Abstract
This paper reviews the current situation concerning nutrition-related noncommunicable diseases (N-NCDs) and the risk factors associated with these diseases in the Eastern Mediterranean region (EMR). A systematic literature review of studies and reports published between January 1, 1990 and September 15, 2011 was conducted using the PubMed and Google Scholar databases. Cardiovascular disease, type 2 diabetes, metabolic syndrome, obesity, cancer, and osteoporosis have become the main causes of morbidity and mortality, especially with progressive aging of the population. The estimated mortality rate due to cardiovascular disease and diabetes ranged from 179.8 to 765.2 per 100,000 population, with the highest rates in poor countries. The prevalence of metabolic syndrome was very high, ranging from 19% to 45%. The prevalence of overweight and obesity (body mass index ≥25 kg/m2) has reached an alarming level in most countries of the region, ranging from 25% to 82%, with a higher prevalence among women. The estimated mortality rate for cancer ranged from 61.9 to 151 per 100,000 population. Osteoporosis has become a critical problem, particularly among women. Several risk factors may be contributing to the high prevalence of N-NCDs in EMR, including nutrition transition, low intake of fruit and vegetables, demographic transition, urbanization, physical inactivity, hypertension, tobacco smoking, stunting of growth of preschool children, and lack of nutrition and health awareness. Intervention programs to prevent and control N-NCDs are urgently needed, with special focus on promotion of healthy eating and physical activity.
Keywords: nutrition transition, noncommunicable diseases, metabolic syndrome, obesity, physical activity, Eastern Mediterranean
Introduction
Nutrition-related noncommunicable diseases (N-NCDs) are the most frequent cause of morbidity and mortality in most countries in the Eastern Mediterranean region (EMR), particularly cardiovascular disease, diabetes, and cancer.1 The main risk factors for these noncommunicable diseases include high blood pressure, high concentrations of serum cholesterol, tobacco smoking, unhealthy eating habits, overweight or obesity, and physical inactivity. Demographic and socioeconomic status is another factor linked with N-NCDs in this region.
The EMR countries are also characterized by large numbers of young people. In fact, the majority of the Arab population (54%) is under the age of 25 years.2 Therefore, future projection of N-NCD prevalence rates might reach epidemic levels, with serious consequences for morbidity and mortality.1 N-NCDs can develop even in the younger age groups, because childhood and adolescent obesity elevates the risk of these diseases. Several studies have indicated a high prevalence of overweight and obesity among children and adolescents in the EMR,3–8 as well as prevalence of other risk factors, such as high blood pressure,9–11 physical inactivity,12–15 unhealthy eating,16–18 and hyperlipidemia.19,20 This review highlights the magnitude of N-NCDs in the EMR and discusses possible factors associated with the prevalence of these diseases.
Methods
The search for this review was based on articles and reports on N-NCDs published between January 1, 1990, and September 15, 2011, using the PubMed and Google Scholar databases, in addition to reports published by regional and international organizations. The search covered the EMR countries, including all Arab countries (except Algeria), Afghanistan, Iran, and Pakistan, according to World Health Organization categorization.
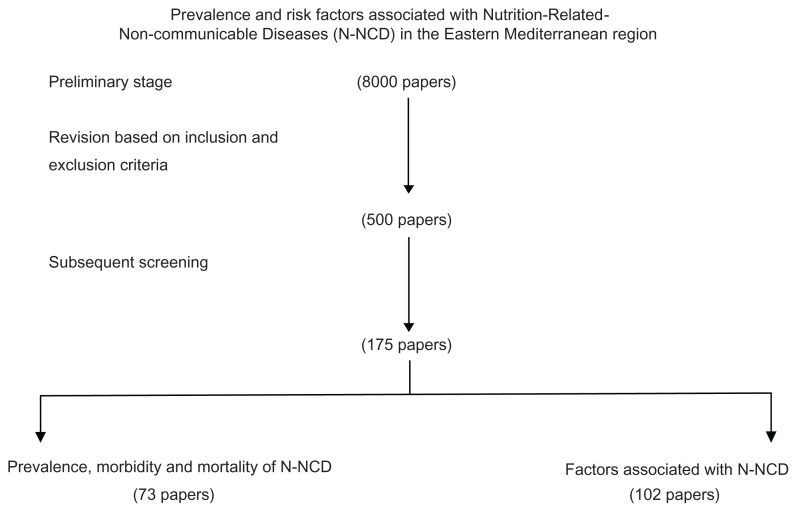
The following key words were used when searching the electronic databases: cardiovascular disease, diabetes, cancer, hypertension, obesity, osteoporosis, dyslipidemia, food consumption, diet, nutrition, physical activity, and smoking, for each country in the region. The titles and abstracts of all articles of potential interest were reviewed for possible inclusion/exclusion in this review. More than 8000 papers were identified at the preliminary stage. The abstracts were then reviewed carefully for final inclusion. The inclusion and exclusion criteria were based on the following:
Only national data on the morbidity and mortality of N-NCDs were included. Therefore, the recent national data provided by the World Health Organization for cardiovascular disease, diabetes, and cancer were considered carefully because these are age-standardized, which make comparison between countries more reliable. For the prevalence of obesity among the various age groups, the search was done on national prevalence published in the literature. When more than one set of national data were available, the most recent one was included.
Morbidity and mortality data from town, rural, urban, and specific ethnic groups, as well as hospital-based data in certain areas, were excluded (most exclusions were in this category)
Studies of an animal or purely biological nature were excluded, because this is an epidemiological review.
For the factors associated with N-NCDs, 1–5 examples were chosen for each disease, as available. The selection of these examples was based on variations between countries, sample size, and year of publication. Consequently, a large number of papers were excluded, especially from countries that had numerous publications on N-NCDs, such as Iran and Saudi Arabia.
Based on the above criteria, we identified 500 papers and reports, and with subsequent screening 175 papers were included in this review (Figure 1).
Figure 1.
Flow chart for processing of the review.
Abbreviation: N-NCD, nutrition-related noncommunicable diseases.
Nutrition-related noncommunicable diseases in EMR
Cardiovascular disease
The EMR is clearly witnessing an increase in cardiovascular disease that is being escalated by the rapidly aging population. 2 In a systematic review on stroke in Arab countries, Benamer and Grosset21 found that the annual stroke incidence ranged from 27.5 to 63 per 100,000 population. Ischemic stroke was the commonest subtype in all series. Hypertension, diabetes mellitus, hyperlipidemia, and cardiac disease were the most common risk factors. Another systemic review in Iran, covering publication dates from 1990 to 2008, reported that the annual stroke incidence of various ages in Iran ranged from 23 to 103 per 100,000 population.22
The estimated mortality from cardiovascular disease and diabetes in the EMR reported by the World Health Organization indicated that poor countries such as Djibouti, Somalia, Sudan, and Yemen have one of the highest mortality rates in the region (ranging from 525.6 to 570.7 for men and from 445.7 to 573.4 for women per 100,000 population). Excluding Jordan and Oman (both of which have a high rate), the mortality rate for men ranged from 179.8 in Qatar to 477.3 in Egypt per 100,000 population. For women, the mortality rate ranged from 239.3 in Qatar to 384 in Egypt per 100,000 population. The high mortality rates for cardiovascular disease and diabetes in poor countries may be due to the health systems in these countries, where treatment of chronic diseases is inadequate due to lack of specialized health personnel, inadequate facilities, lack of intensive care, lack of medicines, and lack of awareness among patients.
