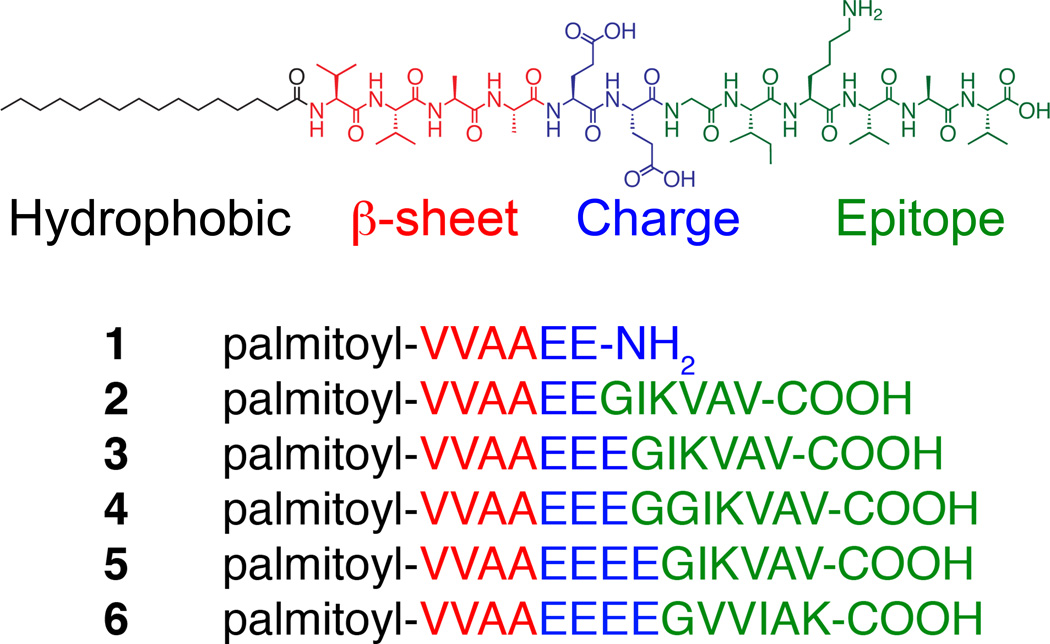
Understanding how to design artificial extracellular matrices that effectively signal and direct cellular responses is essential for the creation of new regenerative medicine therapies.[1]. Injectable, self-assembling biomaterials capable of forming scaffolds in situ around cells are promising therapeutic candidates because of their minimally invasive delivery.[1c, 2] The signalling efficacy of self-assembling bioactive structures will depend not only on molecular structure but also on nanoscale morphology as well.[1b, 3] Peptide amphiphiles (PAs) (Scheme 1) are a class of molecules that spontaneously self-assemble into a variety of nanostructures, including spherical micelles, fibers, and ribbons, and have shown promising therapeutic functions.[1c, 4] Fibers have been found to be particularly bioactive and the PA molecules that form them consist typically of four main segments: (1) a hydrophobic group, commonly an alkyl tail, that drives aggregation through hydrophobic collapse; (2) a β-sheet-forming peptide that promotes nanofiber formation; (3) a peptide segment that contains ionizable side chain residues; and (4) a signaling moiety designed to interact with cellular receptors. These molecules self-assemble into high-aspect ratio nanofibers, forming gels in water at low concentrations when the charges on the ionic side chains are appropriately screened. These cylindrical nanofibers display bioactive sequences perpendicular to their long axis at near Van der Waals density.[5]
Scheme 1.
Peptide amphiphile design includes a hydrophobic group (black), β-sheet forming sequence (red), charged amino acids (blue), and a bioactive epitope (green). 1–5 is a list of synthesized peptide amphiphiles.
We have recently shown that PAs containing the laminin-derived pentapeptide IKVAV can induce differentiation of neural stem cells into neurons, promote neurite outgrowth, and lead to functional improvement after acute spinal cord injury.[5–6] Therefore, structures containing this epitope could also have a profound impact on regenerative therapies requiring new neurons such as Parkinson’s and Alzheimer’s disease, and also help to repair brain tissue following trauma or stroke. The IKVAV epitope has been shown to bind to at least two receptors, a 110 kDa laminin binding protein (LBP110/APP) and nucleolin,[7] although the exact molecular arrangement of this binding and the signal transduction pathways have yet to be elucidated. This IKVAV pentamer contains mostly amino acids with hydrophobic residues that have a strong β-sheet propensity,[8] and peptides containing this epitope have a strong tendency to form amyloid like fibrils.[9] If this IKVAV segment were to exist in a rigid β-sheet conformation with neighboring epitopes, its ability to bind to the target receptor would be highly restricted. In fact, in previous studies on IKVAV covalently grafted to polymer scaffolds, enhanced neurite outgrowth and neuronal differentiation were not observed,[10] possibly due to ineffective epitope presentation. Consequently, an effective supramolecular strategy to control epitope presentation of this and other hydrophobic bioactive signals is required to enhance the signal transduction of biomaterials.
