Abstract
Here we present the results of an experimental study of the effect of ions produced in a dc corona discharge on inactivation of bacteria on the surface of agarose gel. Both positive and negative corona discharges in various gases at different humidities were studied. The measurements in air, O2, N2, Ar and He mixtures show that there is no inactivation in pure N2, pure O2 and an N2–H2O mixture. The best results were achieved in the case of direct treatment, when discharge was ignited in oxygen and water-containing mixtures. We show that neither UV radiation, ozone or H2O2 nor other neutral active species alone produced by corona have an effect on bacteria viability. It is shown that the main role of charged particles may be related to the faster transport of active peroxide species—cluster ions OH−(H2O)n and H3O+(H2O)n. The efficiency of these radicals is much higher than that of the oxygen radicals and ions (including and O3) and that of nitrogen and argon ions.
1. Introduction
Plasma inactivation of bacteria is one of the first demonstrations of biomedical applications of plasmas. Different types of discharges were successfully and effectively applied for the inactivation of Gram-positive and -negative bacteria [1–10], as well as spores [11–15], and have been described in the literature [2, 4, 7], [16–19]. Inactivation efficiency of plasmas was investigated in various chemical systems, such as air, nitrogen, oxygen and the noble gases. The nature of plasma interactions with bacteria is rather complex, and is clearly different for different conditions, e.g. arc discharge at atmospheric pressure and low-pressure glow discharge [7, 16, 17, 19]. In the frame of a new and actively growing field of plasma chemistry, plasma medicine, understanding the mechanisms of interaction of non-thermal atmospheric pressure discharges with living objects becomes extremely important. Here, we have focused on the investigation of role of the different components of corona discharge plasma in the process of bacteria inactivation on the surface.
Non-thermal plasma generated at atmospheric pressure produces a mix of reactive molecules, charges, electric fields and radiation. The role of all these components has been widely studied (see, e.g., [7], [17–19]) and it was noted that inactivation efficiency highly depends on the presence of charged species for room air conditions [2, 3, 7, 17, 18]; however, the effect of radiation is almost always negligible [2, 3, 7, 17, 18]. A convenient discharge to study the role of plasma-produced active components is dc corona, where the stable, uniform and controllable generation of various neutral active species, charges and global electric fields is possible. Some attempts have previously been made to study the effects of corona discharge on bacteria [20–24]. For example, in nitrogen atmosphere both positive and negative corona were shown to have a relatively low inactivation effect, about 1–2 log reduction in tens of minutes of treatment [20, 21]. In contrast, the addition of H2O2 to the air corona discharge system leads to about 103 times higher efficiency of inactivation (with positive ions being slightly more effective than negative) compared to H2O2 only or corona discharge in Ar [24]. In this study, we attempt to classify different types of species created in corona discharge plasma and assess their importance in achieving bacterial inactivation.
2. Materials and methods
2.1. The corona discharge setup
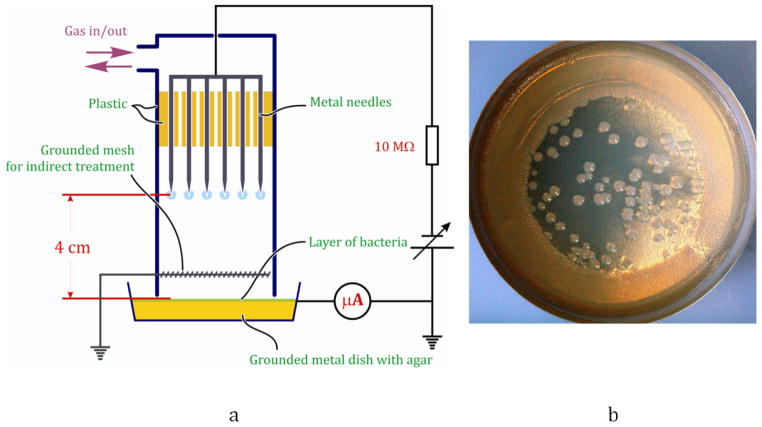