The prevalence of hypercholesterolemia is considerably high in this region, which may contribute to the high risk of cardiovascular disease. It was estimated that elevated total cholesterol levels ranged from 31% to 58% among adults in the region.23 Data in Table 1 indicate that increased total cholesterol was more prevalent among female adults in eight countries of the 10 EMR countries included in the World Health Organization database.
Table 1.
Prevalence (%) of raised blood pressure, raised blood glucose and raised total cholesterol (aged-standardized adjusted estimates) among adults in the Eastern Mediterranean countries
| Country | Raised Blood Pressurea | Raised blood glucoseb | Raised total cholesterolc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Male | Female | Both | Yeard | Male | Female | Both | Yeard | Male | Female | Both | Yeard | |
| Bahrain | 44.3 | 42.5 | 43.7 | 1996 | 13.5 | 12.1 | 13.0 | 1996 | – | – | – | – |
| Egypt | 38.8 | 37.4 | 38.1 | 2002 | 07.0 | 07.4 | 07.2 | – | 33.9 | 45.3 | 39.9 | – |
| Iran | 41.4 | 37.3 | 39.4 | 2007 | 09.3 | 10.5 | 09.9 | 2007 | 49.8 | 58.1 | 54.1 | 2007 |
| Iraq | – | – | – | – | 12.7 | 12.5 | 12.6 | 2006 | 43.7 | 44.1 | 44.0 | 2007 |
| Jordan | 38.0 | 32.0 | 35.1 | 2007 | 17.2 | 18.1 | 17.7 | 2007 | 47.8 | 49.0 | 48.8 | 2007 |
| Kuwait | 40.3 | 34.7 | 38.4 | 2006 | 17.0 | 14.8 | 16.2 | 2006 | 56.2 | 55.7 | 56.2 | 2006 |
| Lebanon | 44.8 | 36.8 | 40.4 | 2008 | 13.0 | 11.0 | 11.9 | – | – | – | – | – |
| Libya | 51.7 | 42.4 | 49.6 | 2008 | 14.5 | 14.4 | 14.4 | 2008 | 34.6 | 31.6 | 35.6 | 2009 |
| Morocco | 43.9 | 46.0 | 45.0 | 2000 | 10.6 | 10.9 | 10.8 | 2000 | 35.3 | 39.0 | 37.2 | 2000 |
| Oman | 43.2 | 38.6 | 41.4 | 2000 | 12.0 | 12.3 | 12.2 | 2000 | – | – | – | – |
| Pakistan | 40.1 | 38.8 | 39.5 | 1992 | 11.7 | 14.1 | 12.9 | 1996 | 30.5 | 31.4 | 31.0 | – |
| Qatar | 44.4 | 33.1 | 42.7 | 2006 | 12.4 | 11.0 | 12.0 | 2008 | – | – | – | – |
| Southern Arabia | 43.1 | 38.9 | 41.4 | 2005 | 22.0 | 21.7 | 21.8 | 20051 | 36.4 | 42.1 | 39.0 | 2005 |
| Tunisia | 42.6 | 41.2 | 42.0 | – | 12.0 | 12.7 | 12.4 | 997 | 37.3 | 43.8 | 40.7 | – |
| UAE | 41.3 | 32.5 | 38.9 | 2000 | 15.3 | 15.8 | 15.5 | 2000 | – | – | – | – |
Notes:
Prevalence of raised blood pressure among adults, defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or on medication for raised blood pressure (%);
prevalence of raised blood glucose among adults, defined as for fasting plasma glucose value ≥7.0 mmol/L (126 mg/dL) or on medication for raised blood glucose (%);
prevalence of raised total cholesterol among adults, defined as total cholesterol ≥5.0 mmol/L or 190 mg/dL (%);
countries with no latest year of data, and the estimate of diseases in these countries was based on a combination of country life tables, causes of death models, regional cause of death patterns, and World Health Organization program estimates for major causes of death.23
Abbreviation: UAE, United Arab Emirates.
Hypercholesterolemia was also reported among children and adolescents in the region, with a high prevalence of obesity and inactivity in these age groups,19,20 so the risk of cardiovascular disease in young and middle-aged adults is expected to rise in the future. A study of 1–6-year-old children in Saudi Arabia revealed an overall range for serum cholesterol of 201–507 mmol/L. A total of 6.9% were at borderline risk and 1.6% were at high risk.24 In Tunisia, the prevalence of hypercholesterolemia in 13–19-year-old adolescents was 8.1%.25 A national survey on risk factors for N-NCD in Egyptian adolescents aged 10–18 years reported 10% with borderline and 6% with high levels (≥200 mg/dL) of total cholesterol. This survey also revealed a low high-density lipoprotein cholesterol levels in 9.4% of adolescents, while 7.5% and 10.3% had high and borderline levels, respectively.26 In Kuwait, the prevalence of dyslipidemia was found to be 10.5% in college-aged students.27
Type 2 diabetes
Most of the studies on type 2 diabetes in the EMR are hospital-based, with a focus on clinical factors linked with diabetes, and few of the published studies represent nationally-based diabetic prevalence surveys utilizing World Health Organization criteria. These studies showed that the prevalence of diabetes among adults ranged from 10% to 15%.28 In general, the prevalence of type 2 diabetes in this region has been increasing dramatically during the past two decades. For example, the prevalence of diabetes in Tunisia has doubled over the past 15 years.29 In Jordan, the prevalence has increased by 31.5% from 1994 to 2006.30 Of the 91 countries in which diabetes prevalence has been studied, five of the ten countries with the world’s highest reported rates have been in the EMR, namely the United Arab Emirates, Saudi Arabia, Bahrain, Kuwait, and Oman. It is no coincidence that all of these countries are experiencing nutrition transition. The prevalence of diabetes in these countries is projected to continue to rise during the 2010–2030 period, if the same trend continues.31 Many EMR countries have been reporting the onset of type 2 diabetes in increasingly younger age groups. Such a pattern of diabetes onset extends the potential burden of therapy to an even younger age group for an even longer period of time. It was estimated that, of the total adult population of 220 million in the region, 17 million people have diabetes, with further dramatic increases expected in the future.21
Age-standardized adjusted estimates for raised blood glucose in the EMR countries showed the highest prevalence among Saudi men and women (20 years and older) at 22% and 21.7%, respectively. The prevalence in Egypt was 7% and in Jordan reached 18%. In general, the prevalence rates in men and women were very close (Table 1). However, the proportion of underdiagnosed diabetes and prediabetes is considerably high, which means that the burden of diabetes on health care systems in this region is substantially large.32 In Iran, for example, 47% of diabetes cases can be attributed to newly diagnosed disease.33
Cancer
Cancer is an increasing problem in the EMR and ranks as the fourth leading cause of death. It is estimated that cancer kills 270,000 people each year in the EMR. Moreover, the largest increase in cancer incidence among the World Health Organization regions in the next 15 years is likely to be in EMR, in which projection modeling predicts an increase between 100% and 180%.34 Half of the cancer cases in the region occur before the age of 55 years, which is 10–20 years younger than in industrialized countries.34 The mortality rate is 70%, which is higher than in the US (40%) and Europe (55%), indicating significantly lower survival rates after diagnosed cancer.32 Estimated data for mortality due to cancer was found to be the highest in Lebanon (151.2 and 113.2 per 100,000 population for men and women, respectively), whereas the lowest mortality rate for men was observed in United Arab Emirates (63.4) and that for women was in Syria (47.2).23
