SUMMARY
Schizophrenia patients suffer from severe cognitive deficits, such as impaired reality monitoring. Reality monitoring is the ability to distinguish the source of internal experiences from outside reality. During reality monitoring tasks, schizophrenia patients make errors identifying “I made it up” items, and even during accurate performance, they show abnormally low activation of the medial prefrontal cortex (mPFC), a region that supports self-referential cognition. We administered 80 hours of computerized training of cognitive processes to schizophrenia patients and found improvement in reality monitoring that correlated with increased mPFC activity. In contrast, patients in a computer games control condition did not show any behavioral or neural improvements. Notably, recovery in mPFC activity after training was associated with improved social functioning six months later. These findings demonstrate that a serious behavioral deficit in schizophrenia, and its underlying neural dysfunction, can be improved by well-designed computerized cognitive training, resulting in better quality of life.
INTRODUCTION
A long-debated and critical question in schizophrenia and other neuropsychiatric illnesses is whether the underlying neural impairments of the disorder are immutably fixed, or whether they can respond in a significant and enduring manner to targeted behavioral interventions. Here, we demonstrate that intensive neuroscience-informed cognitive training can improve brain function in patients who have been ill for decades. Specifically, we show that it can improve a complex and clinically meaningful “reality monitoring” process defined as the ability to distinguish the source of internal experiences (self-generated information) from outside reality (external information) (Bentall et al., 1991; Johnson et al., 1993; Keefe et al., 1999; Morrison and Haddock, 1997; Vinogradov et al., 1997; Vinogradov et al., 2008). Furthermore, we demonstrate that training-induced enhancement of neural activation patterns associated with reality monitoring predict subsequent improvement in longer term social functioning. This study also addresses the fundamental issue of whether “brain training” improves cognitive functions beyond the trained tasks (Owen et al., 2010).
Schizophrenia is a serious and debilitating psychiatric illness that affects 51 million people worldwide. Affected individuals experience a range of disturbing clinical symptoms indicating a break with reality—such as hallucinations and delusions—as well as a range of neurocognitive and social cognitive deficits (Cirillo and Seidman, 2003; Heinrichs and Zakzanis, 1998). Prominent among these deficits are impairments in memory, executive function, and in the assessment of social cues such as facial emotion (Chan et al., 2010; Glahn et al., 2000; Silver et al., 2007). Pharmacologic treatment of schizophrenia targets symptom reduction, but the neurocognitive and social cognitive impairments, which are not improved by current medications, are more predictive of poor functional outcome than are the clinical symptoms of hallucinations and delusions (Evans et al., 2004; Green et al., 2000). Despite an understanding of the strong association between cognitive impairment and long-term disability in patients, the treatment of schizophrenia is at a stalemate (Carter and Barch, 2007; Marder and Fenton, 2004). New cognitive-enhancing medications studied thus far have been disappointing, and conventional psychotherapeutic and psychosocial rehabilitation approaches have been of limited benefit, likely due to the cognitive limitations of the illness (Green et al., 2008; Pilling et al., 2002; Smith et al., 2010).
Informed by the past two decades of systems neuroscience research into the learning mechanisms that drive sustained plastic changes in the cortex (Buonomano and Merzenich, 1998; Jenkins et al., 1990; Karni and Sagi, 1991; Merzenich et al., 1990), we predicted that--in order to improve higher order cognitive functions in human neuropsychiatric illness--computerized training must be designed to intensively target impairments in lower level perceptual processing as well as working memory and executive operations (Adcock et al., 2009; Fisher et al., 2009; Mahncke et al., 2006; Vinogradov et al., 2012). In other words, training must initially target lower level processes in order to increase the accuracy, the temporal and spatial resolution, and the signal strength of auditory and visual inputs to working memory and executive functions, ultimately increasing the efficiency of more complex, higher level cognitive processes in an enduring manner (Vinogradov et al., 2012). Specifically, we predicted that deficits in a type of source memory known as reality monitoring—the ability to distinguish the source of stimuli that have been internally generated from those that have been experienced externally (to separate “inner world” from “outer reality”)-- would respond to intensive training of component aspects of auditory/verbal, visual, and social cognitive processes in patients with schizophrenia. We also predicted that improved reality monitoring in patients would be associated with more normal neural activation patterns in the medial prefrontal cortex. Finally, we hypothesized that training-induced increases in prefrontal activation patterns would predict improved real world social functioning six months later.
In healthy individuals, performance on simple reality monitoring experiments that assess how well someone can distinguish the source of self-generated word items from externally-presented word items-- “I remember that I made that word up” vs. “I remember that you showed it to me”-- is strongly related to the person’s ability to recognize faces and to identify facial and vocal emotion (Fisher et al., 2008). It is also associated with activation of the medial prefrontal cortex (mPFC), a critical node in the neural network that supports the processing of social cognitive information (Frith and Frith, 1999; Gilbert et al., 2007; Heberlein et al., 2008; Hooker et al., 2011; Mattavelli et al., 2011; Northoff et al., 2006; Phan et al., 2002; Sabatinelli et al., 2011; Vinogradov et al., 2006; Vinogradov et al., 2008). In other words, the same neural systems that participate in distinguishing “inner world” from “outside world” also support the representation of “self” and “other”; indeed, the anterior rostral mPFC is particularly implicated in tagging information as being relevant to the “self” (Amodio and Frith, 2006; Ochsner et al., 2004; Ochsner et al., 2005; Vinogradov et al., 2006; Vinogradov et al., 2008). For example, Cabeza et al. (2004) found that mPFC activation was greater when subjects viewed photographs of a building that they themselves had taken (the autobiographical “self” condition) versus when they viewed photographs of the same building taken by another person (the “other” condition).
Not surprisingly, individuals with schizophrenia have particular difficulty recognizing “I made it up” items during reality monitoring experiments, and even during accurate task performance, they show relative underactivation of mPFC (Vinogradov et al., 2008). Further, in schizophrenia, these reality monitoring deficits are associated with a pattern of impairments in attention, memory, executive function, and basic social cognition that is quite different from what is observed in healthy individuals (Fisher et al., 2008). The overall picture is one of reduced efficiency, lower accuracy, and less reliability when individuals with schizophrenia are required to distinguish between “inner world” and “outside reality” and/or to process socially relevant data, with general cognitive abilities contributing to task performance (Fisher et al., 2008). The ongoing real-world consequences of this kind of metacognitive disability are potentially quite profound, and could include a disturbed sense of agency, decreased insight, and abnormal social behavior. These data also suggest, however, that targeted improvement of basic attention, memory, executive, and social cognitive operations could potentially benefit higher-level reality monitoring in schizophrenia.
The present study, therefore, addressed a series of questions fundamental to neuroscience-informed cognitive training and to a “neural systems” approach to the treatment of schizophrenia: 1) Even after years of illness, can intensive computerized training of component perceptual, working memory, executive, and social cognitive processes in schizophrenia patients lead to sustained improvements in reality monitoring? 2) Is training-induced improvement in reality monitoring performance accompanied by an increase in mPFC activation patterns? Do training-induced increases in mPFC activity correlate with improved task performance? 3) Is a training-induced increase in mPFC activity associated with long-term improvements in real world social functioning?
Improvement in reality monitoring in patients with schizophrenia was tested via pre- and post-training assessments of behavioral performance and fMRI activation patterns during a reality monitoring task. We enrolled 31 schizophrenia (SZ) patients and 15 healthy comparison (HC) subjects in a baseline fMRI reality monitoring experiment (Figure 1A). Next, SZ subjects were randomly assigned to either an active training (SZ-AT) or a control condition computer games (SZ-CG) intervention. The SZ-AT group participated in 80 hours of intensive computerized cognitive training, while the SZ-CG group participated in 80 hours of a rotating series of commercial computer games. Both SZ groups participated for approximately 5 hours per week over 16 weeks in the laboratory. The SZ-AT subjects were trained on basic auditory/verbal, visual, facial emotion recognition and theory of mind processes that were embedded within increasingly more complex working memory exercises, with the objective of enhancing the neural systems that support the fidelity and reliability of auditory, visual, verbal and social cognitive working memory (Delahunt et al., 2008; Fisher et al., 2009; Mahncke et al., 2006). After 16 weeks, 15 SZ-AT, 14 SZ-CG, and 12 HC subjects participated in a second fMRI reality monitoring experiment. Six months later, 13 SZ-AT and 12 SZ-CG subjects agreed to return to the laboratory for a follow-up visit and re-assessment of their clinical and functional status.