Experiments were conducted using dc corona discharge at atmospheric pressure. A total of 17 needles were uniformly fixed in a cylindrical plastic enclosure with an inner diameter of 5 cm (figure 1(a)). Grounded metal plates filled with agar or grounded metal coupons were placed 4 cm below the needles, and had the role of a second electrode. The corona needles were powered with either positive or negative dc voltage through a 10 MΩ resistor to produce ion flux. The current associated with the charge impinging on the agar plate was measured by an amperemeter connected in series with the grounding connection. By the variation of applied voltage from 5 to 30 kV, the current of ions was varied from 10 to 250 μA. In order to check the inactivation efficiency of neutral active species generated by the discharge, a grounded metal mesh was introduced into the system in the same way as in [2, 3]. The mesh was placed between the high-voltage electrodes and the treated surface. In these sets of experiments, corona current (current in the high-voltage electrodes—mesh circuit) was kept at 100 μA, while the distance between the mesh and bacteria was 2 mm. In addition, the effect of neutral radicals was studied by corona treatment in reverse room air flow (vacuuming the system) at a rate of 1 slpm. The effects of gas composition and presence of water were studied by introducing either dry or wet (gases were bubbled through distilled water, which was boiled prior to the experiment for ~20 min and then cooled to room temperature) air, O2, N2, Ar and He into the discharge volume at a rate of 10 slpm.
Figure 1.
Schematic representation of the dc corona experimental setup (a) and representative picture of bacteria treated by corona discharge in air after incubation (b).
2.2. Preparation of bacteria
In this study, we have used an Escherichia coli (ATCC 25922) suspension in phosphate-buffered saline (PBS) at the initial concentration of about 3 ×109 cells ml−1. Bacteria were treated either on brain heart infusion (BHI) agar or on aluminum coupons after ~60 min of drying at room air. In the first case, 400 μl of bacteria solution was inoculated on BHI agar prepared in aluminum plates (plate diameter 6.5 cm) and spread on the agar surface to obtain a uniform layer of bacteria. After inoculation plates were allowed to dry in room air for about 30 min to allow evaporation of excess water. In such a case, bacteria were never completely dry and were covered by a tiny amount of water, a condition that we refer to as ‘moist’ [2]. To quantify the number of bacteria per unit area of agar, the bacteria solution was properly diluted and inoculated on agar surface as described above, and after incubation at 37 °C for 15 h, the resulting colonies were counted (see figure 1(b)). For air-dried bacteria treatment experiments, 100 μl drops of bacteria solution were placed on sterile aluminum coupons. Due to relatively low wettability of coupons, drops always had a round shape at the base with a diameter of about 8 mm. then, inoculated drops were dried at room air for about 1 h, and treated with corona discharge or left untreated as controls. After the treatment, bacteria were washed with ~1 ml of PBS, and incubated on BHI agar. Here it is necessary to mention that some of the E. coli after drying were probably killed and the number of colonies observed after incubation decreased for about 90%. Therefore, this loss of bacteria was accounted for in our further experiments, and the actual number of treated bacteria was recalculated correspondingly. All experiments (each experimental point) were repeated at least three times.