Breast cancer occupies the number one prevalence position in most countries of the region. El Salim et al35 reported that cases of breast cancer in Arab countries tend to be young and almost half of the patients are younger than 50 years of age, with a median age of 49–52 years as compared with 63 years in industrialized nations. In a review of cancer registrations in the Arab Gulf countries, breast cancer was the most common cancer among women, ranging from 16.1% in Oman to 35.4% in Bahrain. The age-standardized incidence rate (per 100,000 population) was the highest in Bahrain (46.4), followed by Kuwait (44.3), Qatar (35.5), United Arab Emirates (19.2), Oman (14.4), and Saudi Arabia (12.9). However, these rates are still low compared with most developed countries. 36 Although the incidence of lung cancer is lower than in Western countries, it is ranked number one in two countries (Bahrain and Tunisia) and number two in four countries (Iraq, Jordan, Kuwait, and Lebanon) in the region. Colon cancer is among the top five cancers in most EMR countries.34 A low intake of fruit and vegetables and low physical activity could be the leading factors associated with this type of cancer. Bener37 suggested that the main risk factors that were positively associated with colon cancer in developing countries are physical inactivity, obesity, abdominal adiposity, alcohol consumption, smoking, and high consumption of red meat and fat. Protective factors included consumption of white meat and fiber-rich foods.
Esophageal cancer is also a matter of concern, given that it is among the top five cancers in seven countries. It is the second most common type of cancer in Iran and Yemen. It is believed that the habit of chewing tobacco in Iran and Khat in Yemen may play a role in the high incidence of esophageal cancer in these two countries.34,35 Although there is a lack of studies on the prevalence of cancer among children, it is increasing in the region. In the Arab Gulf countries, it was reported that the rate of pediatric cancer ranged from 4% in Bahrain to 9.5% in Saudi Arabia and the United Arab Emirates.38
Osteoporosis
Osteoporosis is perceived to be rapidly increasing in the EMR, due to the progressive aging of the population. Osteoporosis is currently known as a systemic skeletal disorder that affects bone density and quality, leading to bone fragility and increased risk of fractures. It is defined as a T-score less than −2.5. The T-score is the number of standard deviations separating an individual’s bone mineral density from the peak bone mineral density of young adults. Bone mineral density is considered normal if it is above −1.0. Bone mineral density appears to be lower on average in the EMR compared with industrialized countries, with the T-score being lower by 0.3–0.6.39
Studies conducted in Iran,40 Lebanon,41 Qatar,42 Saudi Arabia,43,44 Tunisia,45 and Morocco46 showed that vitamin D levels and bone mineral density were below the standard established in Western countries. The exception to this is Kuwait,47 where the bone mineral density reference level was similar to that in Western countries. Reduced vitamin D and calcium intake by people in the region may be contributing substantially to the high rates of osteoporosis. In Lebanon, for example, it was found that the average daily vitamin D intake by adults 30–50 years was 100.3 ± 67.9 IU, which is much lower than the recommended daily allowance of 200 IU. This intake was significantly higher (P < 0.001) in men than in women (127.5 ± 79.7 IU versus 87.9 ± 57.9 IU). Dietary calcium intake (average 683 ± 275 mg/day) was also lower than the recommended daily intake of 1000 mg, and the difference between men (778 ± 327 mg/day) and women (237 ± 88 mg/day) was statistically significant (P < 0.001).48 In Saudi Arabia, it was found that 81% of school children aged 12–15 years had low serum vitamin D levels, ranging from 2.2 to 24.0 mmol/dL.49
The main risk factors for osteoporosis in the EMR based on the published literature are low vitamin D and calcium intake, inadequate exposure to sunlight, heavy smoking (>20 cigarettes per day), a family history of osteoporosis, being female and postmenopausal for more than two years, low physical activity, thinness, and small body build.50
Overweight and obesity
The prevalence of overweight and obesity among both children and adults in the EMR has increased markedly during the past three decades. In Saudi Arabia, for example, Al-Hazzaa51 showed that the prevalence of obesity among schoolboys aged 6–14 years increased seven-fold between 1988 and 2005 (from 3.4% to 24.5%, respectively). The average body fat percentage increased by 49.2% for the same period. In general, all anthropometric measurements increased during this period except for lean body mass. In Iran, the overall prevalence of obesity among adults increased from 13.6% in 1999 to 22.3% in 2007 (odds ratio [OR] 1.08 per year, P < 0.001). Overweight prevalence increased during the same period from 32.2% to 36.3% (OR 1.02 per year, P < 0.001).52
A case-control study in 52 countries showed that adult men in the Middle East have the second highest mean body mass index (27.4) after adults in the US (27.7). Mean body mass index in the rest of the regions ranged from 24.0 in Southeast Asia to 27.0 in Australia and New Zealand. In women, the highest body mass index and waist-to-hip ratio were recorded in the Middle East (body mass index 29.5, and waist-to-hip ratio 0.92).53
Data presented in Table 2 and Table 3 indicate that the prevalence of overweight and obesity among adults is considerably high, with the proportion of overweight in men ranging from 19.2% in Libya to 51.7% in Tunisia, while the corresponding proportions in women were 21.1% in Libya and 71% in Tunisia. The prevalence of obesity ranged from 5.7% in Morocco to 39% in Kuwait for men, and from 7.1% in Libya to 53% in Kuwait for women. The prevalence of overweight in EMR among preschool children (0–5 years) ranged from 1.9% in Oman to 21.9% in Syria. Among schoolchildren and adolescents (6–18 years), the prevalence of overweight in males ranged from 5.4% in Iran to 29.3% in Kuwait. Similarly, in females, it ranged from 5.9% in Iran to 32% in Kuwait. With regard to obesity, the prevalence in males ranged from 1.6% in Iran to 24.8% in Kuwait, and a similar pattern was seen in females, with the proportion ranging from 1.3% in Iran to 20% in Kuwait, as shown in Table 2 and Table 3. Unfortunately, the complications of childhood obesity are rarely considered by health authorities in the region, given that they are not clinically apparent until many years after onset of obesity. Clinical studies of obese children indicate development of a vast range of medical consequences, including sleep apnea, asthma, fatty liver disease, menstrual problems and early menarche, type 2 diabetes, hypertension, increased low-density lipoprotein cholesterol, raised serum triglyceride levels, and psychological and social consequences.54
Table 2.