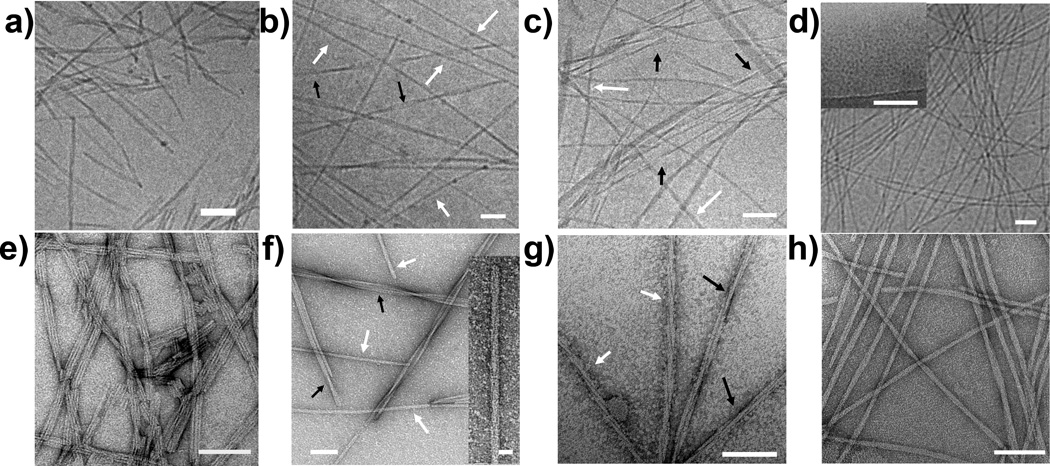
We report here on a design strategy that utilizes electrostatics to control the assembly behavior of PA molecules containing the hydrophobic IKVAV epitope. The PAs in this study contain a palmitic acid tail, a VVAA β-sheet forming region, different numbers of charged glutamic acid residues and glycine residues, and IKVAV (Scheme 1). The key element of the design is the increased number of charged amino acid residues preceding the IKVAV segment, to reduce the propensity for epitope aggregation. PAs were synthesized via solid-phase Fmoc synthesis and purified via high-performance liquid chromatography (Figure S1 in the Supporting Information). We studied the assembly of each molecule under physiologically relevant conditions using cryogenic electron microscopy (cryoTEM) (Figure 1a–d). Here, PAs were dissolved in isotonic salt solutions resembling extracellular fluid (150 mM NaCl, 3 mM KCl, pH 7.4). We also used conventional transmission electron microscopy (TEM) to study the assembly of the various molecules, using samples dissolved in water (Figure 1e–h).
Figure 1.
(a–c, d inset) CryoTEM images of 2–5, in an 150 mM NaCl, 3 mM KCl isotonic salt solution. (d) CryoTEM of 5 dissolved in a 150 mM NaCl, 3 mM KCl, 5 mM CaCl2 salt solution. (e–h, f inset) TEM images of 2–5 from solutions prepared in H2O. In both (b) and (f), black and white arrows refer to trimer bundle motifs, and cylindrical assemblies, respectively. In both (c) and (g), black and white arrows refer to triple helices, and dimer bundle motifs, respectively.
PA 1, which lacks the IKVAV epitope, assembles into canonical cylindrical fibers (Figure S2a) that have an average diameter of 6.6 ± 0.9 nm (Figure S3). This diameter is 87% of twice the length of 1 based on MM+ molecular simulations, thus corresponding approximately to the expected diameter of cylindrical fibers consisting of hydrophobically collapsed β-sheets displaying the epitope on their surfaces (Table 1). In order to compare molecular simulation diameters with those observed from TEM, we scaled the molecular simulation values by 87%. PA 2, which incorporates the IKVAV epitope, forms ribbon morphologies in solution that are comprised of at least three cylindrical fibers 6.0 ± 0.5 nm in diameter side-by-side (Figure 1a,e). The low q region of the small angle x-ray scattering (SAXS) curve (q < 10−1 Å) can be fit to a ribbon morphology with a 6.0 × 18.0 nm rectangular cross-section (Figure 2a, S4). This confirms that ribbons of three bundled cylindrical nanofibers are the dominant supramolecular architecture (Figure S2b). The predicted diameter of fibers of 2 from simulations is 9.7 nm. The reduction in fiber diameter from 1 to 2 suggests that the hydrophobic IKVAV epitopes are interdigitated and forming antiparallel β-sheets with the VVAA core of neighboring fibers.
