Abstract
Background. Chikungunya virus (CHIKV) and related arboviruses have been responsible for large epidemic outbreaks with serious economic and social impact. Although infected individuals clear the virus from the blood, some develop debilitating and prolonged arthralgia.
Methods. We investigated specificity and strength of antibody responses in a longitudinal study on CHIKV-infected patients and analyzed their association with viral load, cytokine profile, and severity.
Results. We found that CHIKV-specific response is dominated by immunoglobulin G3 (IgG3) antibodies. The antibodies were neutralizing, and patients with high viremia rapidly developed high levels of anti-CHIKV antibodies of this specific isotype. Although these patients endured a more severe disease progression during the acute viremic phase, they cleared the virus faster and did not experience persistent arthralgia. However, significant persistent arthralgia was observed in patients with low viremia who developed IgG3 at a later stage.
Conclusions. Absence of early CHIKV-specific IgG3 may therefore serve as a specific marker of patients with increased risk of disease.
In several arthralgia-causing arbovirus outbreaks, morbidity has been unexpectedly high with extensive incapacitation, including some lethal cases [1, 2]. Some were caused by chikungunya virus (CHIKV), first isolated in 1953 in Tanzania from infected patients who often developed a contorted posture caused by debilitating joint pain [3–5]. The reemergence of CHIKV since 2005 has caused millions of cases throughout countries in and around the Indian Ocean and Southeast Asia [6], and sporadic outbreaks are still ongoing in several countries, inflicting naive populations [7]. Singapore, for instance, experienced 2 successive waves of chikungunya fever (CHIKF) outbreaks in January and August 2008 [8–10]. Although there were only 718 laboratory-confirmed cases reported in 2008 and 341 cases in 2009 [11, 12], CHIKF remains a public threat due to the low herd immunity. Therefore, it may represent a major public health problem with severe social and economic impact.
CHIKV is a mosquito-borne virus belonging to the Alphavirus genus of the Togaviridae family. It has a life cycle similar to other alphaviruses and causes sudden onset of fever, rashes, arthritis, and other accompanying symptoms [3, 4]. Following the acute phase of the illness, patients develop severe chronic symptoms lasting from several weeks to months, including fatigue, incapacitating joint pain, and polyarthritis [13, 14]. However, as in many other arthralgia-causing arbovirus infections, the chronic phase is observed only in a fraction of patients [1–5]. A role for both innate and adaptive immunity has been proposed [15], but the mechanisms underlying control of viral replication and dissemination, viral clearance, and acute and chronic disease severity remain poorly defined.
In the current study, we analyzed the timing, specificity, and isotype of the antibody response against CHIKV by longitudinally analysis of 30 patients who were hospitalized for acute CHIKF [9, 10]. By correlating the antibody response with viral load, cytokine profile, and disease progression during the acute and the chronic phases, we show that neutralizing immunoglobulin G3 (IgG3) antibodies clearly dominate the response. Notably, the early induction of anti-CHIKV IgG3 antibodies is strongly associated with protection against persistent arthralgia, whereas a delayed response in patients with low viremia correlates with chronic joint pain. Thus, a strong virus-specific antibody response induced by high virus titers in the viremic phase seems to promote full protection against severity at the later stages of the disease.
MATERIALS AND METHODS
Ethical Approval
Written informed consent was obtained from all participants. This study was approved by the National Healthcare Group’s Domain-Specific Ethics Review Board (DSRB Reference No. B/08/026).
Patients and Plasma Collection
Thirty patients admitted with acute CHIKF to the Communicable Disease Centre at Tan Tock Seng Hospital during the outbreak from 1 August to 23 September 2008 [9, 10] were included in this study. Plasma specimens were collected at 4 time points post–illness onset (PIO): (1) acute phase (median, 4 days PIO); (2) early convalescent phase (median, 10 days PIO); (3) late convalescent phase (4–6 weeks PIO); and (4) chronic phase (2–3 months PIO). Clinical features definition and clinical samples were as described previously [9, 10]. Illness was defined as severe if a patient had a maximum temperature >38.5°C, a maximum pulse rate >100 beats per minute, or a nadir platelet count <100 × 109/L. Arthralgia was defined as having pain in >1 joints, with or without joint inflammation. Patients were later clustered into early and late IgG3 responders based on their IgG3 titer measured at a median of 10 days PIO (Table 1).
Table 1.