National prevalence of overweight and obesity among male preschool children, schoolchildren, adolescents, and adults in Eastern Mediterranean region
| Country | Preschool children (0–5 years) | Schoolchildren and adolescents | Adults (15 years +) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Year of data | % Overweight | Reference | Year of data | Age range (year) | % Overweight | % Obesity | Reference | Year of data | % Overweight | % Obesity | Reference | |
| Afghanistan* | 1997 | 4.0 | 158 | – | – | – | – | – | – | – | – | – |
| Bahrain | 2003 | 7.0 | 159 | 2006 | 15–18 | 15.8 | 13.7 | 165 | 2007 | 34.8 | 32.3 | 174 |
| Djibouti | 2002 | 12.3 | 149 | – | – | – | – | – | – | – | – | – |
| Egypt* | 1995–1996 | 8.6 | 158 | 2004 | 10–18 | 11.5 | 6.5 | 166 | – | – | – | – |
| Iran | – | – | – | 2003–2004 | 6–18 | 5.4 | 1.6 | 167 | 2004–2005 | 42.8 | 11.1 | 175 |
| Jordan | 1990 | 5.7 | 158 | – | – | – | – | – | – | – | – | – |
| Kuwait | 2005 | 5.2 | 160 | 2006 | 10–14 | 29.3 | 14.9 | 168 | 2007 | 38.9 | 39.2 | 176 |
| Lebanon | – | – | – | 1995–1996 | 10–19 | 26.9 | 7.7 | 169 | 1995–1996 | 43.4 | 14.3 | 169 |
| Libya | 2003 | 13.9 | 149 | – | – | – | – | – | 2000 | 19.2 | 5.8 | 177 |
| Morocco | 2003–2004 | 17.8 | 149 | – | – | – | – | – | 1998–1999 | 28.0 | 5.7 | 178 |
| Oman | 1999 | 1.9 | 161 | – | – | – | – | – | 2000 | 30.6 | 15.5 | 179 |
| Pakistan | 1990–1991 | 3.1 | 158 | – | – | – | – | – | – | – | – | – |
| Qatar | 2001 | 8.9 | 162 | 2003–2004 | 10–18 | 27.5 | 7.1 | 170 | – | – | – | – |
| Saudi Arabia | – | – | – | 2005 | 5–12 | 19.9 | 7.8 | 171 | 2005 | 43.0 | 31.5 | 180 |
| Sudan | 2000–2001 | 3.0 | 163 | – | – | – | – | – | – | – | – | – |
| Syria | 2001 | 21.9 | 149 | – | – | – | – | – | – | – | – | – |
| Tunisia | 2006 | 5.6 | 164 | 2005 | 15–19 | 17.4 | 4.1 | 172 | 2005 | 51.7 | 37.0 | 181 |
| UAE | – | – | – | 2005 | 10–19 | 21.2 | 13.2 | 173 | – | – | – | – |
| Yemen | 2003 | 9.3 | 149 | – | – | – | – | – | – | – | – | – |
Note:
Male/female.
Abbreviation: UAE, United Arab Emirates.
Table 3.
National prevalence of overweight and obesity among female preschool children, schoolchildren, adolescents, and adults in Eastern Mediterranean region
| Country | Preschool children (0–5 years) | Schoolchildren and adolescents | Adults (15 years +) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Year of data | % Overweight | Reference | Year of data | Age range (years) | % Overweight | % Obesity | Reference | Year of data | % Overweight | % Obesity | Reference | |
| Afghanistan | 1997 | 4.0 | 158 | – | – | – | – | – | – | – | – | – |
| Bahrain | 2003 | 7.5 | 159 | 2006 | 15–18 | 17.4 | 19.4 | 165 | 2007 | 31.1 | 40.3 | 174 |
| Djibouti | 2002 | 7.9 | 149 | – | – | – | – | – | – | – | – | – |
| Egypt | 1995–1996 | 8.6 | 158 | 2003–2004 | 10–18 | 15.2 | 7.7 | 166 | – | – | – | – |
| Iran | – | – | – | 2003–2004 | 6–18 | 5.9 | 1.3 | 167 | 2004–2005 | 57.0 | 25.2 | 175 |
| Jordan | 1990 | 5.7 | 158 | – | – | – | – | – | – | – | – | – |
| Kuwait | 2005 | 6.8 | 160 | 2006 | 10–14 | 32.1 | 14.2 | 168 | 2007 | 28.9 | 53.0 | 176 |
| Lebanon | – | – | – | 1995–1996 | 10–19 | 14.7 | 2.9 | 169 | 1995–1996 | 30.6 | 15.5 | 169 |
| Libya | 2003 | 12.9 | 149 | – | – | – | – | – | 2000 | 21.1 | 7.1 | 177 |
| Morocco | 2003–2004 | 13.2 | 149 | – | – | – | – | – | 1998–1999 | 33.0 | 18.3 | 178 |
| Oman | 1999 | 01.9 | 161 | – | – | – | – | – | 2000 | 27.2 | 22.3 | 179 |
| Palestine | – | – | – | – | – | – | – | – | 2002 | – | 10.9 | 182 |
| Pakistan | 1990–1991 | 03.1 | 158 | – | – | – | – | – | – | – | – | – |
| Qatar | 2001 | 09.4 | 162 | 2003–2004 | 10–18 | 20.0 | 3.9 | 170 | – | – | – | – |
| Saudi Arabia | – | – | – | 2005 | 5–12 | 19.2 | 11.0 | 171 | 2005 | 28.8 | 50.4 | 180 |
| Sudan | 2000–2001 | 03.9 | 163 | – | – | – | – | – | – | – | – | – |
| Syria | 2001 | 18.4 | 149 | – | – | – | – | – | – | – | – | – |
| Tunisia | 2006 | 07.2 | 164 | 2005 | 15–19 | 20.4 | 4.4 | 172 | 2005 | 71.3 | 13.3 | 181 |
| UAE | – | – | – | 2005 | 15–19 | 21.7 | 11.0 | 173 | – | – | – | – |
| Yemen | 2003 | 8.3 | 149 | – | – | – | – | – | – | – | – | – |
Abbreviation: UAE, United Arab Emirates.