3. Results
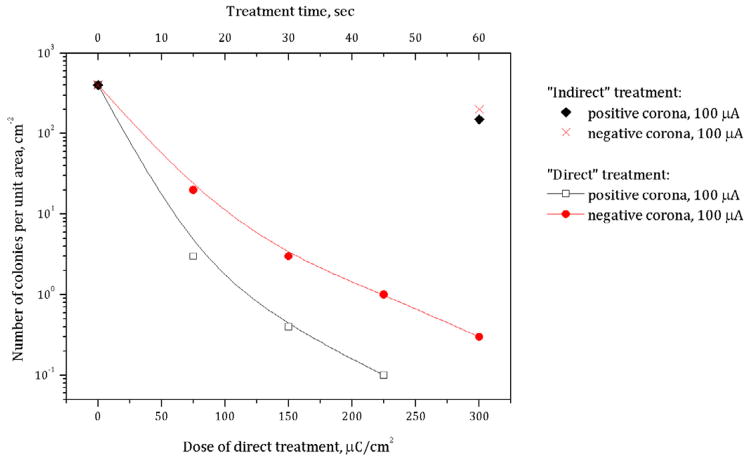
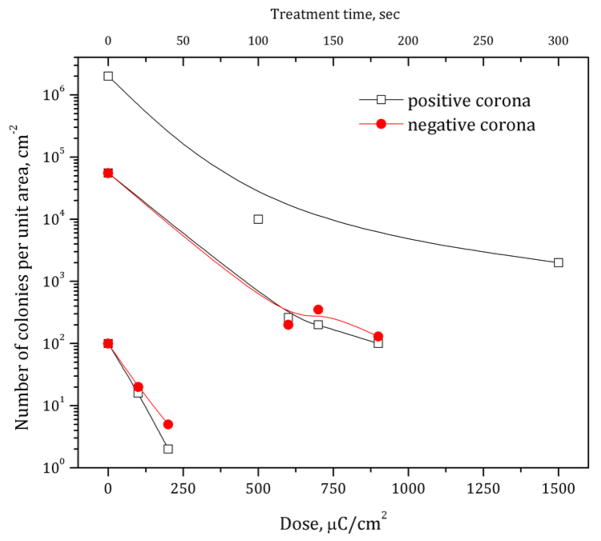
Initial experiments were performed with bacteria of various initial concentrations dried on agar (‘moist’ condition) utilizing corona discharge in room air (~60% humidity) with a constant current of 100 μA. The resulting numbers of colonies on the treated area after incubation were recalculated to a number of colony-forming units (CFU) per cm2 assuming that the area of treatment was about 19.5 cm2. Because it is almost impossible to count a relatively large number of colonies after treatment with low doses, here we considered colony numbers less than ~150 CFU per treated area. To be able to study inactivation kinetics at lower doses, we used initial bacteria surface densities from 107 to ~150 cm−2. The results for the corona of both positive and negative polarities of applied voltage are shown in figure 2 in terms of colony number density as a function of dose, where the exposure time of the treatment area at constant current was recalculated to charge density (μC cm−2). It is shown that positive corona discharge treatment of bacteria dried on the agar surface at a constant current of 100 μA permits up to about 6 log reduction of viable bacteria in about 90 s of treatment. Interestingly, negative corona in general has the same effect on bacteria viability; however, the same level of inactivation may be achieved at 1.5–3 times higher doses of treatment.
Figure 2.
Bacteria inactivation on agar by positive and negative corona discharges in room air.
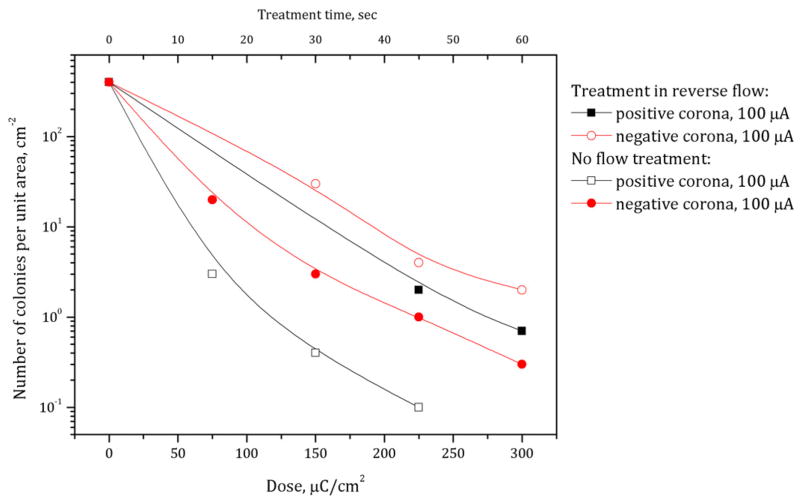
Although the corona discharge used in this study is expected to be relatively weak in terms of production of active neutral species, compared to, for example, dielectric barrier discharges (first of all, due to low discharge power), and to produce mostly ionic flows, here we also studied the effect of neutral radicals. In this set of experiments corona was ignited in room air between powered needle electrodes and a grounded metal mesh placed 2 mm above a bacteria layer dried on agar. The discharge current was fixed at 100 μA. The results (figure 3) show that, indeed, in the present discharge conditions neutral radicals alone probably do not play a major role: no visible effect was observed in the case of indirect treatment. Similar results (figure 4) were achieved by introducing a reverse air flow through the discharge system (the air flow direction was opposite to the ion flux). In this case a large portion of neutral active radicals was moved out by the air flow, which did not result in a significant reduction of bacteria inactivation.