Demographic Characteristics and Immunological and Disease Profiles of Study Patients
| Patient (Sex, Age in y) | Duration of Fever, d | Acute Illness Severitya | Anti-CHIKV IgG Titerb | Anti-CHIKV IgG3 Classificationc | Clinical Outcomed |
| CHIKV 1 (M, 40) | 3 | Severe | Low | Early | Complete recovery |
| CHIKV 2 (M, 23) | 8 | Severe | High | Early | Complete recovery |
| CHIKV 3 (M, 62) | 7 | Severe | High | Early | Lethargy, weakness |
| CHIKV 4 (M, 43) | 5 | Severe | Low | Early | Complete recovery |
| CHIKV 5 (M, 29) | 6 | Severe | Low | Early | Complete recovery |
| CHIKV 6 (M, 35) | 7 | Severe | High | Early | Complete recovery |
| CHIKV 7 (M, 30) | 4 | Severe | Low | Early | Complete recovery |
| CHIKV 8 (M, 35) | 4 | Severe | High | Early | Complete recovery |
| CHIKV 9 (M, 26) | 3 | Severe | High | Early | Complete recovery |
| CHIKV 10 (M, 28) | 4 | Severe | Low | Early | Complete recovery |
| CHIKV 11 (M, 49) | 2 | Severe | Low | Early | Complete recovery |
| CHIKV 12 (M, 50) | 6 | Severe | Low | Early | Complete recovery |
| CHIKV 13 (M, 38) | 3 | Severe | High | Early | Complete recovery |
| CHIKV 14 (M, 60) | 3 | Mild | Low | Early | Complete recovery |
| CHIKV 15 (F, 62) | 7 | Severe | High | Early | Complete recovery |
| CHIKV 16 (M, 45) | 0 | Mild | High | Early | Complete recovery |
| CHIKV 17 (M, 34) | 3 | Mild | High | Late | Complete recovery |
| CHIKV 18 (M, 29) | 2 | Severe | Low | Late | Persistent arthralgia |
| CHIKV 19 (F, 67) | 7 | Mild | High | Late | Complete recovery |
| CHIKV 20 (M, 24) | 3 | Mild | Low | Late | Complete recovery |
| CHIKV 21 (M, 34) | 0 | Mild | High | Late | Complete recovery |
| CHIKV 22 (M, 28) | 7 | Mild | High | Late | Complete recovery |
| CHIKV 23 (M, 42) | 2 | Mild | Low | Late | Complete recovery |
| CHIKV 24 (F, 40) | 6 | Mild | Low | Late | Persistent arthralgia |
| CHIKV 25 (F, 31) | 6 | Mild | Low | Late | Persistent arthralgia |
| CHIKV 26 (M, 46) | 9 | Mild | High | Late | Complete recovery |
| CHIKV 27 (M, 26) | 4 | Mild | Low | Late | Persistent arthralgia |
| CHIKV 28 (M, 28) | 5 | Severe | Low | Late | Complete recovery |
| CHIKV 29 (M, 47) | 8 | Mild | Low | Late | Complete recovery |
| CHIKV 30 (M, 39) | 1 | Mild | High | Late | Complete recovery |
Abbreviations: CHIKV, chikungunya virus; ELISA, enzyme-linked immunosorbent assay; F, female; IgG, immunoglobulin G; M, male.
Severity was defined as having a temperature >38.5°C, pulse rate >100 beats per min, or platelet count <100 × 109 cells/L.
Anti-CHIKV IgG antibody titer was determined by virion-based ELISA from plasma samples collected at 7–10 d post–illness onset. Optical density values > median value of 0.46 were classified as high and the rest were defined as low.
Anti-CHIKV IgG3 isotype titer and response was determined by virion-based ELISA from plasma collected at 7–10 d post–illness onset. During this phase, approximately half of the patient group already had a significant increase of IgG3 antibodies, segregating this cohort into early and late IgG3 responders.
Clinical outcome at chronic phase that is 2–3 months after post–illness onset.
