results of two additional regressions (not shown) comparing sexually abused participants to controls and multiply maltreated participants to controls revealed the same pattern of results, with low-activity genotype being protective against symptoms of dysthymia in females.
The results of additional regressions (not shown) to examine race differences in sexually abused participants compared to controls in the prediction of dysthymia, depression and alcohol abuse showed the same pattern with the genotypic vulnerabilites differing for White and Non-White participants.
Financial Disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Valentina Nikulina, Email: vnikulina@jjay.cuny.edu.
Cathy Spatz Widom, Email: cwidom@jjay.cuny.edu.
Linda M. Brzustowicz, Email: brzustowicz@biology.rutgers.edu.
References
- 1.Widom CS, DuMont KA, Czaja SJ. A prospective investigation of major depressive disorder and comorbidity in abused and neglected children grown up. Arch Gen Psychiatry. 2007;64:176–187. doi: 10.1001/archpsyc.64.1.49. [DOI] [PubMed] [Google Scholar]
- 2.Libby AM, Orton HD, Novis DK, Beals J, Manson SM Al SUPERPFP Team. Childhood physical and sexual abuse and subsequent depressive and anxiety disorders for two American Indian tribes. Psychol Med. 2005;35:329–340. doi: 10.1017/s0033291704003599. [DOI] [PubMed] [Google Scholar]
- 3.Gibb BE, Butler AC, Beck JS. Childhood abuse, depression, and anxiety in adult psychiatric outpatients. Depress Anxiety. 2003;17:226–228. doi: 10.1002/da.10111. [DOI] [PubMed] [Google Scholar]
- 4.Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. Am J Psychiatry. 1991;148:55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Kessler RC, Crum RM, Warner LA, Nelson CB. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- 6.McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AD, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- 7.Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA. Childhood sexual abuse and adult psychiatric and substance use disorders in women: An epidemiological and cotwin control analysis. Arch Gen Psychiatry. 2000;57:953–959. doi: 10.1001/archpsyc.57.10.953. [DOI] [PubMed] [Google Scholar]
- 9.Bifulco A, Brown GW, Adler Z. Early sexual abuse and clinical depression in adult life. Br J Psychiatry. 1991;159:115–122. doi: 10.1192/bjp.159.1.115. [DOI] [PubMed] [Google Scholar]
- 10.Sartor CE, Agrawal A, McCutcheon VV, Duncan AE, Lynskey MT. Disentangling the complex association between childhood sexual abuse and alcohol-related problems: A review of methodological issues and approaches. J Stud Alcohol Drugs. 2008;69:718–727. doi: 10.15288/jsad.2008.69.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson E, Heath A, Madden P, Cooper M, Dinwiddie S, Bucholz K, et al. Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: results from a twin study. Arch Gen Psychiatry. 2002;59:139–145. doi: 10.1001/archpsyc.59.2.139. [DOI] [PubMed] [Google Scholar]
- 12.Brems C, Johnson ME, Neal D, Freemon M. Childhood abuse history and substance use among men and women receiving detoxification services. Am J Drug Alcohol Abuse. 2004;30:799–821. doi: 10.1081/ada-200037546. [DOI] [PubMed] [Google Scholar]
- 13.Fergusson DM, Lynskey MT. Physical punishment/maltreatment during childhood and adjustment in young adulthood. Child Abuse Negl. 1997;21:617–630. doi: 10.1016/s0145-2134(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 14.Galaif ER, Stein JA, Newcomb MD, Bernstein DP. Gender differences in the prediction of problem alcohol use in adulthood: Exploring the influence of family factors and childhood maltreatment. J Stud Alcohol. 2001;62:486–493. doi: 10.15288/jsa.2001.62.486. [DOI] [PubMed] [Google Scholar]
- 15.Wilsnack SC, Vogeltanz ND, Klassen AD, Harris TR. Childhood sexual abuse and women’s substance abuse: National survey findings. J Stud Alcohol. 1997;58:264–271. doi: 10.15288/jsa.1997.58.264. [DOI] [PubMed] [Google Scholar]
- 16.Dube SR, Anda RF, Felitti VJ, Edwards VJ, Croft JB. Adverse childhood experiences and personal alcohol abuse as an adult. Addict Behav. 2002;27:713–725. doi: 10.1016/s0306-4603(01)00204-0. [DOI] [PubMed] [Google Scholar]
- 17.Maxfield MG, Widom CS. The cycle of violence: Revisited six years later. Arch Pediatr Adolesc Med. 1996;150:390–395. doi: 10.1001/archpedi.1996.02170290056009. [DOI] [PubMed] [Google Scholar]
- 18.Widom CS. The cycle of violence. Science. 1989;244:160–166. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- 19.Widom CS, Marmostein NR, White HR. Childhood victimization and illicit drug use in middle adulthood. Psychol Addict Behav. 2006;20:394–403. doi: 10.1037/0893-164X.20.4.394. [DOI] [PubMed] [Google Scholar]
- 20.McGloin JM, Widom CS. Resilience among abused and neglected children grown up. Dev Psychopathol. 2001;13:1021–1038. doi: 10.1017/s095457940100414x. [DOI] [PubMed] [Google Scholar]