Figure 3.
Bacteria inactivation on agar by direct and indirect treatment with positive and negative corona discharges in room air.
Figure 4.
Bacteria inactivation on agar by positive and negative corona discharges in room air with and without reverse gas flow.
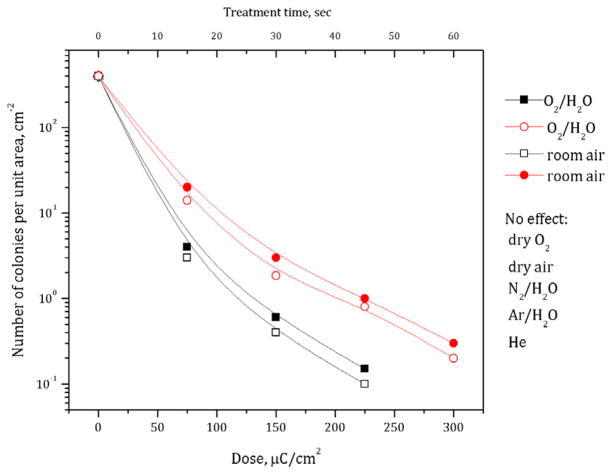
In order to study the effects of different kinds of ions, a series of experiments on bacteria dried on the surface of agar was performed. Here we used He, Ar and N2 flow through the system a rate of 10 slpm. High flow rates and ~60 s exposition prior to treatment (the setup volume was ~0.2 liter) ensured full gas replacement before the experiment. Bacteria were treated with 100 μA corona for up to 60 s, and then immediately exposed to room air and incubated in standard conditions. After the incubation, no visible decrease in viability was noticed, as expected. Similar experiments were performed using oxygen and dry air with the same treatment doses. Surprisingly, in these last two cases, no inactivation was observed. Compared to room air, these experiments were performed using dry gases at zero humidity; therefore the next step was to check the effect of water on the inactivation efficiency of corona discharge. To do so, in three separate experiments nitrogen, argon or oxygen was fed through a bubbler containing distilled water at a flow rate of 10 slpm. The results of the experiments are shown in figure 5. Compared to room air (~60% RH), only in the O2/H2O mixture was the same inactivation efficiency achieved, while no visible inactivation was observed in all other cases. These results, together with the results of ‘indirect’ treatment experiments, show that neither UV radiation, ozone or H2O2 nor other neutral active species alone produced by corona in dry oxygen or other gases with water have an effect on bacteria viability. Also, it is clear that non-oxygen charged species either alone or in combination with water do not provide any bacterial inactivation.
Figure 5.
Effect of gas composition on bacteria inactivation on agar by positive (red symbols) and negative (black symbols) corona discharges.
Possible effects of ions on bacteria inactivation may be classified into two groups: physical effects (e.g. membrane disruption due to accumulation of charges) and chemical effects (e.g. catalysis of membrane peroxidation) [2, 7], [18]. In the first case, there should be a strong dependence on dose rate—the time required to accumulate a certain number of charges on the bacterial outer surface should probably be relatively short, given that agar is a conductive media. In contrast, the chemical mechanism is likely to be much slower. In order to separate these two possibilities, the following experiment was carried out. A 400 μl portion of the bacteria solution was dried on the surface of agar and then exposed to positive corona discharge. The discharge current varied in the following range: 10, 50, 100 and 250 μA, with a maximum treatment time from 700 to 30 s in order to provide the same dose of treatment (number of charges per unit area). The results (figure 6) make it possible to suggest that the inactivation mechanism is provided by chemical effects rather than physical effects.