Immunologic Analyses
Antibody titers were assessed by a virion-based enzyme-linked immunosorbent assay (ELISA). Polystyrene 96-well microtiter plates (MaxiSorp, Nunc) were coated with purified CHIKV (20 000 virions/μL in phosphate-buffered saline [PBS]; 50 μL per well). Wells were blocked with PBS containing 0.05% Tween-20 and 5% nonfat milk (Phosphate buffered saline Tween 20 [PBST]-milk), and plates were incubated for 1.5 hours at 37°C. Plasma samples were then diluted 1:100 to 1:2000 in PBST-milk and incubated for 1 hour at 37°C. Horseradish peroxidase–conjugated mouse antihuman immunoglobulin G (IgG), immunoglobulin G1 (IgG1), immunoglobulin G2 (IgG2), IgG3, immunoglobulin G4 (IgG4), and immunoglobulin M (IgM) (Molecular Probes) were used to detect human antibodies bound to virus-coated wells. Reactions were developed using 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (Sigma-Aldrich) and terminated by Stop reagent (Sigma-Aldrich). Absorbance was measured at 450 nm. Healthy donor samples were used as controls. ELISA readings were done in duplicates. Antigenic responses were detected by immunofluorescence assay as described. HEK 293T cells were seeded on coverslips coated with human plasma fibronectin (Sigma-Aldrich). Virus infection was performed at a multiplicity of infection (MOI) of 10. At 6 hours postinfection, cells were fixed with PBS containing 4% paraformaldehyde. Cells were then permeabilized in PBS containing 0.2% Triton-X and blocked with PBS supplied with 10% fetal bovine serum (FBS). Cells were stained with patients’ plasma diluted in PBS (1:500) containing 1% bovine serum albumin for 1 hour at 37°C. This was followed by incubation with goat antihuman secondary antibody conjugated to Alexa Fluor 488 (Molecular Probes) for 1 hour at 37°C. Cells were washed, mounted, and examined with a confocal laser-scanning microscope (Fluoview FV100; Olympus) using 20× numerical aperture (NA) 0.75 or 60× NA 1.42 objective. Images were collected using FV10-ASW software and processed with Adobe Photoshop software. Levels of cytokines were measured by multiplex bead–based arrays as described previously [16].
Seroneutralization
Neutralizing activity of antibodies from CHIKV-infected patient samples were tested in triplicates and analyzed by immunofluorescence-based cell infection assay in HEK 293T cells. Chikungunya virus was mixed at a MOI of 10 with diluted (1:100, 500, or 1000), heat-inactivated human plasma and incubated for 2 hours at 37°C with gentle agitation (350 rpm). Virus-antibody mixtures were then added to HEK 293T cells seeded in 96-well plates and incubated for 1.5 hours at 37°C. Medium was removed, and cells were replenished with Dulbecco’s modified Eagle’s medium supplied with 5% FBS and incubated for 6 hours at 37°C before fixation with 4% paraformaldehyde, followed by immunofluorescence quantification using the Cellomics ArrayScan V. Percentage of infectivity was calculated according to the equation [% infectivity = 100 × (% responder from seroneutralization group/% responder from virus infection group)].
Affinity Depletion of Anti-CHIKV Antibodies
For affinity depletion of human anti-CHIKV antibodies, purified Chikungunya virions (1 × 106 virions per well) were added to Maxisorp plates (Nunc) and incubated at 4°C for 24 hours in PBS. Human plasma samples were added and incubated for 25 minutes at room temperature for absorption. The unbound portion was collected after 21 rounds of absorption. ELISA analysis was performed to verify the levels of the antibodies during affinity depletion.
Affinity Depletion of Human Isotype IgG3 Antibodies
For affinity depletion of human isotype IgG3 antibodies, mouse biotinylated monoclonal antihuman IgG3 antibodies (30 μg/mL, Molecular Probes) were added to Immobilizer Streptavidin plates (Nunc) and incubated at room temperature for 1 hour in PBS containing 0.02% Tween-20 (0.02% PBST). Human plasma samples were added and incubated for 25 minutes at room temperature for absorption. The unbound portion was collected after 21 rounds of absorption. ELISA analysis was performed to verify the levels of the antibodies during affinity depletion.
Data Analysis
Data are presented as mean ± standard error of the mean (SEM) or as mean ± standard deviation (SD). Differences in responses among groups at various time points and between groups and controls were analyzed using appropriate tests (Mann–Whitney U test, Fisher exact test). Statistics were performed with GraphPad Prism 5.04 software.