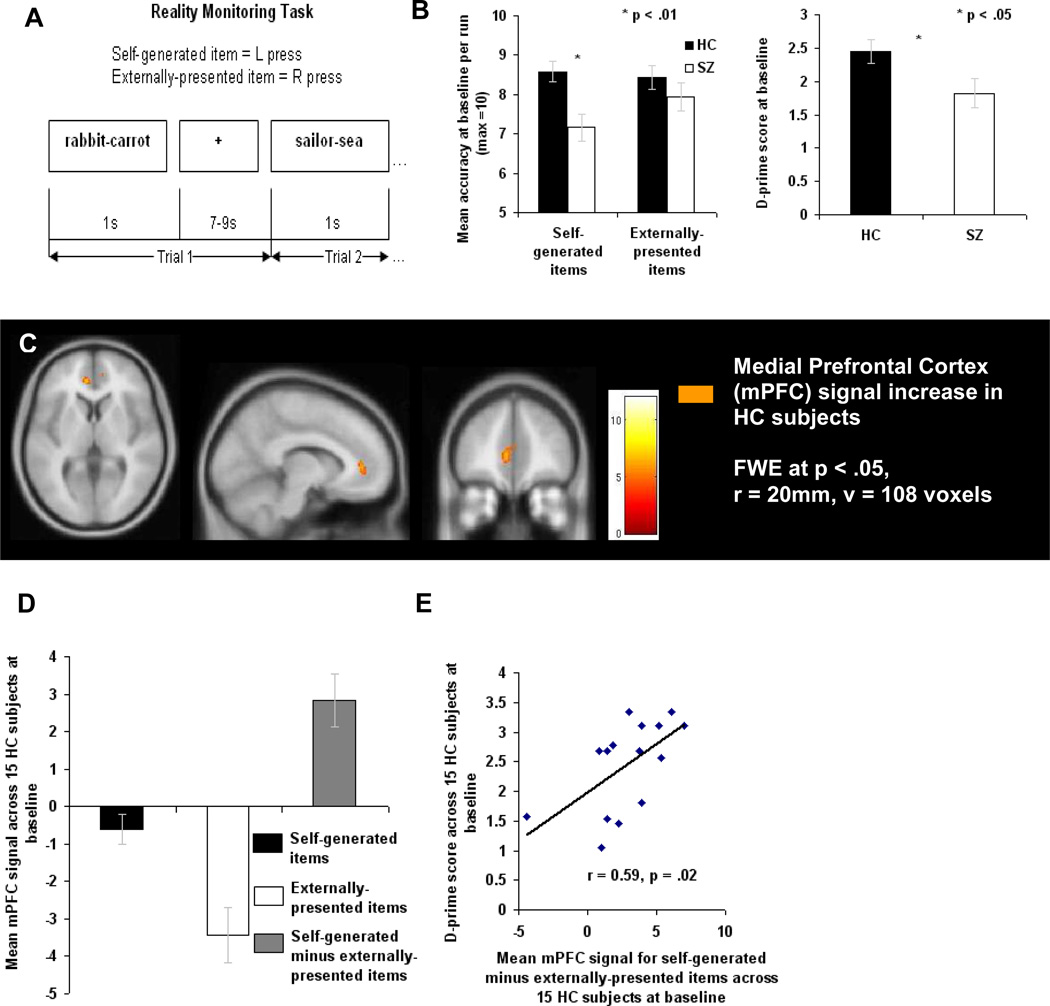
Figure 1. Pre-Training (Baseline) Performance and Brain Activation Differences between Healthy Comparison (HC) and Schizophrenia Subjects (SZ) during a Reality Monitoring Task.
(A) Task Design: example of events within one trial.
(B) Behavior: Mean accuracy averaged across 3 runs for self-generated and externally-presented item-identification is illustrated. One-way ANOVA, comparing mean raw scores for self-generated and externally-presented item identification, reveals significant impairment in SZ subjects during accurate identification of self-generated items, but not for identification of externally-presented items. One-way ANOVA, comparing mean d-prime statistical scores for overall source memory identification of word items between HC and SZ subjects, reveals significant impairment in SZ subjects during overall source-memory accuracy.
(C) Signal increase in mPFC for the self-generated condition vs. the externally-presented condition across 15 HC subjects within the a priori mPFC ROI centered at co-ordinates (−4, 52, 8). (See also Figure S1 and Table S1 for whole brain analyses at baseline in each group, across (A) 15 HC and (B) 31 SZ subjects).
(D–E) Mean mPFC signal is illustrated in beta weights averaged across all voxels within the a priori spherical mPFC ROI. All error bars represent s.e.m.
Each fMRI session consisted of a word-generation phase performed outside the scanner prior to scanning, and a reality monitoring task performed during scanning (Figure 1A). In the word-generation phase, subjects were presented with a list of semantically constrained sentences with the structure “noun-verb-noun.” The final noun was either presented by the experimenter (e.g., The sailor sailed the sea), or left blank for subjects to generate themselves and then recorded by the research assistant (e.g., The rabbit ate the____). During the reality monitoring task in the scanner approximately 45 minutes later, subjects were visually presented with noun pairs from the sentence list (e.g., rabbit-carrot) and had to indicate whether the second word was previously self-generated (“I made it up”) or externally-presented (“You showed it to me”) by making a button-press with their right dominant hand (Vinogradov et al., 2008). In healthy subjects, identification of the source of self-generated word items is associated with greater fMRI activity in the dorsal rostral portion of the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC), relative to identification of externally-presented items (Vinogradov et al., 2006; Vinogradov et al., 2008). In contrast, patients with schizophrenia are known to be significantly less accurate in the identification of the source of self-generated items (but not externally-presented word items), and exhibit no fMRI activity in mPFC and ACC during this reality monitoring task (Vinogradov et al., 2008). We examined both behavioral performance and fMRI neural activity in our subject groups, at baseline and after 16 weeks, contrasting correct trials for identification of self-generated word items with correct trials for externally-presented word items.
RESULTS
Behavioral and FMRI Findings Prior to the Intervention
To examine performance on the reality monitoring task at baseline, accuracy scores were submitted to a repeated measures ANOVA with group (HC, SZ) as a between-subject factor and condition (self-generated, externally-presented) as a within-subject factor. At baseline, consistent with our previous findings (Vinogradov et al., 1997; Vinogradov et al., 2008), we found a significant group by condition interaction (F(2,44) = 3.32, p = .05), driven by the HC subjects who identified significantly more self-generated items than SZ subjects (F(1,45) = 6.78, p = .01), but not more externally-presented items (F(1,45) = 0.71, p = .40) (Figure 1B). In order to correct for any response bias on the reality monitoring task, we utilised signal detection theoretic analyses to compute a d-prime score for overall reality monitoring source memory performance. Signal detection theoretic analysis confirmed that HC subjects performed significantly better than SZ subjects during overall source memory identification of word items (F(1,45) = 4.19, p = .047) (Figure 1B). The effect size of the overall source memory accuracy difference between HC and SZ subjects at baseline was 0.65. After baseline testing, the SZ subjects were randomly assigned to either active training (SZ-AT) or to computer games (SZ-CG). An ANOVA with the three groups at baseline (HC, SZ-CG and SZ-AT subjects) revealed a significant main effect of group in d-prime scores: HC subjects were more accurate than SZ-CG and SZ-AT subjects (F(2,44) = 3.57, p = .037), but there was no accuracy difference in d-prime scores between SZ-CG and SZ-AT subjects (F(1,29) = 2.17, p = .15), indicating that the two patient groups showed similar task performance at baseline.