Figure 6.
Effect of dose rate on bacteria inactivation on agar-positive corona discharge in room air.
Another important question is the role of the water layer which covers bacteria: in all the experiments described above, bacteria were dried on agar (hydrogel) at room air and were therefore covered by a tiny amount of water (‘moist’). To study the effect of this water layer, an experiment similar to the one first described in this paper was performed. To minimize the layer of water covering bacteria, a bacteria-containing solution was dried on stainless-steel coupons as described in section 2.2. Then bacteria were exposed to either positive or negative corona discharge at a constant current of 100 μA (the same conditions as for figure 2). The results are shown in figure 7, where it was taken into account that the total treated area was 19.5 cm−2, but only 0.5 cm−2 was covered with bacteria. From these results, one may conclude that the actual difference between inactivation efficiency of ‘moist’ bacteria is about 2–4 times higher than that of ‘dry’ ones, which is in good agreement with our previous observations when bacteria were treated with dielectric barrier discharge plasma [2]. However, given that the last experiment (treatment of ‘dry’ bacteria) was done in humid room air, the presence of water is still important, and an optimal amount of water would lead to higher efficiency (as in the case of the so-called ‘water chemistry’), and vice versa, excess water leads to dilution of the effect.
Figure 7.
Inactivation of bacteria by positive and negative corona discharges dried on metal in room air.
Using linear parts of inactivation curves from figures 2 and 7, we have recalculated charge doses required for the inactivation of ‘moist’ and ‘dry’ bacteria obtained for both positive and negative corona to obtain the required dose per 1 log reduction and the number of ions (assuming that each ion carries a single charge) per bacterium for different initial concentrations. These curves are shown in figure 8.
Figure 8.
Dose per 1 log reduction and the number of ions per inactivated bacterium for corona discharge treatment. Solid symbols—charge density; open symbols—specific charge per bacterium.
4. Discussion
In this paper we address the problem of direct plasma interaction with bacteria, in particular, the inactivation of viable bacteria. Plasma is a complex medium with the following major active components: charged particles (i.e. electrons and ions), electric fields (both global applied electric field and those associated with charges), photons (and first of all, energetic ones—UV photons) and neutral active species (free radicals, e.g. OH radical, and stable active products, e.g. O3). It was noted [2, 3, 7, 18] that in the case of direct contact of plasma with the treated sample, i.e. when there is a physical charge transfer from plasma to the surface, the effect of plasma treatment is always the highest (here it is necessary to note that this is true for the treatment of the surface and not for the treatment of a liquid in a volume [2]). In contrast, indirect plasma treatment or treatment with afterglow, when only long-living species and in some cases active radicals are able to react with the sample, provides a much lower efficiency. The mechanism of inactivation of microorganisms by cold atmospheric pressure plasmas is undoubtedly complex in nature and should be studied from both biological (i.e. processes that occur in a microorganism after plasma treatment which lead to lethal or sub-lethal effects), and plasma-chemical (i.e. the generation of active components in plasma, the determination of major ones and the study of chemical and physical processes in the water layer surrounding a microorganism) sides. Here we focus on inactivation of viable bacteria (not bacteria in spore form) using direct atmospheric pressure dc corona discharge treatment because of the possibility to separate the effects of different components.
In low-pressure discharges UV radiation often plays a major role in sterilization; however, in atmospheric pressure cold plasmas (such as DBD, corona, ‘plasma needle’, etc) it is usually not the case [2, 7], [17–19]. For corona discharge this suggestion is confirmed by the results of bacteria treatment in various gases, including dry air and oxygen (see figure 5). Also, these results show that global electric fields in our experimental setup also have no effect on inactivation of viable E. coli.