RESULTS
Timing and Isotype Specificity of the Antibody Response
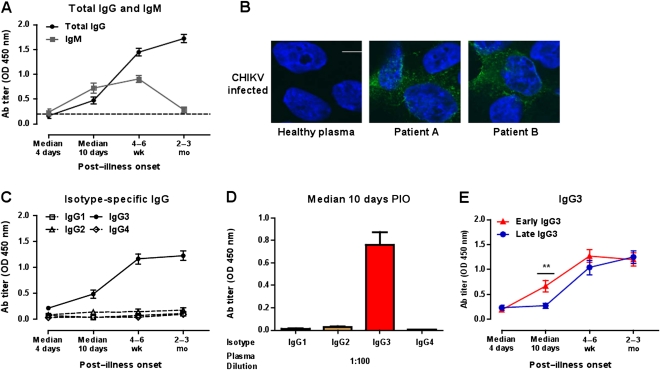
In order to characterize the immune response against CHIKV, we prospectively followed up 30 patients who were admitted for acute CHIKF during the CHIKF outbreak in Singapore between August and September 2008 (Table 1) [9, 10]. CHIKV-specific antibody responses were quantified in the acute phase starting 4 days after infection until the late chronic phase 2–3 months PIO (Figure 1). As expected, IgG levels gradually increased during the early convalescent phase at a median of 10 days PIO, whereas IgM peaked after 4–6 weeks and declined to background levels (Figure 1A). Plasma from these patients was not only reactive to the Chikungunya virion–based ELISA but also specifically detected CHIKV antigens in CHIKV-infected cells by immunofluorescence staining (Figure 1B).
Figure 1.
Antibody (Ab) profiles of chikungunya virus (CHIKV)–infected patients. A, Virus-specific immunoglobulin M (IgM) and immunoglobulin G (IgG) antibody titers in plasma samples (n = 30) at a dilution of 1:2000 were determined by enzyme-linked immunosorbent assay (ELISA) using purified CHIKV virions. B, Detection of CHIKV by plasma from CHIKV-infected patients. HEK 293T cells were infected with CHIKV (SGP11) at a multiplicity of infection of 10, fixed at 6 h postinfection, and stained with 2 representative patients’ plasma at 2–3 months post–illness onset (PIO) using a dilution of 1:500. Healthy plasma was used as a control. CHIKV antigen was detected by antihuman IgG antibody conjugated to Alexa Fluor 488 (green). 4′,6-diamidino-2-phenylindole (DAPI) was used to stain the nucleus. Scale bar: 10 μm. C, Virus-specific IgG isotype titers in plasma samples. Immunoglobulin G1 (IgG1) (□), immunoglobulin G2 (IgG2) (Δ), immunoglobulin G3 (IgG3) (•), or immunoglobulin G4 (IgG4) (⋄) Abs were determined as in (A) using specific secondary antibodies. Each point represents the mean of 30 patients’ samples from the cohort. D, Chikungunya virion–based ELISA was used to determine virus-specific IgG isotype titers in plasma samples (median, 10 d PIO; n = 30) at a dilution of 1:100. Anti-CHIKV IgG1, IgG2, IgG3, or IgG4 Abs were determined using specific secondary Abs. Data are presented as mean ± standard error of the mean (SEM) of 30 patients’ samples and are representative of 2 independent experiments with similar results. E, Profile of IgG3 levels at different times PIO in early (n = 16) and late IgG3 responders (n = 14) according to the pattern of IgG3 titer at median, 10 d PIO. Data are presented as mean ± SEM. Data are representative of 2 independent experiments with similar results. Statistical significance was measured using Mann–Whitney U test. **P < .01. Abbreviation: OD, optical density.
CHIKV-specific IgG antibodies were found to be almost exclusively of the IgG3 isotype. The levels of virus-specific IgG1, IgG2, and IgG4 titers did not increase during the course of infection (Figure 1C) even when a high concentration of plasma was used (Figure 1D). Although IgG3 was the dominant isotype in all members of the cohort, a comparison of the individual titers during the early convalescence phase revealed striking differences within the patient group. At a median of 10 days PIO, only approximately half of the group already had a significant increase of IgG3 (optical density >0.5), segregating this study cohort into early and late IgG3 responders (Figure 1E, Table 1).