To examine mPFC fMRI activity during reality monitoring, we defined an a priori 20 mm (radius) spherical region of interest (ROI) according to Cabeza et al.’s (2004) locus of mPFC activity, for self-referential memory reported in a sample of psychiatrically healthy subjects, centered on −4, 52, 8 Talairach coordinates. We first conducted multiple one-sample t-tests within each group (HC, SZ-CG and SZ-AT) at baseline to assess reality-monitoring activity (i.e., activity for correctly identified self-generated items versus activity for correctly identified externally-presented items) on a voxel-by-voxel basis, using the spherical a priori mPFC ROI as an explicit mask. Multiple comparison corrections were then performed within the mPFC ROI, with the FWE correction of p<.05 and with a cluster extent of 0, using the small volume correction (SVC) implemented in SPM2. Results from these one-sample t-tests revealed that the HC group was the only group at baseline to activate voxels that survived this FWE correction (p<.05) within the mPFC ROI (Figure 1C). Neither the SZ-AT nor SZ-CG groups activated any voxels that survived the FWE correction (p<.05) within the mPFC ROI at baseline. Next, for all group correlations and for all between-group ANOVAs, mean beta weights from the self-generated versus externally-presented comparison were calculated across all voxels within the a priori spherical mPFC ROI for each group. These mean beta weights were submitted to a one-way ANOVA in SPSS to test for differences between the HC, SZ-CG and SZ-AT subject groups. The ANOVA between HC, SZ-CG and SZ-AT subject groups at baseline revealed a significant group effect in mPFC activity for self-generated minus externally-presented items (F(2,43) = 7.52, p = .002). This group effect at baseline was driven by the HC subjects, who revealed significantly more mPFC activity for self-generated items than externally-presented items when compared to the SZ-CG subjects (F(1,28) = 12.75, p = .001) and when compared to the SZ-AT subjects (F(1,29) = 11.08, p = .002). There was no significant difference in mPFC activity between SZ-CG and SZ-AT subjects at baseline (F(1,29) = .90, p = .35). Next, these mean beta weights from the self-generated versus externally-presented comparison that were extracted from the a priori spherical mPFC ROI for each group at baseline were correlated with behavioral performance for each group at baseline. Interestingly, only in HC subjects was mPFC signal level within the a priori ROI significantly correlated with accurate overall source memory identification of word items (r = 0.59, p = .02) (Figures 1D and 1E). This correlation was not significant in the SZ-CG subjects (r = −0.18, p = .53) or in the SZ-AT subjects (r = 0.25, p = .36) at baseline. These data are consistent with the role of mPFC as a critical node in the neural network that supports a range of self-referential processes (Northoff et al., 2006; Ochsner et al., 2004; Ochsner et al., 2005; Vinogradov et al., 2006), and indicate that schizophrenia patients do not show normal recruitment of this network during a reality monitoring task.
Behavioral Performance Changes Due to Intervention
After 16 weeks in which SZ patients participated in either 80 hours of cognitive training or a rotating series of commercial computer games, subjects returned for a second fMRI reality monitoring experiment. A repeated-measures ANOVA revealed a significant group-by-session interaction in d-prime scores for overall source memory identification of word items (F(2,39) = 4.82, p = .013). Specifically, there was a significant group-by-session effect for self-generated word items (F(2,39) = 4.37, p = .02) but not for externally-presented word items (F(2,39) = 2.34, p = .11) (Figures 2A and 2B). The SZ-AT subjects, when compared to the SZ-CG subjects, identified the source of significantly more word items overall at 16 weeks compared to baseline (F(1,28) = 6.98, p = .01) and also specifically identified more self-generated items (F(1,28) = 5.87, p = .02), with a trend effect for externally-presented items (F(1,28) = 3.64, p = .07). The SZ-AT subjects, when compared to the HC subjects, identified the source of more word items overall at 16 weeks compared to baseline (F(1,26) = 4.42, p = .045), identifying more self-generated (F(1,26) = 5.89, p = .02) but not more externally-presented items (F(1,26) = 0.97, p = .33). There were no differences between sessions for HC or SZ-CG subjects on overall source-memory accuracy (F(1,24) = .19, p = .67), on self-generated items (F(1,24) = .04, p = .84) or on externally-presented items (F(1,24) = 1.79, p = .19). After cognitive training compared to baseline, within-group paired t-tests confirmed that SZ-AT subjects identified the overall source of significantly more word items (t(15) = 2.53, p = .02), significantly more self-generated items (t(15) = 2.3, p = .04), and marginally more externally-presented items (t(15) = 2.03, p = .06). A comparison of the change in overall source-memory accuracy from baseline to 16 weeks revealed a large effect size of 0.86 in SZ-AT vs. SZ-CG subjects, and a medium effect size of 0.61 in SZ-AT vs. HC subjects. In contrast, neither HC nor SZ-CG subjects showed significant improvement in overall source memory accuracy at 16 weeks compared to baseline (HC: t(11) = 0.23, p = .82; SZ-CG: t(13) = 1.11, p = .29). These results indicate that improvement in reality monitoring performance was specific to schizophrenia patients who engaged in 16 weeks of computerized cognitive training.
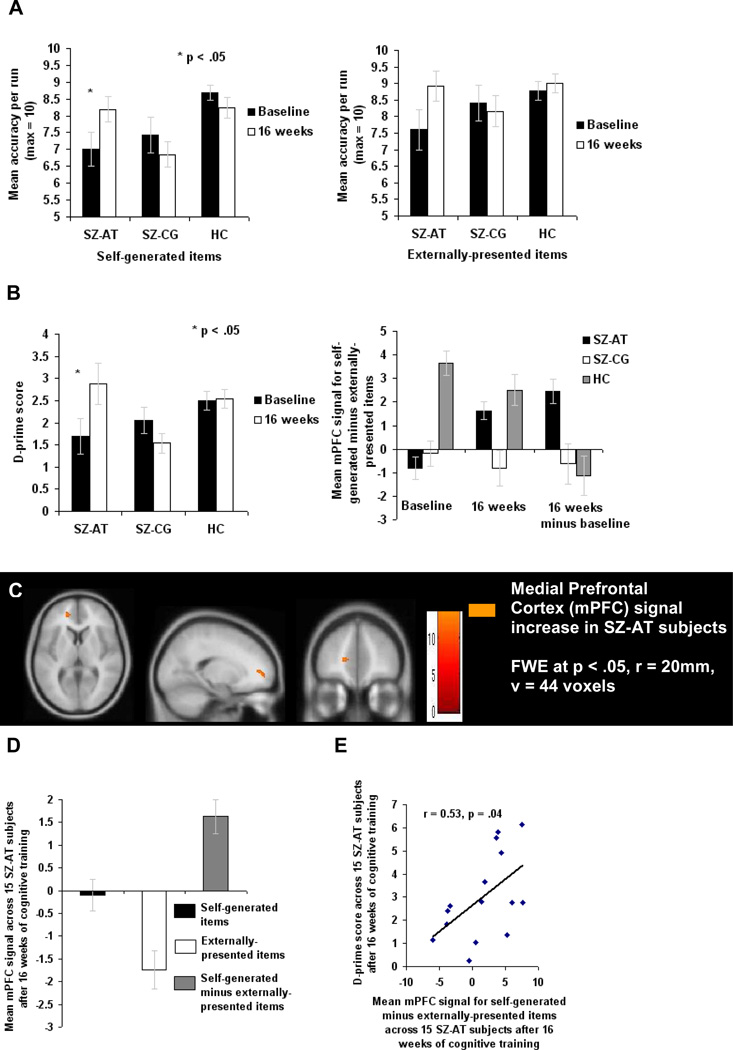
Figure 2. Cognitive Training Effects: Performance and Brain Activation Differences (at 16 Weeks) between Active Training Schizophrenia Subjects (SZ-AT), Control Condition Computer Games Schizophrenia Subjects (SZ-CG), and Healthy Comparison Subjects (HC).
(A–B) Mean accuracy averaged across 3 runs for self-generated and externally-presented item-identification, d-prime scores and mean mPFC signal are illustrated. Repeated measures ANOVAs reveal group differences at 16 weeks compared to baseline in: (A) Self-generated item accuracy, but not externally-presented item accuracy, and (B) D-prime scores for overall source memory identification of word items, as well as in mPFC reality monitoring signal averaged across all voxels within the a priori spherical ROI.
(C) Reality monitoring activity in mPFC across 15 SZ-AT subjects after cognitive training compared to baseline within the a priori mPFC ROI (See also Figure S2 and Table S2 for the whole brain analyses of reality monitoring mPFC activity at 16 weeks versus baseline in each group, across (A) 15 SZ-AT, (B) 14 SZ-CG, and (C) 12 HC subjects).
(D–E) Mean mPFC signal is illustrated in beta weights averaged across all voxels within the a priori spherical ROI in the SZ-AT group after training. All error bars represent s.e.m.