- 21.DuMont KA, Widom CS, Czaja SJ. Predictors of resilience in abused and neglected children grown-up: The role of individual and neighborhood characteristics. Child Abuse Negl. 2007;31:255–274. doi: 10.1016/j.chiabu.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Collishaw S, Pickles A, Messer J, Rutter, Shearer C, Maughan B. Resilience to adult psychopathology following childhood maltreatment: Evidence from a community sample. Child Abuse Negl. 2007;31:211–229. doi: 10.1016/j.chiabu.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Martin JL, Anderson JC, O’Shea ML, Mullen DE. Factors that mediate between child sexual abuse and adult psychological outcome. Psychol Med. 1995;25:127–142. doi: 10.1017/s0033291700028154. [DOI] [PubMed] [Google Scholar]
- 24.Cicchetti D, Rogosch FA, Lynch M, Holt KD. Resilience in maltreated children: Processes leading to adaptive outcomes. Dev Psychopathol. 1993;5:629–647. [Google Scholar]
- 25.Cicchetti D, Rogosch FA. The role of self-organization in the promotion of resilience in maltreated children. Dev Psychopathol. 1997;9:797–815. doi: 10.1017/s0954579497001442. [DOI] [PubMed] [Google Scholar]
- 26.Heller SS, Larrieu JA, D’Imperio R, Boris NW. Research on resilience to child maltreatment: Empirical considerations. Child Abuse Negl. 1999;23:321–338. doi: 10.1016/s0145-2134(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 27.Jaffee S, Caspi A, Moffitt TE, Polo-Tomas M, Taylor A. Individual, family, and neighborhood factors distinguish resilient from non-resilient maltreated children: A cumulative stressors model. Child Abuse Negl. 2007;31:231–253. doi: 10.1016/j.chiabu.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy ER, Powell JF, Buckle VJ, Hsu YP, Breakfield XO, Craig IW. Localization of human monoamine oxidase-A gene to Xp11.23-11.4 in situ hybridization: Implications for Norrie disease. Genomics. 1989;5:368–370. doi: 10.1016/0888-7543(89)90072-4. [DOI] [PubMed] [Google Scholar]
- 29.Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase: A gene promotor. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- 30.Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, et al. Excess of high activity monoamine oxidase A gene promotor alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- 31.Denney RM, Koch H, Craig IW. Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promotor-associated variable number tandem repeat. Hum Genet. 1999;105:542–551. doi: 10.1007/s004399900183. [DOI] [PubMed] [Google Scholar]
- 32.Shih JC, Thompson RF. Monoamine oxidase in neuropsychiatry and behavior. Am J Hum Genet. 1999;65:593–598. doi: 10.1086/302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keltikangas-Jarvinen L, Salo J. Dopamine and serotonin systems modify environmental effects on human behavior: A review. Scandinavian Journal of Psychology. 2009;50:574–582. doi: 10.1111/j.1467-9450.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- 34.Horwitz AV, Widom CS, McLaughlin J, White HR. The impact of early childhood abuse and neglect on adult mental health: A prospective study. J Health Soc Behav. 2001;42:184–201. [PubMed] [Google Scholar]
- 35.Allen B. An analysis of the impact of diverse forms of childhood psychological maltreatment on emotional adjustment in early adulthood. Child Maltreat. 2008;13:307–312. doi: 10.1177/1077559508318394. [DOI] [PubMed] [Google Scholar]
- 36.Widom CS, White HR, Czaja SJ, Marmorstein NR. Long-term effects of child abuse and neglect on alcohol use and excessive drinking in middle adulthood. J Stud Alcohol Drugs. 2007;68:317–326. doi: 10.15288/jsad.2007.68.317. [DOI] [PubMed] [Google Scholar]
- 37.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 38.Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HM, Kuhn J, et al. Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson KW, Sjoberg RL, Damberg M, Leppert J, Ohrvik J, Alm PO, et al. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry. 2006;59:121–127. doi: 10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 41.Widom CS, Brzustowicz LM. MAOA and the ‘cylce of violence’: Childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 42.Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, et al. MAOA genotype, maltreatment, aggressive behavior: The changing impact of genotype at varying levels of trauma. Biol Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prom-Wormley ES, Eaves LJ, Foley DL, Archer KJ, Wormley BK, Maes HH, et al. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychol Med. 2009;39:579–590. doi: 10.1017/S0033291708004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huzinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, et al. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biol Psychiatry. 2005;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 45.van der Vegt EJM, Oostra BA, Atrias-Vasquez A, van der Ende J, Verhulst FC, Tiemeier H. High activity of Monoamine oxidase A is associated with externalizing behaviour in maltreated and nonmaltreated adoptees. Psychiatr Genet. 2009;19:209–211. doi: 10.1097/YPG.0b013e32832a5084. [DOI] [PubMed] [Google Scholar]
- 46.Taylor A, Kim-Cohen J. Meta-analysis of gene-environment interactions in developmental psychopathology. Dev Psychopathol. 2007;19:1029–1037. doi: 10.1017/S095457940700051X. [DOI] [PubMed] [Google Scholar]