Metabolic syndrome
The metabolic syndrome is a complex disorder and composed of several metabolic abnormalities, each of which is considered to be a risk factor for cardiovascular disease.53 With the large increase in obesity rates among both adolescents and adults, the metabolic syndrome is also increasing. A high prevalence of metabolic syndrome has been reported in most EMR countries, ranging from 19% to 45%.55–63 For example, the prevalence of metabolic syndrome in the Arab Gulf countries was some 10%–15% higher than in most developing countries, with a higher prevalence among women. The proportion of metabolic syndrome in the Arab Gulf countries ranged from 20.7% to 37.2% using the third Adult Treatment Panel (ATP III) definition, and from 29.6% to 36.2% using the International Diabetes Federation definition.64
There has been a limited number of studies related to pediatric metabolic syndrome in EMR countries. Two studies were carried out in Iran using modified ATP III criteria for metabolic syndrome, and indicated a high prevalence of metabolic syndrome. Esmaillzadeh et al65 found that the prevalence of metabolic syndrome among Iranian adolescents aged 10–19 years was 10.1% (95% confidence interval [CI] 9.0–11.1), whereas Kelishadi et al66 reported a prevalence of 14.1% among Iranian adolescents aged 6–18 years. In Saudi Arabia, Al-Daghri67 found that the overall prevalence of metabolic syndrome among children aged 10–18 years was 9.4% (95% CI 7.8–11.0). A combination of interacting genetic, environmental, and behavioral factors may be responsible for the occurrence of metabolic syndrome in both children and adults.68 Research on the association between dietary habits in children and metabolic syndrome in the EMR is mostly absent. One study has reported a correlation between metabolic syndrome and dietary intake in children and adolescents from this region. It was found that the risk of metabolic syndrome among Iranian children and adolescents (aged 14–19 years) rose with the consumption of solid hydrogenated fat and white bread. The frequent intake of sweets/candies increased the risk of metabolic syndrome in both genders. The frequency of eating fast foods increased this risk in boys but not in girls, and vice versa for carbohydrate-rich foods. In both genders, the intake of fruit, vegetables, and dairy products decreased the risk of metabolic syndrome.69
Factors associated with N-NCDs in EMR
Nutrition transition
Nutrition transition, documented by several studies in the EMR,70–74 is characterized by considerable changes in food intake and dietary behavior in the population. The shift from a traditional diet to a more westernized diet, with a high energy and fat-rich food intake, eating out, and an increase in food portion sizes are apparent in most countries in the EMR. These changes may increase the risk for obesity and other metabolic disorders. Data calculated from the Food Balance Sheet75 showed that there has been a decrease in the consumption of red meat by 4.3% and an increase in the consumption of poultry meat by 51.9% in the EMR during the period 1990–2005. However, the daily per capita availability of fruit and vegetables in the region has declined by 7.8% and 0.3%, respectively. It is worth mentioning that there was a great variation between countries in respect to changes in patterns of food consumption.
In order to test the relationship between change in dietary intake and obesity, Esmaillzadeh and Azadbakht76 examined the association of obesity with three dietary patterns (healthy, Western, and Iranian) in 486 Iranian women aged 40–60 years. They found that women in the upper category of the healthy pattern scores were less likely to be obese, based on body mass index (OR 0.28, 95% CI 0.14–0.53) or be centrally obese, based on waist circumference (OR 0.30, 95% CI 0.16–0.55). Women in the upper quintile of the Western dietary pattern had greater odds for general obesity (OR 2.73, 95% CI 1.46–5.08) and for central obesity (OR 5.74, 95% CI 2.99–10.99). The association between dietary patterns and general obesity in Iran was not statistically significant. However, subjects in the highest quintile of Iranian dietary patterns had greater odds of being centrally obese either before (OR 2.15, 95% CI 1.18–3.90) or after (OR 2.08, 95% CI 1.09–3.65) control of potential confounders.
High income and urbanization are the main drivers of nutrition transition and emergence of N-NCDs in some countries in the EMR. With the rapid improvement in the economy, people are becoming affluent and are consuming diets high in saturated fat, cholesterol, salt, and refined carbohydrates, and low in polyunsaturated fats and fiber, along with a marked sedentary lifestyle and increased stress.77 High fat consumption has been reported by several studies in this region,78–80 and saturated fats represent a high proportion of the daily energy intake. In Egypt, for instance, Mahmood81 found that total fat was contributing 37% of total energy intake in women.
Unhealthy eating habits and high fat intake have also been reported among school children and adolescents in the region. Unhealthy eating habits among school children aged 6–18 years were studied nationwide in Iran.82 It was found that hydrogenated solid fat was the main type of fat frequently consumed at home (73.8%), about 58% of the children consumed white bread, and 79.3% admitted to adding salt to the food they consumed. The average frequency of deep-fried foods consumed at home was four times per week. Fruit, vegetables, and dairy products were consumed twice a day. Using three categories of a healthy eating index, ie, poor diet, needs improvement, and good diet, it was found that 74%, 23%, and 3%, respectively, of adolescents in Tehran were in these categories. There was a significant negative correlation between healthy eating score and fat intake (r = −0.2, P < 0.001), percentage of saturated fatty acid (r = −0.2, P < 0.05) and cholesterol intake (r = −0.4, P < 0.05), and the ratio of polysaturated/saturated fatty acids in the diet (r = 0.2, P < 0.05).83
Data on the frequency and quantity of food eaten outside the home is another factor which could be associated with obesity.84 The increase in family income and the higher proportion of working women may have contributed substantially to the increase in eating out, the nutritional quality of which is of concern. The increasing frequency of eating out at restaurants and fast food outlets has had a great impact on the dietary habits of people in the region.8 Bashour85 showed that 67.4% of male adolescents (13–18 years) in Syria frequently eat outside the home compared with 54.5% of female adolescents (P < 0.001). Food eaten outside the home was more likely to be high in total energy, total fat, saturated fat, cholesterol, and sodium, but be lower in dietary fiber and calcium. In Iran, Hejazi and Mazloom86 found a significant difference in mean daily energy intake (P < 0.007) between adolescents (12–16 years) who ate at least one meal away from home (1618 kCal) and those who did not eat outside their homes (1472 kCal).