Brain Imaging Changes Due to Intervention
We performed one-way within-subject ANOVAs to compare reality-monitoring activity (i.e., activity for correctly identified self-generated items versus activity for correctly identified externally-presented items) on a voxel-by-voxel basis before and after intervention within each group, using the spherical a priori mPFC ROI as an explicit mask. Multiple comparison corrections were then performed within the mPFC ROI with the FWE correction of p<.05 and with a cluster extent of 0, using the small volume correction (SVC) implemented in SPM2. Results from the one-way within-subject ANOVAs revealed that only the SZ-AT group showed increased mPFC activation during reality-monitoring that survived the FWE correction (p<.05) at 16 weeks versus baseline (Figure 2C). Next, in order to investigate between-group differences at 16 weeks versus baseline, mean beta weights from the self-generated versus externally-presented comparison were extracted across all the voxels within the a priori spherical mPFC ROI for each group and for each session (i.e., at baseline, and at 16 weeks). These mean beta weights were submitted to a repeated-measures ANOVA in SPSS to test for differences between the HC, SZ-CG and SZ-AT groups in mPFC signal change from baseline to 16 weeks. There was a significant group-by-session interaction in mPFC reality monitoring activity (F(2,38) = 3.49, p = .04). This group-by-session effect was driven by the SZ-AT subjects, who had significantly more mPFC signal after the intervention than the SZ-CG subjects (F(1,27) = 4.07, p = .05) and than the HC subjects (F(1,25) = 4.48, p = .04). There were no differences between sessions for HC or SZ-CG subjects in mPFC signal for the self-generated item minus externally-presented item comparison (F(1,24) = .01, p = .91).
Next, these mPFC mean beta weights from the self-generated versus externally-presented comparison that were extracted across the a priori spherical mPFC ROI for each group at 16 weeks were correlated with behavioral performance for each group at 16 weeks. Importantly, in the SZ-AT subjects, mPFC signal within the a priori ROI after training was correlated with task accuracy after training (r = 0.53, p = .04) (Figures 2D and 2E), similar to the correlation we observed in HC subjects at baseline. These results indicate that, after 16 weeks of intensive training of component cognitive processes, the SZ-AT subjects began to “normalize” their brain-behavior associations during performance of an untrained higher-order reality monitoring task, so that they more closely resembled healthy subjects. These brain-behavior associations were not observed in the SZ-CG subjects after 16 weeks of computer games (r = 0.12, p = .68).
Association of Reality Monitoring Task Performance and mPFC Activation Levels with Neuropsychological Measures after Cognitive Training
The effects of this form of cognitive training on standard neuropsychological outcome measures in a larger sample of schizophrenia subjects have been previously reported by us (Sacks et al.,(2012); Fisher et al., 2009, 2010). In brief, this form of intensive computerized cognitive training drives significant improvements in processing speed, verbal learning and memory, and general cognition in patients with schizophrenia. Here, we describe the associations we observed after training between improved reality monitoring and standard outcome measures of verbal memory and of executive function, as these are both known to contribute to source memory performance (e.g., Fisher et al., 2008).
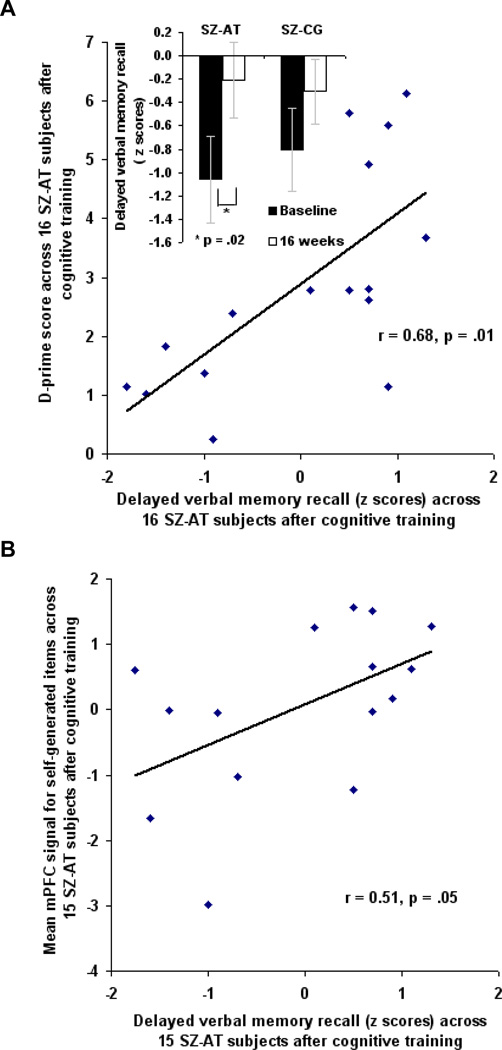
After cognitive training, SZ-AT subjects performed significantly better on delayed verbal memory recall (Neuropsychological Assessment Battery, NAB; Stern and White, 2003) compared to baseline (t(15) = 2.70, p = .02; Figure 3A), but no such improvement was found for the SZ-CG group (delayed recall: t(13) = 1.08, p = .30). After training, accuracy for overall source memory identification of word items in the SZ-AT subjects was significantly correlated with better delayed verbal memory recall, even after controlling for age, education and IQ (delayed recall: r = 0.68, p = .01) (Figure 3A); however, no such association was present at baseline (delayed recall: r = 0.23, p = .45). Furthermore, after cognitive training, mPFC signal within the a priori ROI was significantly correlated with verbal memory scores at 16 weeks (Figure 3B); however, mPFC signal within the a priori ROI in the SZ-AT subjects at baseline did not correlate with delayed recall at baseline (r = −0.04, p = .89). No such associations were found in SZ-CG subjects after the intervention (task performance with delayed recall: r = −0.18, p = .53; mPFC signal with delayed recall: r = −0.14, p = .64). These data indicate that correlations between verbal memory and reality monitoring performance, and between verbal memory and mPFC signal, are the result of the computerized cognitive training.
Figure 3. Association of Reality Monitoring Performance and Medial Prefrontal Activation Levels with Verbal Memory in Active Training Schizophrenia Subjects (SZ-AT) after 16 Weeks of Intervention.
(A) Partial two-tailed correlations, controlling for age, education and IQ, show a significant association in SZ-AT subjects after 16 weeks of cognitive training (n=16) between overall source memory accuracy on the reality monitoring task and delayed verbal memory recall,
(B) Two-tailed correlations show a significant association in SZ-AT subjects after 16 weeks of cognitive training (n=15) between mPFC signal within the a priori ROI after 16 weeks with delayed verbal memory recall.
After cognitive training, the SZ-AT subjects performed significantly better on a measure of executive functioning (Tower of London task; Keefe et al., 2004) compared to baseline (t(15) = 2.47, p = .03), a finding not seen in the SZ-CG subjects (t(13) = 0.15, p = .89). In SZ-AT subjects, overall source memory identification of word items after training was significantly correlated with performance on executive functioning, even after controlling for age, education and IQ (r = 0.59, p = .03), though this association was not present at baseline (r = 0.29, p=.28). However, mPFC signal within the a priori ROI at 16 weeks was not associated with executive functioning at 16 weeks (r = 0.31, p = .27). No associations between task performance and executive functioning were seen after the intervention in SZ-CG subjects (r = 0.05, p = .85). These data indicate that cognitive training induces an improvement in executive function in SZ-AT subjects which is associated with better reality monitoring, but not with greater activation in mPFC.
Clinical symptoms were assessed with the Positive and Negative Syndrome Scale (PANSS) which rates each symptom-- such as delusions or hallucinations-- on a scale of 1 (absent) to 7 (extreme) (Kay et al., 1987). Overall mean symptom ratings were low in this clinically stable group of SZ participants (slightly over 2, mild) at baseline and at 16 weeks (Table 3). There was no significant change in mean symptom ratings at 16 weeks compared to baseline in either the SZ-AT group (t(15) = 0.58, p = .57) or in the SZ-CG group (t(13) = 1.62, p = .13). Source memory accuracy was not correlated with any reduction in symptom ratings at 16 weeks in the SZ-AT group (r = 0.27, p = .30) or in the SZ-CG group (r = 0.31, p = .27).
Table 3.