Table 1.
Observed morphologies from TEM, simulated molecular length, scaled simulated diameter, and observed diameter of individual fibers of 1–5 from TEM.
| PA | Assembled Morphology |
Simulated Molecular Length (Å) |
Scaled, Simulated Diameter (Å) |
Observed Fiber Diameter (Å) |
|---|---|---|---|---|
| 1 | Cylindrical fiber | 38.0 | 66 | 66 ± 9 |
| 2 | Trimer ribbon | 55.5 | 97 | 60 ± 5 |
| 3 | Trimer ribbon | 59.6 | 104 | 71 ± 10 |
| 3 | Cylindrical assembly | 59.6 | 104 | 128 ± 14 |
| 4 | Dimer bundle | 63.5 | 110 | 77 ± 9 |
| 4 | Triple helix | 63.5 | 110 | 81 ± 10 |
| 5 | Cylindrical fiber | 60.1 | 105 | 108 ± 15 |
Figure 2.
(a) SAXS spectra of synthesized PA molecules at 1 wt% dissolved in 150 mM NaCl, 3 mM KCl, and when noted, 5 mM CaCl2. Curves were shifted vertically for visually convenience. (Inset) Fit of 5 to a spherical micelle model. (b) CD curves of 1 wt% PA solutions dissolved in the same salt solutions.
When three glutamic acids are incorporated into the charged region, a mixture of interdigitated assemblies is observed. CryoTEM and conventional TEM (Figure 1b,f) showed that 3 assembled into a mixture of trimeric ribbons with fiber diameters of 7.1 ± 1 nm as well as cylindrical assemblies with a 12.8 ± 1.3 nm diameter. For the trimer ribbon motif, this increase in radius of ~0.5 nm from 2 to 3 corresponds closely to the simulated increase in molecular length upon addition of an amino acid (~0.4 nm). The structure that appears as a cylindrical assembly does not correspond to a conventional cylindrical aggregate; the diameter is larger than the length expected from molecular simulations (10.4 nm), and there is a helical twist 8–12° along the vertical axis of the fiber (Figure 1f inset), supporting the notion that this supramolecular assembly is larger than two molecules. The difficulty in preparing solutions containing only cylindrical assemblies prevents elucidation of the structure from NMR. PA 4, containing an additional glycine residue between the charged EEE and the IKVAV segments, also forms a mixture of aggregated fibers, including bundled dimers (Figure S2c) consisting of 8.1 ± 1.0 nm fibers, triple helices (Figure S2d) comprised of 7.7 ± 0.9 nm diameter fibers, and larger aggregates (Fig 1c,g).
To disrupt interdigitation, a fourth glutamic acid was added to the negatively charged domain in 5. Surprisingly, this additional charged amino acid disrupted β-sheet formation and prevented self assembly into nanofibers under unscreened conditions. Only spherical micelles (Figure S2e) having a diameter of 5.3 ± 1.1 nm were observed in CryoTEM micrographs of 5 (Figure 1d inset). Furthermore, the SAXS curve can be fit with a core-shell spherical micelle model with a diameter of 5.7 ± 1.2 nm (Figure 2a inset, S5). Dynamic light scattering data confirmed further that solutions were comprised of spherical micelles with a hydrodynamic radius of 8.6 nm. The lack of positive ellipticity in the CD spectrum indicates that 5 has a random coil peptide structure in the supramolecular aggregates it forms (Figure 2b). There is also no β-sheet peak at 4.7 Å in the wide angle x-ray scattering (WAXS) curve (Figure S6). However, when a 1 wt % solution was dropcast and dried onto a TEM grid, cylindrical nanofibers with an average diameter of 10.8 ± 1.5 nm were the only structures observed (Figure 1h). This diameter corresponds to 88% of twice the simulated extended length of the molecule. The change in supramolecular structure is likely the result of an increase in concentration of PA and salt impurities during the drying process involved in the preparation of the TEM grid. This observation suggests that assembly of 5 into cylindrical nanostructures can occur when the charge on the glutamic acids are properly screened. Indeed, when 5 mM of divalent CaCl2 was added to the isotonic salt solution, CryoTEM revealed the existence of the cylindrical structures having a diameter of 9.9 ± 1.1 nm (Figure 1d). Furthermore, the CD spectrum became positive at 210 nm, indicating the presence of β-sheet structure (Figure 2b). A weak β-sheet peak at 4.7 Å was also observed in the WAXS spectrum (Figure S6). The addition of calcium is physiologically relevant as the cerebrospinal fluid of the nervous system contains 1.05–1.35 mM of Ca2+.[11] No significant change in morphology was observed in the SAXS data or CryoTEM for 1–4 when 5 mM CaCl2 was added to the isotonic salt solution.