A common hypothesis of the bacterial inactivation mechanism by air plasmas is related to oxidative damage by neutral active species, either stable ones, e.g. ozone and hydrogen peroxide, or short-living reactive oxygen species, such as hydroxyl radical and atomic oxygen. These neutrals may be formed directly in plasma, in the water layer surrounding bacteria or inside bacteria. Dc corona discharges were studied to produce ROS, ozone, H2O2, etc; however, it was shown that ozone and H2O2 alone require much longer treatment times than direct plasma treatment (see, e.g., [2]). Our experiments show that the treatment of bacteria with corona discharge ignited in oxygen, where ozone is formed, resulted in no visible inactivation.
Hydroxyl radicals (and hydrogen peroxide as a result of recombination of two OH molecules) are formed in discharges ignited in humid atmosphere (e.g. Ar/H2O mixture):
where M+ is any ion with ionization energy above the H2O ionization threshold. Hydroxyl radicals can then react with nearby organics, leading to chain oxidation and thus damage of cellular membranes and other cell components (here R is any organic molecule):
Hydrogen peroxide can travel through liquid and is relatively easily transferred through the cell membrane and, in appropriate conditions inside the cell, is converted back to hydroxyl radicals through a natural reaction chain termed the Fenton mechanism:
Bacteria are known to have protective mechanisms to ‘inactivate’ hydrogen peroxide through enzymes, e.g. catalase and peroxidase, which convert H2O2 into water and oxygen: 4H2O2 → 2H2O + O2 · 2H2O2 → 2H2O + O2 + 2H2O2. Here we have experimentally shown that these two active species, OH and H2O2, alone do not play a major role in the process of bacteria inactivation.
Charged species were shown to have a great influence on efficiency of bacteria inactivation, and several hypotheses were proposed. First, it should be mentioned that energetic ions may be responsible for membrane etching; however, this probably happens mostly in low-pressure plasmas or in streamers of direct DBD. Another possible effect of ions is traditionally associated with superoxide ( ), which in the presence of superoxide dismutase (SOD) is readily converted into hydrogen peroxide: , causing oxidative damage to the cell. Positive ions, as was mentioned above, also may be converted into OH and hydrogen peroxide directly on the bacterial membrane. The third hypothesis is related to mechanical stress caused by electrostatic charging of bacteria [7, 18]. It was considered that bacteria charged in plasma (analogous to particle charging in ‘dusty’ plasmas) under certain conditions may be ruptured due to mechanical stress. Although in this model only spherical Gram-negative bacteria were considered, the proposed electrostatic rupture mechanism may be applied to non-spherical objects, which may be killed even easier. Another hypothesis is related to neutralization of bacterial surface charge, which leads to cytoplasm leakage and cell death (analogous to surface disinfectants) [18]. These two last physical mechanisms of electrostatic inactivation of microorganisms may take place under conditions of an electrically isolated surface, where there is no leakage of transferred charge but accumulation of these charges. In this work, bacteria were placed on the surface of conductive agar that was grounded, and therefore no charge accumulation may be expected. The results of our study clearly show that inactivation of bacteria cannot be explained by the effect of only neutral active species or charged particles acting independently. Moreover, the presence of oxygen and water molecules in the discharge is absolutely necessary. Therefore a mechanism of peroxidation of bacterial membrane catalyzed by charges is proposed [2] where
both positive and negative ions have relatively the same effect;
the effect of charged species is chemical and not related to such physical phenomena as sheer stress, ion bombardment damage or thermal effects;
ions catalyze peroxidation processes of a bacterial membrane composed of polysaccha-rides;
the presence of oxygen and water is necessary and reactive oxygen species (ROS) play a crucial intermediate role.
Chain ion-radical mechanisms are well-known chemistry of hydrocarbons, where reactions of oxidation, peroxidation, hydrolysis, etc of organic molecules are catalyzed (and sometimes initiated) by cation or anion molecules (ions). It is well known that these reactions can create long chains for both positive and negative ions and these chains may be much longer than the OH oxidation of organic molecules discussed above. Careful biophysical studies [25] show that the greater catalytic effect of charged species is related to the various hydrogen atom transfer reactions, and could be due to both transition-state and product stabilization by increased solution ionic strength. At the same time, radicals derived from O2 are believed to be important in the initiation of lipid peroxidation-associated oxidative damage in biological systems [25]. The role of water vapors in the plasma system may be related to charged water clusters which carry both charge and radicals (e.g. OH and H2O2), resulting in simultaneous delivery of all active components to bacteria. Another possibility is related to production of highly reactive HO2 ions, which in the present work are effectively transported to the target inside protective water clusters.