As a result of nutrition transition, the intake of sugar-sweetened beverages has increased and that of whole grain food has decreased. More and more evidence has been reported on the negative health impact of sugar-sweetened beverages, because regular consumption of these beverages not only contributes to obesity but also to an increased risk of type 2 diabetes and metabolic syndrome.87 The consumption of sugar-sweetened beverages in the EMR is relatively high among both children and adults.88–91 In Kuwait, a high proportion of children (11–13 years) reported consuming sweets (42%), soft drinks (43%), and cakes (31%) several times a day. Further, when all the associated variables were analyzed together using the logistic regression model, lifestyle, school satisfaction, and self-esteem factors appeared to have a stronger association with frequent sugar consumption than did gender, grade, or nationality.88 A cross-sectional study of 9433 Saudi Arabian schoolchildren aged 10–19 years demonstrated that waist circumference and body mass index were positively correlated with sugar-sweetened carbonated beverage intake in boys only (P < 0.0001). However, the intake of these beverages was positively associated with poor dietary choices in both males and females. Correlations between sugar-sweetened drinks and fast foods, savory snacks, iced desserts, and total sugar in boys were 0.39, 0.13, 0.10, and 0.52, respectively (P < 0.001), while the corresponding relationships in girls were 0.45, 0.23, 0.16, and 0.55, respectively (P < 0.001).89
Whole grain cereal intake is associated with many health benefits, including lower body mass index, and lower incidences of type 2 diabetes, cardiovascular disease, and colorectal cancer.92 In general, the contribution of complex carbohydrates (a rich source of dietary fiber and other nutrients) decreased in the 1971–2005 period, and the percentage of this contribution decreased as the per capita income of a country rose. For example, the contribution of complex carbohydrates to daily energy supply was 60%–70% in low-income countries but decreased to 50%–60% and 45%–50% in middle-income and high-income countries, respectively, in the EMR.75 Mean consumption of whole grains and refined grains by Iranian adults (18–74 years) was 93 ± 29 g/day and 201 ± 57 g/day, respectively. Whole grain intake was inversely associated and refined grain was positively associated with the risk of hypertriglyceridemia. Moreover, the proportion of obesity was lower among subjects in the highest quintile of whole grain intake and higher among those in the highest quintile of refined grain intake compared with the corresponding lower quintiles.93
Low intake of fruit and vegetables
Low intake of fruit and vegetables was reported in children and adolescents,94,95 as well as in adults 96,97 in many countries in the EMR. Lock et al98 calculated fruit and vegetable intake worldwide, and showed that this was inadequate in EMR countries. In general, the intake was lower than the recommended daily allowance (>400 g) in all age groups and in both genders, except in men aged 45 years and over in poor countries, in addition to Egypt, Iraq, and Morocco. The intake of fruit and vegetables increased with age. For example, the daily consumption of fruit and vegetables ranged from 174 g to 218 g among children aged 0–4 years, increased to 296–348 g among those aged 15–29 years, and to 336–442 g among subjects aged 70–79 years.
There is good evidence that an adequate intake of fruit and vegetables is protective against certain chronic diseases, including obesity, cardiovascular disease, and some types of cancer.99,100 Al-Roomi et al101 conducted a case-control study of lifestyle factors associated with acute myocardial infarction in adults aged 20–79 years in Bahrain. After adjusting for several demographic and health variables, the odds of having a first myocardial infarction in subjects with an infrequent intake of fruit (<3 times/week) was 1.57 (95% CI 0.61–2.53) and in those with an infrequent intake of vegetables (<3 times/week) was 1.40, (95% CI 0.77–2.52), with both rates being higher than in control subjects. After adjusting for several potentially confounding factors, the fruit and vegetable intake in Iranian women aged 18–74 years was found to be significantly and inversely associated with cardiovascular disease.102 In a community-based case-control study in Iran, it was found that a high intake of allium vegetables (OR 0.35) and fruit (OR 0.37) were significantly protective against gastric cancer. However, consumption of red meat (OR 0.34) and dairy products (OR 2.28) was positively associated with risk of gastric cancer. People with a higher reported salt intake (OR 3.10) and drinking strong (OR 2.64) and hot (OR 2.85) tea were at higher risk.103
Kelishadi et al104 found that fruit and vegetable intake had a negative association with obesity among adolescents (11–18 years) in Iran. Adolescents of normal weight (body mass index <85th percentile) were more likely to consume fruit (6.2 ± 1.8 times/week) and vegetables (5.9 ± 1.4 times/week) than overweight/obese adolescents (≥85th percentile), whose intakes were 4.2 ± 1.1 times/week and 3.8 ± 1.2 times/week, respectively. The difference was statistically significant (P < 0.04 and P < 0.03, respectively). A study in university students in Kuwait revealed that regular consumption of fruit and vegetables is protective against obesity (OR 0.55, 95% CI 0.20–1.40 and OR 0.55, 95% CI 0.30–2.24, for fruit and vegetables, respectively).
Demographic transition
The decreasing fertility rate in EMR countries is due to many factors. In poor-income and middle-income countries, economic hardship has introduced a different stimulus for fertility decline. The demographic transition appears to be a product of both development and underdevelopment. For rich countries (such as the Arab Gulf states), more recent changes in the fertility rate are prompted by both the high cost of living and a marked improvement in education for women.105
Excluding Oman, poor countries such as Afghanistan, Djibouti, Pakistan, Palestine, Somalia, Sudan, and Yemen showed the highest decline in fertility during the 2000–2005 period. The average fertility rate for the EMR countries is expected to decrease from 4.2 in 2000–2005 to 2.8 per 1000 population in 2025–2030 (a decrease of 33%), with a further decline to 2.2 is projected for 2045–2050 (a decrease of 21%). Life expectancy is expected to increase sharply in 2000–2050, especially in poor countries, and is already high in the high-income and middle-income countries. The average life expectancy in 2000–2050 will increase from 66.7 to 76.6 years, representing a 15% rise. However, the life expectancy will range from 62.4 years in Afghanistan to 81.1 years in Kuwait during 2045–2050. Consequently, the percentage of the aged population (≥60 years) will increase. The average percentage increase for all the EMR countries will be from 5.8% in 2000–2005 to 15% of the total population in 2045–2050, ranging from 5.8% in Djibouti to 25.7% in Kuwait.106
Urbanization
There is a growing shift in the urban-rural population balance in the EMR. According to recent Arab human development report, the region has been characterized by large movements from rural to urban areas. This rapid urbanization is coupled with increased growth of large cities.2 In fact, in the Arab Gulf states, the vast majority of the population is already living in urban areas, while urbanization is growing fast in middle-income and low-income countries in the region.107 Urbanization means a shift from traditional foods to fast and convenience foods, more female participation in the workforce, more reliance on cars and other types of transportation, higher income, more television viewing, more use of computers and the Internet, a more sedentary lifestyle and less physical activity, and ultimately a more stressful life. Therefore, urbanization will intensify the burden of N-NCDs in the region.108