Protective Capacity of Anti-CHIKV Antibodies From Patients
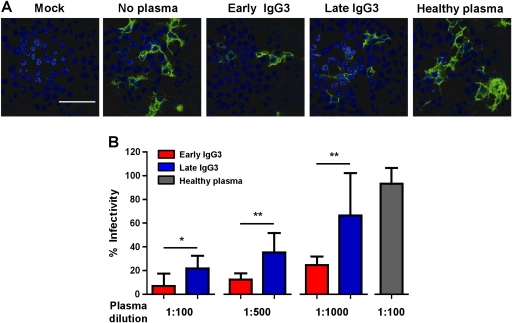
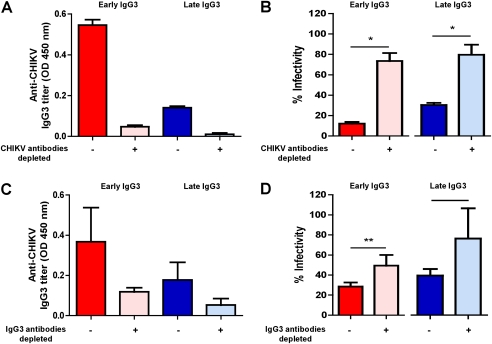
To determine if the antibodies also have protective capacity, in vitro infections of HEK 293T cells with CHIKV were carried out in the presence of plasma from patients or healthy donors (Figure 2). The experiments revealed that plasma samples collected at a median of 10 days PIO effectively inhibited CHIKV infection (Figure 2A). Preincubation of CHIKV with plasma samples induced a clear and dose-dependent reduction in the detection of CHIKV antigens (Figure 2A and 2B). In line with the observed differences in IgG3 titer, plasma from early IgG3 responders showed a higher neutralizing activity than plasma from the late IgG3 responders (Figure 2B). To confirm the protective role of anti-CHIKV IgG3 antibodies, CHIKV-infected patient plasma samples were depleted of antibodies against the purified Chikungunya virion (Figure 3A). Removal of anti-CHIKV IgG3 antibodies led to a marked decrease in neutralization for both early and late IgG3 responders (Figure 3B). In addition, the partial removal of IgG3 from the plasma of CHIKV patients by plate-bound anti-IgG3 reduced the IgG3 titer by 70%–80% (Figure 3C) and led to a marked decrease in neutralization for both early and late IgG3 responders (Figure 3D), confirming the importance of IgG3 antibodies in virus neutralization.
Figure 2.
Neutralizing capacity of anti–chikungunya virus (CHIKV) immunoglobulin G3 (IgG3) antibodies. A, Visualization of CHIKV by immunofluorescence in infected cultures after seroneutralization. Virus samples were preincubated with patients’ plasma collected at median 10 d post–illness onset (PIO) from early and late IgG3 groups before being added to the cells. Noninfected (mock) or virus samples preincubated with healthy donor plasma were used as controls. Analysis was performed at 6 h postinfection. Images were captured with 60× magnification. Scale bar: 50 μm. Representative microscopic images per treatment condition are illustrated. B, In vitro neutralizing activity against CHIKV from plasma samples of early and late IgG3 responders. Plasma samples (median, 10 d PIO) were tested in triplicates at different dilutions. Healthy plasma was used as a control and performed in the same conditions. Dilution at 1:100 is shown. Results are presented as mean ± SD of percentage control infection. Data are representative of 3 independent experiments. Statistical significance was measured using Mann–Whitney U test. *P < .05. **P < .01.
Figure 3.
Role of anti–chikungunya virus (CHIKV) immunoglobulin G3 (IgG3) in in vitro neutralizing activity against CHIKV from plasma samples of early and late IgG3 responders. A, Pooled plasma samples collected at median 10 d post–illness onset (PIO) (early IgG3, n = 16; late IgG3, n = 14) were added to plates precoated with purified Chikungunya virion for depletion of anti-CHIKV antibodies. Depleted samples were subjected to anti-CHIKV IgG3 antibody detection with virion-based enzyme-linked immunosorbent assay (ELISA). B, Depleted samples were subjected to in vitro neutralizing activity detection with a seroneutralization assay. C, IgG3 antibodies from pooled plasma samples collected at a median of 10 d PIO (early IgG3, n = 16; late IgG3, n = 14) were depleted as described in the “Methods” section and measured for anti-CHIKV IgG3 antibodies with virion-based ELISA. D, Depleted samples were subjected to in vitro neutralizing detection in a seroneutralization assay. All samples assayed were performed at 1:500 dilution (n = 3). Plasma from healthy donors was used as negative controls. Data are presented as mean ± SD. Data are representative of 3 independent experiments. Statistical significance was measured using Mann–Whitney U test. *P < .05. **P < .01.