In summary, it is shown that for effective and fast inactivation of bacteria on a surface by a plasma discharge, simultaneous presence of oxygen and hydrogen-containing compounds (e.g. water vapor) is required. It is well known that in a discharge ignited in this atmosphere, highly biologically active peroxides, peroxide radicals and their ions (e.g. H2O2, HO2, OH− and H3O+) are produced. The significant difference in inactivation efficiency observed in the experiments with the direct and indirect plasma treatment setups demonstrates the importance of charged particles. The efficiency does not strongly depend on the polarity of ions: both positive and negative charges are important. In the O2–H2O system, these may be cluster ions OH−(H2O)n and H3O+(H2O)n. We show in experiments with pure oxygen (mainly and ions) and pure nitrogen (electrons and ions) atmospheres that ions probably play a secondary role and the chemical composition is the most important factor. Therefore we come to the conclusion that the role of charge in the process of bacteria inactivation is probably related to the active radical transport to the treated surface.
Our estimations show that in an electric field of E/n ~ 30 Td that is created in the discharge gap, the ion (mainly OH− (H2O)n and H3O+(H2O)n) drift time to the surface is about 100–200 μs. In contrast, convective transport with a typical speed of about 1 cm s−1 for the same distance of 4 cm requires about 4 s. Therefore, presence of charged particles provides about 4 orders of magnitude faster transport of active radicals to the treated surface, while the transport does not depend on polarity of ions. Thus, recombination losses are significantly reduced and the inactivation efficiency is increased.
This mechanism explains that bacteria inactivation efficiency does not depend on the discharge polarity (in fact, the difference between positive and negative coronas is related to different peroxide radical ions—H3O+ and OH−), the weak effect of the treatment with the discharge ignited in a dry atmosphere (the role of ions is related to fast radical transport to the surface), and the difference between direct and indirect treatments (charged radical clusters OH−(H2O)n and H3O+(H2O)n are effectively trapped by the grounded metal mesh, which prevents their transport to the bacteria).
5. Conclusion
In this study, we investigated the effects of various components of dc atmospheric pressure corona discharge on inactivation of bacteria. The best results were achieved in the case of direct treatment when discharge was ignited in O2–H2O mixtures. It was shown that the main role of charged particles may be related to the faster transport of active peroxide species—cluster ions OH−(H2O)n and H3O+(H2O)n. The efficiency of these radicals is much higher than that of the oxygen radicals and ions (including , and O3) and of nitrogen and argon ions. The proposed mechanism allows us to explain all the data that were obtained for the bacteria inactivation on surfaces by both corona and DBD discharges.