Urbanization and increased income has led to increasing consumption of white bread, sugar, and excess fat, and decreasing consumption of oils, grains, legumes, and vegetables. This has led to higher consumption of saturated fat, increased total energy and carbohydrate intake, and decreased intake of vitamin C and fiber.109 In Egypt, the ratio of saturated fat to total energy was 11.9 ± 5.2 and 7.4 ± 4.6 (P < 0.0001) among urban and rural men, respectively. The corresponding proportions for women were 10.7 ± 4.3 and 8.5 ± 4.6 (P < 0.013), respectively.110 In Iraq, statistically significant differences between urban and rural populations have been identified in preference for sweetness and sugar consumption. The urban groups consumed a mean of 4.1 teaspoons of sugar per cup of tea versus 1.7 teaspoons per cup for rural groups (P < 0.001). The urban group consumed 62.3 g of sugar in tea per day, compared with 16.6 g for the rural groups. The longer the subjects had been living in the city, the greater was their preference for sweetness (r = 0.43, P < 0.001). Daily consumption of sugar in tea increased from 10 g/day for those living in the city for 2 years or less to 182 g/day for those who had lived for more than 15 years in the city (r = 0.68, P < 0.001).111 A comparison of dietary habits in Pakistani schoolchildren aged 10–12 years from six groups of rural and urban areas showed different patterns. With urbanization, the intake of fat and sugar increased steadily, while that of carbohydrates and fiber decreased.112
Inactivity and sedentary lifestyle
There is growing evidence that inactivity and a sedentary lifestyle are associated with increased risk of N-NCDs.113 Changes to labor-saving occupations, a high dependence on cars and buses for transportation, massive urbanization, propagated satellite television, and increased reliance on computers and telecommunication technology have all led to a more sedentary lifestyle and reduced energy expenditure in most EMR countries. Therefore, leisure activities have changed from outdoor play to indoor entertainment.107 Data on trends in occupational activity in this region are scarce. However, mere observation shows that there has been a great shift from manual work to office work, especially in high-income and middle-income countries. In the Arab Gulf states, for example, people have shifted from farming, pearl diving, and fishing to less physically demanding office work such as banking, selling, accounting, management, teaching, and other administrative work. Manual work in these states has been delegated to migrants, especially from the Indian-subcontinent and the Far East.114
In the majority of countries in the region, there is a paucity of reliable data on physical activity, especially for children and adolescents. Surprisingly, national surveillance data on physical activity are lacking, and most available physical activity/inactivity data are included as one item and as part of wider surveillance of health or noncommunicable diseases. Thus, existing physical activity research in the EMR is hampered by limited reliable data, varying methodology, and different physical activity instruments used by different researchers.115 Some national and local studies have surveyed only leisure-time physical activity and sports,116,117 while a few studies have assessed all domains of physical activity, including occupational, transport, household, yard/garden, and leisure/sports.118 Therefore, it is difficult to make any meaningful comparison between findings from these studies, except for the World Health Organization STEPwise approach to noncommunicable disease surveillance, where similar methodology and instrumentation were used.
Using the stepwise approach of the World Health Organization119 to study the risk factors for N-NCDs in eight EMR countries, it was found that the prevalence of low daily physical activity (less than 10 minutes per day) among adults aged 15 years and above was high. Among men, the prevalence was highest in Sudan (75.9%) and lowest in Syria (21.3%), with the comparable percentages in women being 94.8% and 41% in Sudan and Syria, respectively (Table 4).
Table 4.
National prevalence of risk factors for diet-related chronic noncommunicable diseases in selected Eastern Mediterranean countries in persons aged ≥15 years using the STEPwise approach to surveillance
| Country (year) | Gender | Hypertension (BP ≥ 140/90 mmgHg; %) | Hypercholesterolemia cholesterol level (≥5.2 mmol/dl; %) | Smoking (current daily smokers; %) | Low physical daily activity (≤10 minutes; %) | Low intake of fresh fruit and vegetables (≤5 servings/day; %) |
|---|---|---|---|---|---|---|
| Egypt (2005–2006) | M | 26.3 | 15.7 | 34.6 | – | – |
| F | 27.1 | 23.0 | 0.70 | – | – | |
| Total | 26.7 | 19.4 | 18.0 | 70.4 | 79.0 | |
| Iraq (2005) | M | 43.0 | 38.9 | 41.5 | 61.8 | 91.2 |
| F | 38.3 | 36.5 | 06.9 | 52.7 | 91.5 | |
| Total | 40.4 | 37.5 | 21.6 | 56.7 | 91.4 | |
| Iran (2005) | M | 17.1 | 41.0 | 24.0 | 58.8 | – |
| F | 12.5 | 46.0 | 01.9 | 76.3 | – | |
| Total | 14.8 | 43.6 | 13.0 | 67.3 | – | |
| Jordan (2007) | M | 30.9 | 35.6 | 49.6 | 54.0 | 61.9 |
| F | 21.5 | 36.5 | 05.7 | 49.0 | 52.5 | |
| Total | 25.5 | 36.0 | 29.0 | 51.0 | 57.0 | |
| Kuwait (2006) | M | 21.3 | 40.0 | 37.8 | 57.9 | 79.2 |
| F | 19.7 | 37.2 | 03.0 | 71.7 | 82.8 | |
| Total | 20.5 | 38.6 | 20.6 | 64.7 | 81.0 | |
| Saudi Arabia (2007) | M | 24.2 | 18.6 | 20.9 | 60.9 | 91.6 |
| F | 18.5 | 19.7 | 01.2 | 74.3 | 95.3 | |
| Total | 21.3 | 19.2 | 11.0 | 67.7 | 93.5 | |
| Sudan (2005) | M | 24.8 | 19.6 | 24.7 | 75.9 | – |
| F | 22.7 | 19.9 | 02.9 | 94.8 | – | |
| Total | 23.6 | 19.8 | 12.0 | 86.8 | – | |
| Syria (2003) | M | 30.4 | 36.5 | 48.0 | 21.3 | 95.8 |
| F | 26.4 | 31.6 | 08.9 | 41.0 | 95.6 | |
| Total | 28.4 | 34.0 | 24.7 | 31.2 | 95.7 |
Note: World Health Organization/Regional Office for the Eastern Mediterranean.119
Abbreviation: BP, blood pressure.
The Global School Health Survey from 2003–2007120 defined sedentary behavior as the percentage of adolescents spending three or more hours per day sitting, watching television, playing computer games, or talking to friends. In this survey, the proportion of sedentary activity reported for schoolchildren in some EMR countries ranged from 22.4% for boys in Egypt to 44% for adolescents in Jordan. In general, there does not seem to be a large difference between the percentages of sedentary males and females. In addition, the Global School Health Survey findings indicated that schoolchildren from the United Arab Emirates had the lowest prevalence of walking or riding a bicycle to school of all the countries surveyed.
Recently, a multicenter epidemiological survey has been in progress among adolescents in the major Arab cities, ie, the Arab Teens Lifestyle Study, which is using standardized methodology and a valid instrument for the assessment of physical activity, sedentary behavior, and dietary habits among adolescents in the major Arab cities. It is hoped that this project will provide much needed lifestyle information on Arab adolescents.121
Decreased physical activity among schoolchildren and adolescents has been suggested to be a result of increased time spent watching television, playing computer games, and using the Internet, and decreased opportunities for physical activity in schools and communities.82 Several studies in the region have demonstrated that obesity is more prevalent among children watching television for more than 2 hours a day, compared with those who watch television for less than two hours a day.122–124 In Iran, Kelishadi et al82 indicated that the mean length of time per day spent by schoolchildren aged 16–18 years watching television and/or at the computer (playing games or on the Internet) was 4.6 hours and 4.1 hours for boys and girls, respectively, and there was a significant (P < 0.0001) correlation with obesity in both genders (r = 0.61 and 0.48, respectively).