Neuropsychological Measures and Clinical Symptom Ratings (mean, SD) at Baseline and after 16 Weeks of Intervention in Active Training SZ Subjects (SZ-AT) and Control Condition Computer Games SZ Subjects (SZ-CG)
| SZ-AT (N=16) | SZ-CG (N=14) | |||
|---|---|---|---|---|
| Baseline | 16 weeks | Baseline | 16 weeks | |
| Delayed Verbal Memory Recall (z-score) | −1.06 (1.54) | −0.21a (1.27) | −0.80 (1.33) | −0.31 (1.04) |
| Tower of London (z-score) | −0.13 (1.12) | 0.39b (0.86) | −0.10 (0.86) | −0.25 (1.08) |
| Clinical Symptom Ratings (1=absent, 7=extreme) | 2.57 (0.67) | 2.59 (0.68) | 2.48 (0.53) | 2.30 (0.52) |
Paired two-tailed t-tests indicate that SZ-AT subjects showed significant improvement on delayed verbal memory recall at 16 weeks compared to baseline (p = .02); SZ-CG subjects showed no significant improvement in this measure (p = .30).
SZ-AT subjects also showed significant improvement on the Tower of London task at 16 weeks compared to baseline (p = .03); SZ-CG subjects showed no significant improvement in this measure (p = .89).
Findings Six Months After the Intervention
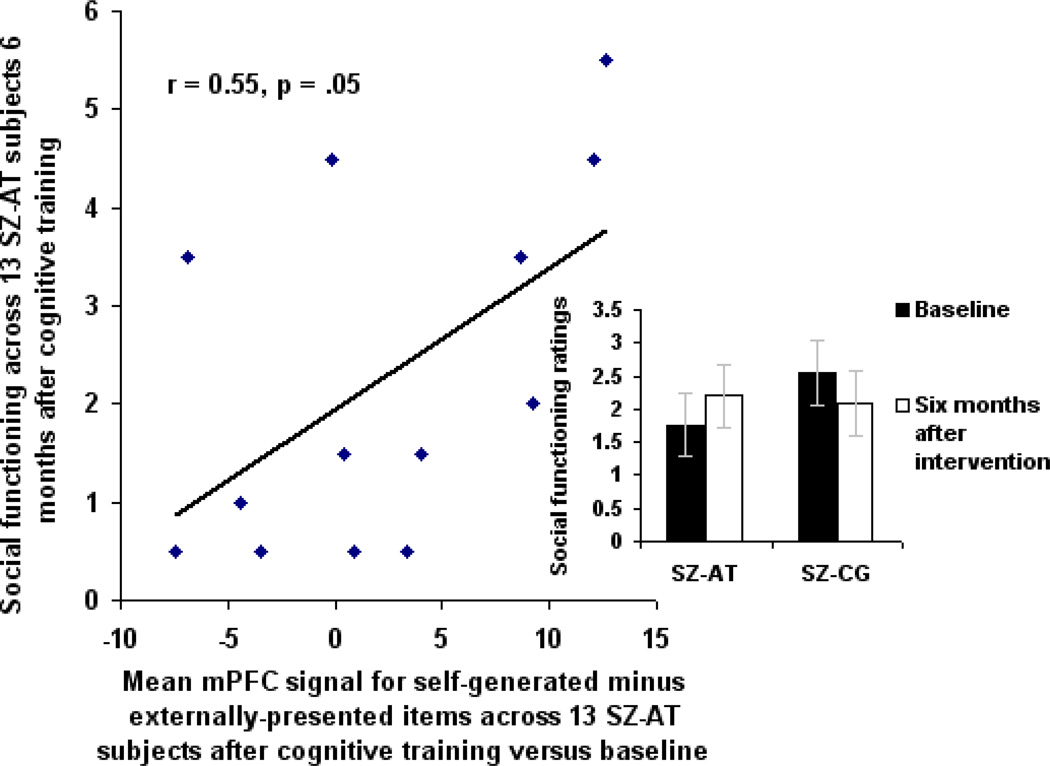
In the 13 SZ-AT subjects who returned for re-assessment six months later, there was no overall change in social functioning at a group level (t(12) = .49, p = .63) as measured by the QLS Social Functioning Subscale (Bilker et al., 2003). However, the level of reality monitoring signal within the a priori spherical mPFC ROI immediately after training was significantly correlated with ratings of social functioning at the six month follow-up (Figure 4). Reality monitoring signal within the a priori mPFC ROI at baseline did not correlate with ratings of social functioning at baseline (r = −0.02, p = .94). In the 12 SZ-CG subjects who returned for re-assessment six months later, reality monitoring signal within the a priori mPFC ROI at 16 weeks did not correlate with social functioning at six month follow-up (r = .04, p = .90). There was no association between mPFC signal within the a priori ROI after training and mean clinical symptom ratings 6 months later (r = .12, p = .69). These results suggest that SZ patients who show higher training-induced recruitment of mPFC during reality monitoring also demonstrate better real-world social functioning six months later.
Figure 4. Social Functioning Six Months after Cognitive Training.
Two-tailed correlations show a significant association in SZ-AT subjects (n=13) between reality monitoring mPFC signal within the a priori ROI at 16 weeks, and social functioning ratings assessed 6 months after cognitive training was completed.
DISCUSSION
Schizophrenia patients who received intensive computerized training of component auditory/verbal, visual, and social cognitive processes, compared to patients who played computer games, showed: 1) A significant improvement in their accuracy performing a complex reality monitoring task that was not part of the training exercises (i.e., generalization of training effects); 2) A significant increase in mPFC activation during performance of this task; 3) A significant association between the level of mPFC activation and task performance (findings that were not present at baseline); and, 4) A significant relationship between mPFC activation after training and better social functioning six months later. Our findings are consistent with prior work indicating that medial prefrontal dysfunction is associated with poor self-reflection processes, poor social cognition, and poor social functional status in schizophrenia (Holt et al., 2011; Lee et al., 2006; Park et al., 2008), but indicate that—rather than being a static deficit—this neural system impairment is responsive to an intensive cognitive training intervention.
To our knowledge, this is the first time that a complex higher order cognitive process in a serious neuropsychiatric illness—in this case, the ability to distinguish the source of information generated by the “self” from information generated by the “other”-- has been the targeted outcome of a neuroscience-informed cognitive training strategy. Our study is also the first-in-kind demonstration that, for patients with schizophrenia, training of component cognitive processes generalizes to an untrained higher order operation and produces a significant improvement in its neural correlates, such that patients begin to demonstrate more “normal” brain-behavior associations, which in turn predict better social functioning several months later. Thus, it is possible to significantly improve brain function in schizophrenia, even in patients who have been ill for an average of twenty years, and it appears that these improvements set the stage for an enduring improvement in social functioning that occurs even in the absence of other psychosocial therapies. Of note, schizophrenia participants showed a range of responses to the intervention, and even after 80 hours of intensive training, and despite significant increases in mPFC activation, they still did not demonstrate the same activation levels as those observed in healthy comparison subjects. Though not all patients respond equally well to cognitive training, a successful response appears to open a critical window for further functional gains, consistent with our previous finding that patients with higher general cognitive improvement after training show significantly better overall quality of life ratings at 6 months (Fisher et al., 2010).