To assess the ability of the molecules to promote neurite outgrowth, we quantified the average neurite length from neurons cultured in gels composed of networks of the filamentous structures formed by PAs 1–5. Pluripotent murine P19 embryonal carcinoma cell line were differentiated into neurons. These neurons were then homogeneously distributed into isotonic salt solutions composed of 0.8 wt% 1 mixed with 0.2 wt% 2–5 and gelled with 25 mM CaCl2 (Figure 3a,b). Mixtures of 1 with 2–5 were used in biological experiments in order to form robust gel matrices capable of supporting cells in culture. After two days of culture, the cells were fixed and the neurites and nuclei were fluorescently labelled green and blue using β-III-tubulin immunostaining and Hoechst staining, respectively, then imaged by confocal microscopy (Figure 3a,b). Neurites were traced and measured, and average neurite lengths were calculated (Figure 3c). Surprisingly, we did not observe enhancement of neurite outgrowth when neurons were cultured in networks formed by 2. However, in networks formed by PAs with additional glutamic acid residues in the charged domain of the peptide neurite outgrowth was significantly increased. Cells cultured in gels containing 0.2 wt% 3 and 5 revealed a 42±13% and 88±13% increase in neurite outgrowth, respectively.
Figure 3.
Representative flattened-stack confocal fluorescence images of neurons cultured in peptide amphiphile gels containing 0.8 wt% 1 with (a) 0.2 wt% 2 and (b) 0.2 wt% 5. Green = β-III-tubulin, Blue = Hoechst. Scale bar = 20 µm. C) Average neurite lengths of neurons cultured in peptide amphiphile gels. n > 59. * p < 0.01 to 1, ^ p < 0.05 to 3.
Data obtained in this work demonstrate that surface display of the IKVAV sequence in supramolecular filaments is critical for strong bioactivity. IKVAV-bearing nanostructures that were less likely to form interdigitated bundles of cylindrical fibers (3, 5) promoted neurite outgrowth, while those that bundled (2, 4) did not. Less bundling of fibers and thus less masking of the IKVAV epitopes from receptors leads to higher effective concentration of IKVAV epitopes available for binding. To test if concentration of epitope affected neurite outgrowth, gels containing half the concentration (0.1 wt%) of 5 were also assayed, resulting in an increase in neurite length of 57±14%, slightly more than half the effect in gels with 0.2 wt% 5 (Figure 3c). This further supports the notion that by forming less aggregated, cylindrical nanostructures, 5, and to a lesser degree 3, present higher effective concentrations of IKVAV epitopes capable of interacting with target receptors. To consider if surface area determines the concentration of IKVAV available for binding, we estimated the dimer and trimer bundles to have 63% and 52% of the surface area per unit length compared to isolated nanofibers (See Supporting Information). That 0.1 wt% of 5 still produces a significant enhancement in neurite outgrowth, whereas 0.2 wt% 2 and 4 did not suggests that the IKVAV segment in 5 is also more effective at binding to receptors. Since 4, which has the same molecular length as 5, did not show any bioactivity, the enhanced coulombic repulsion 5 is essential to prevent inter- and intra-fiber β-sheet formation between neighboring IKVAV epitopes, fostering a better interaction with the target receptor.
In summary, we have demonstrated that PA nanofibers displaying a hydrophobic epitope such as IKVAV on their surfaces have a high propensity to interdigitate into bundles. This bundling naturally reduces the bioactivity of these self-assembling materials. We have also shown that nanofiber bundling can be suppressed by electrostatic forces. Our results demonstrate that the design of bioactive materials requires not only the incorporation of molecular signals, but also strategies to control their effective display on the nanoscale.
Experimental Section
Detailed synthesis and purification, peptide content analysis, high resolution mass spectrometry, molecular simulations, WAXS analysis, calculation of the percentage of nanostructure surface area that is not interdigitated, and cell culture protocol is included in supporting information.