- 47.Sjoberg RL, Nilsson KW, Wargelius H, Leppert J, Lindstrom L, Oreland L. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosocial factors. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- 48.Aslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson K. Maltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behavioral Genetics. 2010;41:262–272. doi: 10.1007/s10519-010-9356-y. [DOI] [PubMed] [Google Scholar]
- 49.Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: Depressive symptomatology among adolescents from low socioeconomic status backgrounds. Dev Psychopathol. 2007;19:1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- 50.Beach SRH, Brody GH, Gunter TD, Packer H, Wernett P, Philibert RA. Child maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorder. J f Fam Psych. 2010;24:12–20. doi: 10.1037/a0018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, et al. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson KW, Wargelius H, Sjoberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAOA-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug Alcohol Depend. 2008;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 53.Nilsson K, Comasco E, Aslund C, Nordquist N, Leppert J, Oreland L. MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict Biol. 2010;6:347–355. doi: 10.1111/j.1369-1600.2010.00238.x. [DOI] [PubMed] [Google Scholar]
- 54.Nilsson KW, Sjoberg RL, Wargelius H, Leppert J, Lindstrom L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- 55.Widom CS. Child abuse, neglect and adult behavior: Research design and findings on criminality, violence, and child abuse. Am J Orthopsychiatry. 1989;59:355–367. doi: 10.1111/j.1939-0025.1989.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 56.Edvardsen J, Torgersen S, Roysamb E, Lygren S, Skre I, Onstad S, et al. Unipolar depressive disorders have a common genotype. J Affect Disord. 2009;117:30–41. doi: 10.1016/j.jad.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Hollingshead AB. Four-factor index of social status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 58.Robins LN, Helzer JE, Cottler L, Goldring E. National Institute of Mental Health Diagnostic Interview Schedule, Version III Revised (DIS-III-R) St. Louis, MO: Washington University; 1989. [Google Scholar]
- 59.Robins LN, Helzer JE, Croughan JL, Ratcliff KS. National Institute of Mental Health diagnostic interview schedule: Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 60.VonKurff M, Shapiro S, Burke JD, Teitlebaum M, Skinner EA, German P, et al. Anxiety and depression in a primary care clinic. Arch Gen Psychiatry. 1987;44:152–156. doi: 10.1001/archpsyc.1987.01800140058008. [DOI] [PubMed] [Google Scholar]
- 61.Day IN, Humphries SE. Electrophoresis for genotyping: microtiter array diagonal gel electrophoresis on horizontal polyacrylamide gels, hydrolink, or agarose. Anals of Biochemistry. 1994;222:389– 395. doi: 10.1006/abio.1994.1507. [DOI] [PubMed] [Google Scholar]
- 62.Cohen P, Cohen J, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3. Hillsdale, New Jersey: Routledge Academic; 2002. [Google Scholar]
- 63.IBM SPSS Software Version 19
- 64.Baker GB, Coutts RT, McKenna KF, Sherry-McKenna RL. Insights into the mechanisms of action of the MAO inhibitors Phenelzine and Tranylcypromine: A review. J Psychiatry Neurosci. 1992;17:206–214. [PMC free article] [PubMed] [Google Scholar]
- 65.Kersting A, Kroker K, Horstmann J, Baune BT, Hohoff C, Mortensen LS, et al. Association of MAO-A variant with complicated grief in major depression. Neuropsychobiology. 2007;56:191–196. doi: 10.1159/000120624. [DOI] [PubMed] [Google Scholar]
- 66.Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. Elevated monoamine oxidase A levels in brain. An explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 67.Ou X, Chen K, Shih JC. Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by R1 and Sp1. Jorn Biol Chem. 2006;281:21512–21525. doi: 10.1074/jbc.M600250200. [DOI] [PubMed] [Google Scholar]
- 68.Sjoberg RL, Ducci F, Barr CS, Newman TK, Dell’Osso L, Virkkunen M, et al. A Non-Additive Interaction of a Functional MAO-A VNTR and Testosterone Predicts Antisocial Behavior. Neuropsychopharmacology. 2008;33:425–430. doi: 10.1038/sj.npp.1301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Collaer ML, Hines M. Human behavioral sex differences: A role of gonadal hormones during early development? Psychol Bull. 1995;118:55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- 70.Carrel RJ, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 71.Sjöberg RL, Nilsson KW, Wargelius H-L, Leppert J, Lindström L, Oreland L. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosocial factors. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2007;114B:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds S. Gene influences the impact of maternal smoking on children’s behavioral problems. NIDA Publications. 2011;23:1–5. [Google Scholar]
- 73.Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse: Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.