We do not know which aspects of the cognitive training were most responsible for the behavioral and neural improvements we observed. The reality monitoring task had a strong verbal memory component, and the auditory/verbal learning exercises we employed (for 50 hours of the training) have been shown to improve verbal learning and memory in schizophrenia subjects in a prior study (Fisher et al., 2009) and in the current study (Figure 3A). Indeed, after training, both overall reality monitoring task performance and mPFC signal within the a priori ROI were significantly associated with better verbal memory (Figures 3A and 3B). These findings suggest that training of auditory/verbal learning and memory processes contributes to significant behavioral improvement in reality monitoring as well as improvement in the underlying neural systems that facilitate reality monitoring. However, basic social cognition performance is also strongly correlated with reality monitoring abilities and with activation in mPFC (Benoit et al., 2010; Heberlein et al., 2008; Hooker et al., 2011; Mattavelli et al., 2011; Ochsner et al., 2004; Ochsner et al., 2005; Phan et al., 2002; Ray et al., 2010; Sabatinelli et al., 2011). Thus, the 10 hours of computerized training in facial emotion recognition and theory of mind we provided may have also contributed significant stimulation to mPFC-related neural system function. Finally, as an ensemble, the exercises were designed to enhance attention, working memory, and the representational fidelity of verbal and visual stimuli, and in so doing they may have resulted in more efficient executive functioning in participants in a manner that contributed to improved reality monitoring, as is suggested by our finding of a significant correlation between better executive functioning after training and reality monitoring task performance. Taken together, our data indicate that intensive training of component cognitive processes generalized to increase the efficiency of a complex reality monitoring source memory operation in clinically stable but persistently ill patients with schizophrenia. These results do not appear to be due to non-specific effects of attention, motivation, or engagement, since the patients in the computer games control condition were fully engaged in the intervention and also rated their experiences as highly enjoyable and beneficial (Fisher et al., 2009),
Our results have several far-reaching implications for the treatment of neurocognitive disorders in general and serious psychiatric illness more specifically. First, significant improvements in cognitive and neural function in schizophrenia can be induced by a neural systems based behavioral intervention. Second, the training of component cognitive processes in schizophrenia generalizes to improvement on an untrained complex and higher-order reality monitoring operation. While this is a promising finding, additional research must determine the necessary and sufficient elements of training; whether this training reveals generalization effects beyond the trained tasks in healthy populations; and finally whether it can induce the desired behavioral outcomes of improved quality of life and community functioning (Fisher et al., 2010; Green et al., 2000). Finally, intensive cognitive training can begin to “normalize” abnormal brain-behavior associations in schizophrenia (see also Haut et al., 2010), and such improvements predict better social functioning six months later. This research, therefore, raises the exciting likelihood that the neural impairments in schizophrenia—and undoubtedly other neuropsychiatric illnesses—are not immutably fixed, but instead may be amenable to well-designed interventions that target restoration of neural system functioning.
EXPERIMENTAL PROCEDURES
Subjects
Subjects in this study were thirty-one clinically stable, persistently ill, volunteer schizophrenia patients (SZ: mean age = 40; education = 13 years; IQ = 103; illness duration = 19.4 years) drawn from our randomized clinical trial of cognitive-training (ClinicalTrials.gov NCT00312962) and 16 healthy comparison subjects matched to the SZ subjects at a group level in age, gender, and education (HC: mean age = 45; education = 14 years; IQ = 115) (Table 1). SZ subjects were recruited from community mental health centers and outpatient clinics, and HC subjects were recruited via advertisement. Inclusion criteria were Axis I diagnosis of schizophrenia (determined by the Structured Clinical Interview for DSM-IV [SCID]) (First et al., 2002) or, for HC subjects, no Axis I or Axis II psychiatric disorder (SCID—Nonpatient edition), no substance dependence or current substance abuse, good general physical health, age between 18 and 60 years, and English as first language. All subjects gave written informed consent and underwent a series of baseline behavioral assessments and imaging. One HC provided only behavioral data because he was too claustrophobic to be scanned. All others participated in a baseline fMRI session. SZ subjects were then stratified by age, education, gender, and symptom severity and randomly assigned to either 80 hours of active training (SZ-AT) or 80 hours of a computer games control condition (SZ-CG). SZ subjects were blind to group assignment. There were no significant differences between the two patient groups at baseline in antipsychotic medications (1st generation, 2nd generation, multiple, or none), in Cogentin or Chlorpromazine equivalents, or in the number of subjects in each group taking antidepressants, mood stabilizers, benzodiazepines, or anticholinergic medications (Table 2). All SZ subjects had outpatient status for 3 months prior to study entry and no significant medication changes (dosage change <10%) during the study. One SZ-CG subject withdrew from the study for personal reasons between baseline and 16 weeks; one SZ-AT subject felt too anxious to complete the reality monitoring experiment in the scanner at 16 weeks, and thus performed the task outside the scanner, providing only behavioral data. Thirteen out of 15 SZ-AT subjects and 12 out of 14 SZ-CG subjects returned to the laboratory six months later to receive follow-up clinical assessments. The 2 SZ-AT subjects and the 2 SZ-CG subjects who did not return were unavailable and/or unwilling to be involved in further study participation. None of the 13 SZ-AT or the 12 SZ-CG subjects had participated in any new psychosocial treatment program during the no-contact period.
Table 1.
Demographics (mean, SD) of Healthy Comparison (HC) and Schizophrenia (SZ) Subjects
| HC (N=16) |
SZ (N=31) |
|
|---|---|---|
| Age | 45 (11.6) | 40 (1.17) |
| Gender | 11M, 5F | 26M, 5F |
| Education (years) | 14 (1.35) | 13 (0.89) |
| IQ | 115 (11.45) | 103a (11.5) |
There was a statistically significant difference in IQ between HC and SZ subjects at baseline (t = 2.01, p = .052); therefore we used partial correlations for all behavioral training effects in order to control for any baseline differences in age, education and IQ.
Table 2.
Medication Profiles of Active Training Schizophrenia Patients (SZ-AT) and Control Condition Computer Games SZ Patients (SZ-CG)
| Antipsychotic Medicationa | SZ-AT (N=16) | SZ-CG (N=15) | p value |
|---|---|---|---|
| 1st Generation (N) | 0 | 2 | 0.23 |
| 2nd Generation (N) | 11 | 12 | 0.69 |
| Multiple (N) | 1 | 0 | 0.99 |
| No antipsychotic (N) | 4 | 1 | 0.33 |
| Other Psychiatric Medication | |||
| Antidepressants or Mood Stabilizers (N) | 9 | 5 | 0.48 |
| Benzodiazepines (N) | 4 | 6 | 0.46 |
| Anticholinergics (N) | 2 | 3 | 0.65 |
| Mean Chlorpromazine (CPZ) Equivalentsb | 478 (SD =380) | 419 (SD=453) | 0.63 |
| Mean Cogentin Equivalentsc | 0.73 (SD=0.82) | 1.35 (SD=2.95) | 0.45 |
Fisher’s Exact test (2-tailed) revealed no significant differences in the number of Antipsychotic Medication or Other Psychiatric Medication between the two patient groups at baseline. 1st generation antipsychotic medication = thioridazine; 2nd generation antipsychotic medication = aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone.
Two sample two-tailed t-tests revealed no significant differences in mean CPZ Equivalents between groups (Andreasen et al., 2010);
Two sample two-tailed t-tests revealed no significant differences in mean Cogentin Equivalents (Minzenberg et al., 2004). All patients were clinically stable, and there was no change in medication at 16 weeks compared to baseline in either group.
Assessments
All SZ subjects received clinical and cognitive assessments at baseline and after training. Clinical symptoms were assessed with the Positive and Negative Syndrome Scale (Kay et al., 1987), which rates each symptom on a scale of 1 (absent) to 7 (extreme). Verbal memory and executive functioning was assessed via the Neuropsychological Assessment Battery (NAB) Daily Living Memory Scale (Stern and White, 2003) and BACS Tower of London test (Keefe et al., 2004) (Table 3). Raw scores were converted to age-adjusted z scores using normative data, published by the test authors. Social functioning was assessed with the Quality of Life Scale-Abbreviated Version (QLS) six months after the cognitive training was completed (Bilker et al., 2003) (Table 4). The QLS is a semi-structured interview that assesses functioning during the preceding four weeks on a scale of 0 = virtually absent to 6 = adequate functioning. Researchers who randomized subjects were independent from assessment personnel, and all assessment staff were blind to subjects’ group assignment. The PANSS and QLS were conducted by two assessment staff with inter-rater reliabilities greater than 0.85 (intraclass correlation coefficients) for the PANSS and QLS total and subscale scores.
Table 4.
Ratings on QLS Social Functioning Subscale (mean, SD) for Active Training SZ Subjects (SZ-AT) and Control Condition Computer Games SZ Subjects (SZ-CG) at Baseline and 6 Months after the Intervention was Completed
| SZ-AT (N=13) |
SZ-CG (N=12) |
|
|---|---|---|
| Baseline | 1.76 (1.70) | 2.54 (1.74) |
| Social functioning 6 months after intervention (0=virtually absent, 6=adequate functioning) | 2.20 (1.77) | 2.08 (1.84) |
Statistical Analyses: Behavioral Data
Signal detection theoretic d-prime analyses for the reality monitoring task were conducted on overall accuracy in source memory identification of word items by calculating the hit rate and the false alarm rate for self-generated and externally-presented items, then converting each measure to z-scores, and subtracting the false alarm rate from the hit rate in order to differentiate sensitivity during accurate performance from response bias. For all behavioral correlations, partial two-tailed correlation coefficients were used to measure the strength of the linear relationship between the two variables after cognitive training, controlling for age, education and IQ. Effect sizes were used to quantify the magnitude of the difference in overall source memory identification of word items between HC and SZ subjects at baseline, as well as to quantify the change in accuracy at 16 weeks compared to baseline between SZ-AT and SZ-CG subjects and between SZ-AT and HC subjects.