Association Studies
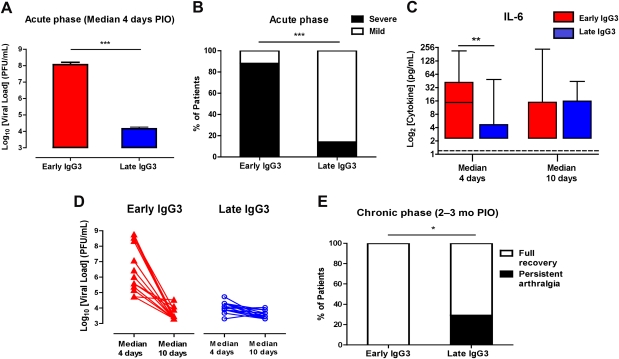
Because CHIKV IgG3 plays a key role in the control of CHIKV infections, we examined viral load and disease progression in early and late IgG3 responders. First, we observed a much higher viral load in the early IgG3 responders compared with the late IgG3 responders (Figure 4A). This was particularly evident on median day 4 PIO, suggesting that the high IgG3 titers of early IgG3 responders are indeed induced by a high viremia.
Figure 4.
Association of immunoglobulin G3 (IgG3) antibody responses with disease progression. A, Viral load in early and late IgG3 responders during the acute phase of disease (median, 4 d post–illness onset [PIO]). Data are presented as mean ± standard error of the mean. Statistical significance was measured using Mann–Whitney U test. ***P < .001. Abbreviation: PFU, plaque-forming units. B, Disease severity in early and late IgG3 responders during the acute phase of disease. Severity was previously defined. Histogram shows the percentage of patients with mild (n = 14) or acute severe clinical phenotypes (n = 16). Statistical significance was measured using 2-sided Fisher exact test between the number of patients with severe phenotype in 2 responder groups. ***P < .0001. C, Interleukin 6 levels in early and late IgG3 responders were determined using a multiplex-bead based assay. Horizontal dotted lines represent median values of healthy controls. Statistical significance was measured using Mann–Whitney U test. **P < .01. D, Viral clearance from a median of 4 d to a median of 10 d PIO in early and late IgG3 responders. E, Persistent arthralgia in early and late IgG3 responders during the chronic phase of disease (2–3 mo PIO). Histogram shows the percentage of patients with full recovery or persistent arthralgia. Statistical significance was measured using 2-sided Fisher exact test between the patients who have fully recovered and patients with persistent arthralgia in the 2 responder groups. *P = .04.
High viremia was also previously shown to correlate with increased disease severity during the acute phase of infection [17]. In line with this notion, we also observed that 90% of early IgG3 responders developed severe disease during the acute phase of the infection compared with <10% of late IgG3 responders (Figure 4B). In this cohort, disease severity was previously shown to be associated with increased plasma levels of 2 known endogenous pyrogens, interleukin 1β and interleukin 6 (IL-6) [16, 17]. Interestingly, high levels of IgG3 in early IgG3 responders also correlated with higher IL-6 levels, especially during the initial phase of infection (median, 4 days PIO) (Figure 4C). This finding may be explained by IL-6 being one of the major B-cell growth factors [18] and a known inducer of IgG3 [19].
Comparison of the viral load on median 4 and 10 days PIO indicated that early IgG3 responders exhibited a very efficient clearance of CHIKV. Although the average viral load on day 4 differed by > 3 logs, they reached similar low levels as the late IgG3 responders after completing the acute phase of infection at median 10 days pio (Figure 4D). Thus, the early increase of IgG3 is apparently associated with an efficient clearance of the virus.
Notably, although early IgG3 responders develop more severe symptoms during the acute phase, they completely recovered from the infection. None of them developed any persistent arthralgia (Figure 4E). This, however, was not the case for late IgG3 responders. Despite having a low viremia, approximately 30% of this group developed arthralgia during the later stage of the disease (Figure 4E). This suggests that a strong early IgG3 response triggered by a high viral load is needed to fully protect against chronic long-term effects of the CHIKV infection.
DISCUSSION
We have conducted the first detailed longitudinal analysis of the antibody response in a cohort of patients detected early during a CHIKF outbreak. The study revealed that antibodies of the IgG3 isotype dominate the humoral response against CHIKV. This bias toward IgG3 production to virus infections has also been previously reported in other pathogen infections [20, 21]. Long-term IgM production associated with persistence of CHIKV [22] was not detected in this cohort.