Footnotes
The authors acknowledge James Hulvat, Liam Palmer, Hongang Cui, Shantanu Sur, and Sunitha Suresh for helpful discussions. We acknowledge the following Northwestern University facilities: IBNAM Peptide Core, IBNAM Cleanroom Core, Keck, BIF, MRC, Cell Imaging Facility (generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center) DLS: C. Shad Thaxton lab. J. B. Cohen X-Ray Diffraction Facility - MRSEC program, National Science Foundation (DMR-0520513). WAXS: Argonne National Laboratory at the BioCARS 14-BM-C beamline. Additional support from the Ben Gurion University in Negev, Israel is also acknowledged in the form of a postdoctoral fellowship for R.B. This project was supported by the Army Research Office (W911NF-09-1-0044). The project described was supported by Award Number F32EB007131 and 5 R01EB003806-05 from the National Institute Of Biomedical Imaging And Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Biomedical Imaging And Bioengineering or the National Institutes of Health.
Supporting information for this article is available on the WWW under http://www.angewandte.org
Contributor Information
Joshua E. Goldberger, Department of Chemistry, Northwestern University, Evanston, IL 60208 (USA) Fax: (+1) 847-491-3010.
Eric J. Berns, Department of Biomedical Engineering, Northwestern University, Evanston, IL 60208 (USA).
Ronit Bitton, Institute for BioNanotechnology in Medicine, Northwestern University, Chicago, IL 60611 (USA).
Christina J. Newcomb, Department of Materials Science and Engineering, Northwestern University, Evanston, IL 60208 (USA)
Samuel I. Stupp, Email: s-stupp@northwestern.edu, Department of Chemistry, Northwestern University, Evanston, IL 60208 (USA) Fax: (+1) 847-491-3010; Department of Materials Science and Engineering, Northwestern University, Evanston, IL 60208 (USA); Feinberg School of Medicine, Northwestern University, Evanston, IL 60208 (USA).
References
- 1.a) Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]; b) Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nat. Mater. 2009;8:543. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]; c) Webber MJ, Kessler JA, Stupp SI. J. Intern. Med. 2009;267:71. doi: 10.1111/j.1365-2796.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer LC, Stupp SI. Acc. Chem. Res. 2008;41:1674. doi: 10.1021/ar8000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Kiessling LL, Gestwicki JE, Strong LE. Angew. Chem. Int. Ed. 2006;45:2348. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Muraoka T, Koh C-Y, Cui H, Stupp SI. Angew. Chem. Int. Ed. 2009;48:5946. doi: 10.1002/anie.200901524. [DOI] [PubMed] [Google Scholar]
- 4.a) Cui H, Webber MJ, Stupp SI. Biopolymers. 2010;94:1. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]; c) Yu Y-C, Berndt P, Tirrell M, Fields GB. J. Am. Chem. Soc. 1996;118:12515. [Google Scholar]
- 5.Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, Stupp SI. Science. 2004;303:1352. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 6.Tysseling-Mattiace VM, Sahni V, Niece KL, Birch D, Czeisler C, Fehlings MG, Stupp SI, Kessler JA. J. Neurosci. 2008;28:3814. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Kibbey MC, Jucker M, Weeks BS, Neve RL, Van Nostrand WE, Kleinman HK. Proc Natl Acad Sci U S. 1993;90:10150. doi: 10.1073/pnas.90.21.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kibbey MC, Johnson B, Petryshyn R, Jucker M, Kleinman HK. J. Neurosci. Res. 1995;42:314. doi: 10.1002/jnr.490420305. [DOI] [PubMed] [Google Scholar]
- 8.a) Kim CA, Berg JM. Nature. 1993;362:267. doi: 10.1038/362267a0. [DOI] [PubMed] [Google Scholar]; b) Levitt M. Biochemistry. 1978;17:4277. doi: 10.1021/bi00613a026. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M, Kadoya Y, Kasai S, Kato K, Mochizuki M, Nishi N, Watanabe N, Kleinman HK, Yamada Y, Nomizu M. FEBS Lett. 2002;530:48. doi: 10.1016/s0014-5793(02)03393-8. [DOI] [PubMed] [Google Scholar]
- 10.a) Saha K, Irwin EF, Kozhukh J, Schaffer DV, Healy KE. J. Biomed. Mater. Res. Part A. 2007;81A:240. doi: 10.1002/jbm.a.30986. [DOI] [PubMed] [Google Scholar]; b) Tong YW, Shoichet MS. Biomaterials. 2001;22:1029. doi: 10.1016/s0142-9612(00)00338-0. [DOI] [PubMed] [Google Scholar]
- 11.Smith SV, Forman DT. Clin Lab Sci. 1994;7:32. [PubMed] [Google Scholar]