Computerized Cognitive Training
Complete details on the computerized cognitive training exercises are presented in the Supplemental Experimental Procedures. In brief, cognitive training consisted of a module of auditory processing exercises (http://www.positscience.com/our-products/brain-fitness-program), a module of visual processing exercises (http://www.positscience.com/our-products/demo), and a module of computerized emotion identification exercises, composed of training in facial emotion recognition and theory of mind (MindReading, MicroExpressions Training Tool, Subtle Expressions Training Tool; Baron-Cohen et al., 2003; Eckman, 2003). The SZ-AT subjects participated in auditory exercises for 1 hr a day for a total of 50 hours (10 weeks), and then participated in visual exercises for 1 hr a day for a total of 30 hours (6 weeks) that were combined with 15 minutes per day of emotion identification exercises (total of 10 hours). In the exercises, patients were driven to make progressively more accurate discriminations about the spectro-temporal fine-structure of auditory and visual stimuli under conditions of increasing working memory load, or of basic social cognitive stimuli under progressively briefer presentations, and to incorporate and generalize those improvements into working memory rehearsal and decision-making. The auditory and visual exercises were continuously adaptive: they first established the precise parameters within each stimulus set required for an individual subject to maintain 80% correct performance, and once that threshold was determined, task difficulty increased systematically and parametrically as performance improved. The social cognition training was partially adaptive, in that difficulty level increased progressively as participants successfully completed blocks of trials at a given difficulty level. The design and implementation of this approach was informed by research demonstrating impairments in schizophrenia in basic auditory and visual perceptual processes, as well as in higher-order working memory, and social cognitive functions (e.g., Green, 1996; Javitt, 2009; Javitt et al., 2000). In the computer games control condition, SZ-CG subjects systematically rotated through 16 different commercially available computer games (i.e., clue-gathering and visual-spatial puzzle games such as Hangman, Tic-Tac-Toe, Tetris) for a total of 80 hours over 16 weeks. The control condition was designed to allow for non-specific motivation, engagement, and deployment of attentional and executive functioning resources, without providing constrained, intensive, and adaptive training on specific cognitive operations. Subjects rated both conditions as equally entertaining on self-report questionnaires, and subjectively found both conditions to be equally beneficial; a prior study found excellent maintenance of the study blind with this protocol (Fisher et al. 2009).
FMRI Stimulus Presentation
Visual fMRI stimuli were presented with E-Prime (http://www.pstnet.com/eprime.cfm) and back-projected using an LCD projector onto a screen at the foot of the scanner table. Subjects viewed the screen using a mirror attached to the head coil and made finger-press responses on a fibre-optic 8 channel response pad (Lightwave Medical Industries Ltd., Vancouver B.C.). The response pad device collected scanner TTL pulses generated at the onset of MR acquisition. Subject responses and scanner signals were recorded by the E-Prime presentation program, allowing for precise retrospective temporal synchronization of stimulus events and image acquisition.
Image Acquisition
fMRI activity was measured on a 3 Tesla General Electric Signa LX 15 scanner and eight channel head coil. Functional imaging consisted of blood oxygen-level-dependent (BOLD) sensitive images acquired during performance of the experimental task, using a spiral sequence (TR=1s; TE=30 ms; flip angle=60, matrix = 64 × 64, FOV = 22cm, 14 slices, 6mm thickness). Stimulus duration was 1s, with a 7–9s variable interstimulus interval during which subjects fixated on a cross. Image analysis was performed using MATLAB (Mathworks Inc.) and SPM2 software (www.fil.ion.ucl.ac.uk/spm).
Statistical Analyses: FMRI Data
Images were realigned to correct for motion artifacts using a 6-parameter rigid body affine transformation. The resulting images were normalized to a standard stereotaxic space (Montreal Neurological Institute (MNI) Template) using a 12 parameter affine/non-linear transformation and spatially smoothed with a 10mm full-width half maximum isotropic Gaussian kernel. Data were submitted to a General Linear Model analysis, fitting a reference canonical hemodynamic response function (hrf) to each event. Correct and incorrect trials were modelled separately. Image intensity was scaled to the mean global intensity of each time series. To examine mPFC fMRI activity during reality monitoring, we defined an a priori 20 mm (radius) spherical region of interest (ROI) according to Cabeza et al.’s (2004) locus of mPFC activity, for self-referential memory reported in a sample of psychiatrically healthy subjects, centered on −4, 52, 8 Talairach coordinates. We first conducted multiple one-sample t-tests within each group (HC, SZ-CG and SZ-AT) at baseline to compare reality-monitoring activity (i.e., activity for correctly identified self-generated items versus activity for correctly identified externally-presented items) on a voxel-by-voxel basis, using the spherical a priori mPFC ROI as an explicit mask. Multiple comparison corrections were then performed within the mPFC ROI, with the FWE correction of p<.05 and with a cluster extent of 0, using the small volume correction (SVC) implemented in SPM2. This comparison between self-generated and externally-presented items was used because the deficit in correctly identifying the source of self-generated information is one of the most striking clinical findings in schizophrenia; furthermore, prior studies indicate that schizophrenia patients are significantly impaired at identifying the source of self-generated items but not externally-presented items, compared to healthy subjects (Bentall et al., 1991; Vinogradov et al., 2008). Next, mean beta weights (i.e. signal levels) from the self-generated versus externally-presented contrast, were extracted across all voxels within the a priori spherical mPFC ROI for each group at baseline. These mean beta weights were submitted to a one-way ANOVA in SPSS to test for mPFC signal differences between the HC, SZ-CG and SZ-AT subject groups at baseline. These mPFC mean beta weights from the self-generated versus externally-presented comparison that were extracted across the a priori spherical mPFC ROI for each group at baseline were then correlated with behavioral performance for each group (HC, SZ-CG and SZ-AT) at baseline.
Next, on a voxel-by-voxel basis, we performed one-way within-subject ANOVAs to compare reality-monitoring activity before and after intervention for each group, using the spherical a priori mPFC ROI as an explicit mask. Multiple comparison corrections were then performed within the mPFC ROI, with the FWE correction of p<.05 and with a cluster extent of 0, using the small volume correction (SVC) implemented in SPM2. These voxel-based analyses were used to reveal within-group intervention-based effects at 16 weeks versus baseline for each group. Next, in order to investigate whether any between-group differences in mPFC signal were specifically associated with the cognitive training, mean beta weights for the self-generated versus externally-presented contrast were extracted across all voxels within a priori spherical mPFC ROI for each group (HC, SZ-CG and SZ-AT) and for each session (i.e., at baseline and at 16 weeks). These mPFC mean beta weights within the a priori spherical mPFC ROI were submitted to a repeated measures group-by-session ANOVA in SPSS to test for differences between the HC, SZ-CG and SZ-AT groups in mPFC signal change from baseline to 16 weeks. Next, these mPFC mean beta weights from the self-generated versus externally-presented comparison that were extracted across the a priori spherical mPFC ROI for each group at 16 weeks were correlated with behavioral performance for each group at 16 weeks. (See Figure S1 and Table S1 for whole brain analyses of the self-generated condition vs. the externally-presented condition at baseline in (A) HC and (B) SZ subjects, and see Figure S2 and Table S2 for whole brain signal change at 16 weeks versus baseline in (A) SZ-AT, (B) SZ-CG, and (C) HC subjects).