The analysis of the cohort data revealed a clear correlation between efficient viral clearance and clinical protection against persistent arthralgia and the early production of IgG3 antibodies. A putative explanation may be that late IgG3 responders established elevated levels of virus-specific IgG3 only at late phase, a time when virus was no longer detectable in the blood. In joint biopsies of patients with chronic arthralgia, CHIKV was detected in cells such as macrophages [23]. This observation has also been confirmed by studies in a nonhuman primate model [24]. It is plausible that the viruses in these cells are nonreplicative, so that only few virions are released. These 2 studies have therefore proposed that viral reservoirs existed in the afflicted joints, suggesting that CHIKV harboring at these sites may be protected from the neutralization action of the anti-CHIKV IgG3 antibodies. Late IgG3 responders would therefore be more prone to persistent complications.
The early increase of CHIKV IgG3 was associated with an efficient viral clearance in vivo, an effect presumably mediated by an inhibition of virus invasion and/or replication in host cells. The neutralizing effect of IgG3 antibodies was also evident in in vitro infection assays. Exposure of CHIKV to IgG3-depleted patient plasma partly prevented its inhibitory effect on the viral infection of HEK 293T cells. Although the elevated titers of early CHIKV-specific antibodies are apparently induced by high viremia, the isotype selection seems to be linked to IL-6. The early increase of IgG3, apparently induced by a high viremia, was clearly associated with a higher production of the cytokine, which is known to be a major B-cell growth factor [18] and an inducer of IgG3 [19].
Due to the explosive nature of CHIKV outbreaks and the unpreparedness of the healthcare system in countries where they occurred, no longitudinal studies on anti-CHIKV immune responses of this manner have been performed previously. It will be of interest to confirm our findings with cohorts from different parts of the world where CHIKV outbreaks have been reported. The association of anti-CHIKV IgG3 with clinical severity will allow for more cost-effective patient management [25, 26], because a single determination during acute phase would help predict severity.
Last, these studies, viewed in the broader context of immune markers of protection against viral diseases, suggest that the production of protective IgG3 antibodies correlate with the virus titer. The paradoxical situation emerges in which a high viral load during the acute phase is beneficial to establish full protection for the chronic phase. Low viremia, in contrast, which causes less severe symptoms during the initial phase, was proposed to be associated with persistent arthralgia at later stages of the disease. Thus, the timely induction of high titers of neutralizing IgG3 seems to be crucial to prevent persistent complications arising from chronic viral infections. Although this has important implications for prevention and treatment of CHIKF, it remains to be seen if this can also be observed for other pathogens causing severe and lasting symptoms.
Notes
Author contributions.
All authors discussed the results and commented on the manuscript. L. F. P. N., Y. W. K., and L. R. conceived and designed the experiments. Y. W. K., D. S., Z. H., T. S. T., and E. K. S. O. performed the experiments. Y. W. K., L. F. P. N., and L. R. analyzed the data. A. C. and Y. S. L. contributed materials. L. F. P. N., Y. W. K., L. R., and Y. S. L. wrote the paper.
Acknowledgments.
We thank the study participants and healthy volunteers for their participation; research staff from the Communicable Disease Centre/Tan Tock Seng Hospital, namely, Meng-Li Teo for assistance in blood sample preparation and Clement Kan, Amy Chan , and Mar-Kyaw Win for patient enrollment, study coordination, and data entry; clinical staff of the Communicable Disease Centre/Tan Tock Seng for patient enrollment and care; and Catharina Svanborg from Lund University and Jean-Laurent Casanova from Rockefeller University for critical reading of this manuscript.
Financial support.