References
- 1.Cheng C, et al. Development of a new atmospheric pressure cold plasma jet generator and application in sterilization. Chin Phys. 2006;15:1544. [Google Scholar]
- 2.Dobrynin D, Fridman G, Friedman G, Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys. 2009;11:115020. [Google Scholar]
- 3.Fridman G, Brooks AD, Manjula B, Fridman A, Gutsol A, Vasilets VN, Ayan H, Friedman G. Comparison of direct and indirect effects of non-thermal atmospheric pressure plasma on bacteria. Plasma Process Polym. 2007;4:370–75. [Google Scholar]
- 4.Gaunt LF, Beggs CB, Georghiou GE. Bactericidal action of the reactive species produced by gas-discharge nonthermal plasma at atmospheric pressure: a review. IEEE Trans Plasma Sci. 2006;34:1257–69. [Google Scholar]
- 5.Goree J, et al. Killing of S. mutants bacteria using a plasma needle at atmospheric pressure. IEEE Trans Plasma Sci. 2006;34:1317–24. [Google Scholar]
- 6.Gostev V. Cold Plasma in Biological Investigations. NATO Advanced Study Institute (ASI): Plasma Assisted Decontamination of Biological and Chemical Agents (Cesme, Turkey) 2007. [Google Scholar]
- 7.Laroussi M, Leipold F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom. 2004;233:81–6. [Google Scholar]
- 8.Martines E, et al. A novel plasma source for sterilization of living tissues. New J Phys. 2009;11:115014. [Google Scholar]
- 9.Lee MH, Park BJ, Jin SC, Kim D, Han I, Kim J, Hyun SO, Chung K-H, Park J-C. Removal and sterilization of biofilms and planktonic bacteria by microwave-induced argon plasma at atmospheric pressure. New J Phys. 2009;11:115022. [Google Scholar]
- 10.Moreau M, Orange N, Feuilloley MG. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008;26:610–7. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Birmingham, Joseph G. Mechanisms of bacterial spore deactivation using ambient pressure nonthermal discharges. IEEE Trans Plasma Sci. 2004;32:1526–31. [Google Scholar]
- 12.Hury S, et al. A parametric study of the destruction efficiency of Bacillus spores in low pressure oxygen-based plasmas. Lett Appl Microbiol. 1998;26:417–21. doi: 10.1046/j.1472-765x.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- 13.Boudam MK, Moisan M, Saoudi B, Popovici C, Gherardi N, Massines F. Bacterial spore inactivation by atmospheric-pressure plasmas in the presence or absence of UV photons as obtained with the same gas mixture. J Phys D: Appl Phys. 2006;39:3494–507. [Google Scholar]
- 14.Dobrynin D, Fridman G, Mukhin YV, Wynosky-Dolfi MA, Rieger RJ, Gutsol AF, Fridman A. Cold plasma inactivation of bacillus cereus and bacillus anthracis (anthrax) spores. IEEE Trans Plasma Sci. 2010;38:1878–84. [Google Scholar]
- 15.Laroussi M, Minayeva O, Dobbs FC, Woods J. Spores survivability after exposure to low-temperature plasmas. IEEE Trans Plasma Sci. 2006;34:1253–6. [Google Scholar]
- 16.Moisan M, et al. Low-pressure cold plasma sterilization at atmospheric pressure. Vide Sci Tech Appl. 2002;57 [Google Scholar]
- 17.Stoffels E. Gas plasmas in biology and medicine. J Phys D: Appl Phys. 2006;39 [Google Scholar]
- 18.Stoffels E, Sakiyama Y, Graves DB. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans Plasma Sci. 2008;36:1441–57. [Google Scholar]
- 19.Stoffels E. Biomedical applications of electric gas discharge. High Temp Mater Process. 2000;5:191–202. [Google Scholar]
- 20.Noyce JO, Hughes JF. Bactericidal effects of negative and positive ions generated in nitrogen on starvedPseudomonas veronii. J Electrostat. 2003;57:49–58. [Google Scholar]
- 21.Noyce JO, Hughes JF. Bactericidal effects of negative and positive ions generated in nitrogen onEscherichia coli. J Electrostat. 2002;54:179–87. [Google Scholar]
- 22.Fletcher LA, Gaunt LF, Beggs CB, Shepherd SJ, Sleigh PA, Noakes CJ, Kerr KG. Bactericidal action of positive and negative ions in air. BMC Microbiol. 2007;7:32. doi: 10.1186/1471-2180-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korachi M, Turan Z, Senturk K, Sahin F, Aslan N. An investigation into the biocidal effect of high voltage AC/dc atmospheric corona discharges on bacteria, yeasts, fungi and algae. J Electrostat. 2009;67:678–85. [Google Scholar]
- 24.Yamamoto M, Nishioka M, Sadakata M. Sterilization by H2O2 droplets under corona discharge. J Electrostat. 2002;56:173–87. [Google Scholar]
- 25.Dix TA, Aikens J, Thomas A. Effect of solution ionic strength on lipid peroxidation initiation by the perhydroxyl (xanthine oxidase-derived) and peroxyl radicals. Chem Res Toxicol. 1992;5:263–7. doi: 10.1021/tx00026a018. [DOI] [PubMed] [Google Scholar]