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIMH through grant RO1MH82818 to Sophia Vinogradov and R01 grants DC4855 and DC6435 to Srikantan Nagarajan. Two of the authors have financial disclosures to declare: Gregory Simpson is a Senior Scientist at Brain Plasticity Institute, Inc. and Sophia Vinogradov is a consultant to Brain Plasticity Institute, Inc., which has a financial interest in computerized cognitive training programs. We thank Kasper Winther Jorgensen, Stephanie Sacks, Arul Thangavel, Adelaide Hearst, Coleman Garrett, Mary Vertinski, Christine Holland, Alexander Genevsky, Christine Hooker, Daniel H. Mathalon, Michael M. Merzenich, and Gary H. Glover for their assistance and input on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35:1132–1141. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Hill J, Wheelwright S. Mind Reading: the interactive guide to emotions. London and New York: University of Cambridge, Kingsley Publishers; 2003. [Google Scholar]
- Benoit RG, Gilbert SJ, Volle E, Burgess PW. When I think about me and simulate you: Medial rostral prefrontal cortex and self-referential processes. Neuroimage. 2010;50:1340–1349. doi: 10.1016/j.neuroimage.2009.12.091. [DOI] [PubMed] [Google Scholar]
- Bentall RP, Baker GA, Havers S. Reality Monitoring and Psychotic Hallucinations. Brit J Clin Psychol. 1991;30:213–222. doi: 10.1111/j.2044-8260.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Bilker WB, Brensinger C, Kurtz MM, Kohler C, Gur RC, Siegel SJ, Gur RE. Development of an abbreviated schizophrenia quality of life scale using a new method. Neuropsychopharmacology. 2003;28:773–777. doi: 10.1038/sj.npp.1300093. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophr Bull. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Li H, Cheung EF, Gong QY. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178:381–390. doi: 10.1016/j.psychres.2009.03.035. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- Delahunt P, Hardy JL, Brenner DF, Chan SC, Dewey JA, Mahncke HW, Wade TW, Merzenich MM. InSight. Scientific principles of a brain-plasticity based visual training program. Posit Science Corporation. 2008 [Google Scholar]
- Eckman P. Micro Expressions Training Tool and The Subtle Expressions Training Tool (METT AND SETT) Venice, CA: MOZGO Media; 2003. [Google Scholar]
- Evans JD, Bond GR, Meyer PS, Kim HW, Lysaker PH, Gibson PJ, Tunis S. Cognitive and clinical predictors of success in vocational rehabilitation in schizophrenia. Schizophr Res. 2004;70:331–342. doi: 10.1016/j.schres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fisher M, McKoy K, Poole J, Vinogradov S. Self and Other in Schizophrenia: A Cognitive Neuroscience Perspective. American Journal of Psychiatry. 2008;165:1465–1472. doi: 10.1176/appi.ajp.2008.07111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-Based Cognitive Training in Schizophrenia: An Interim Report on the Effects 6 Months Later. Schizophr Bull. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Williamson ID, Dumontheil I, Simons JS, Frith CD, Burgess PW. Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Soc Cogn Affect Neurosci. 2007;2:217–226. doi: 10.1093/scan/nsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Cannon TD, Gur RE, Ragland JD, Gur RC. Working memory constrains abstraction in schizophrenia. Biol Psychiatry. 2000;47:34–42. doi: 10.1016/s0006-3223(99)00187-0. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiat. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Kern RS, Baade LE, Fenton WS, Gold JM, Keefe RS, Mesholam-Gately R, Seidman LJ, Stover E, et al. Functional co-primary measures for clinical trials in schizophrenia: results from the MATRICS Psychometric and Standardization Study. Am J Psychiatry. 2008;165:221–228. doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- Haut KM, Lim KO, Macdonald A., 3rd Prefrontal Cortical Changes Following Cognitive Training in Patients with Chronic Schizophrenia: Effects of Practice, Generalization, and Specificity. Neuropsychopharmacology. 2010;35:1850–1859. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein AS, Padon AA, Gillihan SJ, Farah MJ, Fellows LK. Ventromedial frontal lobe plays a critical role in facial emotion recognition. J Cogn Neurosci. 2008;20:721–733. doi: 10.1162/jocn.2008.20049. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, Gabrieli JD, Moran JM. An Anterior-to-Posterior Shift in Midline Cortical Activity in Schizophrenia During Self-Reflection. Biol Psychiatry. 2011;69:415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry. 2011;70:1169–1178. doi: 10.1016/j.biopsych.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Recanzone G. Neocortical representational dynamics in adult primates: implications for neuropsychology. Neuropsychologia. 1990;28:573–584. doi: 10.1016/0028-3932(90)90035-m. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychol Bull. 1993;114:3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proc Natl Acad Sci U S A. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (Panss) for Schizophrenia. Schizophrenia Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Arnold MC, Bayen UJ, Harvey PD. Source monitoring deficits in patients with schizophrenia; a multinomial modelling analysis. Psychological Medicine. 1999;29:903–914. doi: 10.1017/s0033291799008673. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Lee KH, Brown WH, Egleston PN, Green RD, Farrow TF, Hunter MD, Parks RW, Wilkinson ID, Spence SA, Woodruff PW. A functional magnetic resonance imaging study of social cognition in schizophrenia during an acute episode and after recovery. Am J Psychiatry. 2006;163:1926–1933. doi: 10.1176/ajp.2006.163.11.1926. [DOI] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- Marder SR, Fenton W. Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res. 2004;72:5–9. doi: 10.1016/j.schres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Mattavelli G, Cattaneo Z, Papagno C. Transcranial magnetic stimulation of medial prefrontal cortex modulates face expressions processing in a priming task. Neuropsychologia. 2011;49:992–998. doi: 10.1016/j.neuropsychologia.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Recanzone GH, Jenkins WM, Grajski KA. Adaptive mechanisms in cortical networks underlying cortical contributions to learning and nondeclarative memory. Cold Spring Harb Symp Quant Biol. 1990;55:873–887. doi: 10.1101/sqb.1990.055.01.082. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Morrison AP, Haddock G. Cognitive factors in source monitoring and auditory hallucinations. Psychol Med. 1997;27:669–679. doi: 10.1017/s003329179700487x. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D'Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Howard RJ, Ballard CG. Putting brain training to the test. Nature. 2010;465:775–778. doi: 10.1038/nature09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Park HJ, Chun JW, Kim EY, Kim JJ. Dysfunctional modulation of emotional interference in the medial prefrontal cortex in patients with schizophrenia. Neurosci Lett. 2008;440:119–124. doi: 10.1016/j.neulet.2008.05.094. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Martindale B, Orbach G, Morgan C. Psychological treatments in schizophrenia: II. Meta-analyses of randomized controlled trials of social skills training and cognitive remediation. Psychol Med. 2002;32:783–791. doi: 10.1017/s0033291702005640. [DOI] [PubMed] [Google Scholar]
- Ray RD, Shelton AL, Hollon NG, Matsumoto D, Frankel CB, Gross JJ, Gabrieli JD. Interdependent self-construal and neural representations of self and mother. Soc Cogn Affect Neurosci. 2010;5:318–323. doi: 10.1093/scan/nsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Sacks SA, Fisher M, Alexander P, Holland C, Rose D, Genevsky A, Garrett C, Hooker C, Vinogradov S. A pilot study of computer-based neurocognitive and social-emotional training in schizophrenia. Clinical Schizophrenia and Related Psychoses. 2012 doi: 10.3371/CSRP.SAFI.012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver H, Goodman C, Bilker WB, Knoll G, Gur R, Povar G. Suboptimal processing strategy and working-memory impairments predict abstraction deficit in schizophrenia. J Clin Exp Neuropsychol. 2007;29:823–830. doi: 10.1080/13803390601125963. [DOI] [PubMed] [Google Scholar]
- Smith T, Weston C, Lieberman J. Schizophrenia (maintenance treatment) Am Fam Physician. 2010;82:338–339. [PubMed] [Google Scholar]
- Stern RA, White T. The Neuropsychological Assessment Battery (NAB): development and psychometric properties. Arch Clin Neuropsych. 2003;18:805–805. [Google Scholar]
- Vinogradov S, Willis-Shore J, Poole JH, Marten E, Ober BA, Shenaut GK. Clinical and neurocognitive aspects of source monitoring errors in schizophrenia. Am J Psychiatry. 1997;154:1530–1537. doi: 10.1176/ajp.154.11.1530. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Simpson GV, Schulman BJ, Glenn S, Wong AE. Brain activation patterns during memory of cognitive agency. Neuroimage. 2006;31:896–905. doi: 10.1016/j.neuroimage.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Schulman BJ, Simpson GV. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cereb Cortex. 2008;18:2532–2539. doi: 10.1093/cercor/bhn028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive Training for Impaired Neural Systems in Neuropsychiatric Illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.