This work was supported by the Biomedical Research Council, A*STAR. Z. H. is supported by the President’s Graduate Fellowship from the Yong Loo Lin School of Medicine, National University of Singapore.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–77. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 2.Higgs S. The 2005–2006 chikungunya epidemic in the Indian Ocean. Vector Borne Zoonotic Dis. 2006;6:115–6. doi: 10.1089/vbz.2006.6.115. [DOI] [PubMed] [Google Scholar]
- 3.Lumsden WH. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. II. General description and epidemiology. Trans R Soc Trop Med Hyg. 1955;49:33–57. doi: 10.1016/0035-9203(55)90081-x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 5.Kondekar S, Gogtay NJ. Why chikungunya is called chikungunya. J Post Med. 2006;52:307. [Google Scholar]
- 6.Renault P, Solet JL, Sissoko D, et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77:727–31. [PubMed] [Google Scholar]
- 7.ProMED-mail. Chikungunya virus, humans—New Caledonia. ProMED-mail; 2011. http://www.promedmail.org. Accessed 29 April 2011. [Google Scholar]
- 8.Leo YS, Chow AL, Tan LK, Lye DC, Lin L, Ng LC. Chikungunya outbreak, Singapore, 2008. Emerg Infect Dis. 2009;15:836–7. doi: 10.3201/eid1505.081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Win MK, Chow A, Dimatatac F, Go CJ, Leo YS. Chikungunya fever in Singapore: acute clinical and laboratory features, and factors associated with persistent arthralgia. J Clin Virol. 2010;49:111–4. doi: 10.1016/j.jcv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Ng KW, Chow A, Win MK, et al. Clinical features and epidemiology of chikungunya infection in Singapore. Singapore Med J. 2009;50:785–90. [PubMed] [Google Scholar]
- 11.Ministry of Health, Singapore. Communicable disease surveillance in Singapore 2008. 2009. pp. 21–48. http://www.moh.gov.sg/content/moh_web/home/Publications/Reports/2009/communicable_diseasessurveillanceinsingapore2008.html. Accessed 01 February 2012. [Google Scholar]
- 12.Ministry of Health, Singapore. Communicable disease surveillance in Singapore 2009. 2010. pp. 21–44. http://www.moh.gov.sg/content/moh_web/home/Publications/Reports/2010/communicable_diseasessurveillanceinsingapore2009.html. Accessed 01 February 2012. [Google Scholar]
- 13.Brighton SW, Prozesky OW, de la Harpe AL. Chikungunya virus infection. A retrospective study of 107 cases. S Afr Med J. 1983;63:313–5. [PubMed] [Google Scholar]
- 14.Simon F, Parola P, Grandadam M, et al. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine (Baltimore) 2007;86:123–37. doi: 10.1097/MD/0b013e31806010a5. [DOI] [PubMed] [Google Scholar]
- 15.Kam YW, Ong EK, Renia L, Tong JC, Ng LF. Immuno-biology of chikungunya and implications for disease intervention. Microbes Infect. 2009;11:1186–96. doi: 10.1016/j.micinf.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Ng LF, Chow A, Sun YJ, et al. IL-1beta, IL-6, and RANTES as biomarkers of chikungunya severity. PLoS One. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow A, Her Z, Ong EK, et al. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203:149–57. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano T, Taga T, Nakano N, et al. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2) Proc Natl Acad Sci U S A. 1985;82:5490–4. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994;153:4948–58. [PubMed] [Google Scholar]
- 20.Narita M, Yamada S, Matsuzono Y, Itakura O, Togashi T, Kikuta H. Measles virus-specific immunoglobulin G subclass response in serum and cerebrospinal fluid. Clin Diagn Virol. 1997;8:233–9. doi: 10.1016/s0928-0197(97)10007-1. [DOI] [PubMed] [Google Scholar]
- 21.Rzepczyk CM, Hale K, Woodroffe N, et al. Humoral immune responses of Solomon Islanders to the merozoite surface antigen 2 of Plasmodium falciparum show pronounced skewing towards antibodies of the immunoglobulin G3 subclass. Infect Immun. 1997;65:1098–100. doi: 10.1128/iai.65.3.1098-1100.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver M, Grandadam M, Marimoutou C, et al. Persisting mixed cryoglobulinemia in chikungunya infection. PLoS Negl Trop Dis. 2009;3:e374. doi: 10.1371/journal.pntd.0000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, et al. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–27. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 24.Labadie K, Larcher T, Joubert C, et al. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120:894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuzzubbo AJ, Endy TP, Nisalak A, et al. Use of recombinant envelope proteins for serological diagnosis of dengue virus infection in an immunochromatographic assay. Clin Diagn Lab Immunol. 2001;8:1150–5. doi: 10.1128/CDLI.8.6.1150-1155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marot-Leblond A, Nail-Billaud S, Pilon F, Beucher B, Poulain D, Robert R. Efficient diagnosis of vulvovaginal candidiasis by use of a new rapid immunochromatography test. J Clin Microbiol. 2009;47:3821–5. doi: 10.1128/JCM.01168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]