Television occupies much leisure time for both children and adults. Television affects food consumption and lifestyle in three ways, ie, lengthy watching of television has been cited as a contributing factor to higher intake of energy and fat, exposure to television advertising, especially commercials for fast foods and convenience foods, may influence the food choices of viewers towards high intake of fat and energy, and more lengthy watching of television means less physical activity and a more sedentary lifestyle.8,84
Hypertension
Hypertension affects 26% of the total population in the EMR. Prehypertension (blood pressure 120–139/80–89 mmHg) doubles the risk of developing hypertension and should be treated essentially with lifestyle modification. Lowering blood pressure reduces stroke by 35%–40%, myocardial infarction by 20%–25%, and heart failure by more than 50%.125 Hypertension, in general, is underdiagnosed and undertreated in the EMR, meaning the current prevalence of hypertension is likely to be underestimated in these countries. In the United Arab Emirates, it was found that 33% of people aged 40–60 years were underdiagnosed for hypertension and 76% were undertreated.126 In general, the prevalence of elevated blood pressure in EMR countries is considerably high, ranging from 38.8% in Egypt to 51.7% in Libya for male adults. The corresponding range for female adults is 32.5% in the United Arab Emirates and 46.0% in Morocco. With the exception of Morocco, women were less likely to have hypertension than men (Table 1).
A sedentary lifestyle, unhealthy eating habits, a high salt intake, and a relatively high prevalence of obesity among adolescents in the EMR may lead to early occurrence of high blood pressure in this age group.127 In a nationwide study which included 16,226 Saudi children and adolescents from birth to 18 years, systolic blood pressure rose steadily with age in both boys and girls. The annual increase in systolic blood pressure was 1.66 mmHg for boys and 1.44 mmHg for girls, while that for diastolic blood pressure was 0.83 mmHg for boys and 0.77 mmHg for girls. Diastolic blood pressure rose sharply in boys at the age of 18 years.128 In Iraq, the prevalence of hypertension was shown to be 1.8-fold higher among obese school children aged 6–12 years (4.7%) than in their nonobese counterparts (2.6%, P < 0.05). The overall prevalence of hypertension was 1.7%, with significantly more high systolic (1.1%) than high diastolic (0.6%, P < 0.05) blood pressure.129 The prevalence of elevated blood pressure among Tunisian adolescents aged 15–19 years was 37.4% ± 1.8% (44.7% ± 2.7% for males versus 30% ± 2.3% for females, P < 0.0001), and that of hypertension was 5.2% ± 1.0% (5% ± 1.4% for males versus 5.3% ± 1.0% for females, P < 0.096).109
Tobacco smoking
Tobacco smoking is a well documented risk factor for many chronic diseases, especially cardiovascular disease, diabetes, and lung cancer.130,131 The prevalence of smoking in EMR countries in males and females aged 15 years and above has been reported by the World Health Organization and the Regional Office for the Eastern Mediterranean.34 For prevalence among males, countries can be divided into three categories, ie, countries with a very high prevalence of tobacco smoking (57%–77%), including Djibouti, Tunisia, and Yemen; countries with a moderate prevalence of tobacco smoking (30%–48%), including Egypt, Iraq, Jordan, Kuwait, Lebanon, Morocco, Pakistan, Palestine, Qatar, and Syria; and countries with a relatively low prevalence of tobacco smoking (<30%), include the rest of the EMR countries. For females, two countries reporting a high prevalence were Lebanon (35%) and Yemen (29%), while the prevalence in other countries ranged from 1.0% to 9%. However, it is worth noting that the prevalence of smoking among women is mostly under-reported, due to cultural factors and, therefore, the actual prevalence may be higher than what is documented.
The proportion of tobacco smoking in the region was also found to be high among schoolchildren and adolescents,132–136 medical students,137,138 and physicians.139 For example, 25% of Iraqi male adolescents in Kurdistan were reported to be currently smokers, compared with 2.7% in females.132 In Tunisia, the prevalence of current smoking was higher (33%) among adolescent males aged 13–15 years compared with 4.8% among females.133 Similar patterns were noticed among secondary school students in Lebanon,134 Saudi Arabia,135 and Iran.136 Among medical students, Almerie et al137 showed that 15.8% of male medical students in Syria were current smokers compared with 3.3% of females. The prevalence of smoking in the western region of Saudi Arabia was 24.8% in male medical students compared with 9.1% in females.138 Behbehani et al139 reported a study of smoking among physicians in Bahrain and Kuwait, finding that 14.6% and 13.4% of physicians, respectively, in these two countries were current smokers.
The high prevalence of smoking among secondary schoolchildren and women in this region could be attributed to high use of water-pipe tobacco smoking. Water-pipe smoking in the EMR countries is traditionally the domain of men; however, this type of smoking has increased dramatically among both men and women in the past 15 years. A recent systematic review on the prevalence of tobacco smoking reported a concerning high use of water-pipe smoking among school and university students in the Middle Eastern countries as well as among Middle Eastern immigrants living in Western countries.140 A multinational study carried out among school students (13–15 years) in Gulf Cooperative Council countries showed that the prevalence of water-pipe smoking was 15% in Bahrain, 16% in Kuwait, 9% in Oman, 14% in Qatar, 15% in the United Arab Emirates, and 15% in Yemen. The study found that boys were significantly more likely to smoke a water-pipe than girls, except in Qatar, and water-pipe smoking among these groups was more common than cigarette smoking in most of the countries studied.141 Similar patterns of water-pipe smoking were observed in university students in the region, with a prevalence rate for ever use ranging from 8% to 64% in males and 3% to 37% in females.142–146 About 6.4% of physicians in Bahrain were current water-pipe smokers, while the prevalence was 12% in their Kuwaiti counterparts.139
Several factors may explain the steady increase in water-pipe smoking in the region: first, the social acceptance of water-pipe smoking, especially in women; second, the misperception that water-pipe smoking is less harmful than cigarette smoking; third, the widespread emergence of coffee shops which provide water-pipes for both men and women; and fourth, the lack of legislation to control water-pipe smoking in public.
The very high smoking rate (77%) reported for Yemen could be linked with khat chewing. Khat is a leafy green plant containing cathinone and cathine as active ingredients. The khat-chewing habit is widespread in Yemen and Somalia. The erroneous belief that khat chewing does not have any negative effects on health has played an important role in the high khat chewing rates in these countries. Ali et al147 showed that khat chewing carries a significantly higher risk of cardiogenic shock, stroke, and mortality. After adjustment of baseline variables, khat chewing was an independent risk factor for inhospital mortality (OR 1.9, 95% CI 1.3–2.7, P < 0.001) and stroke (OR 2.7, 95% CI 1.3–5.9, P < 0.01).
Stunting
Recent findings showed that stunting (short height for age) is comorbid with overweight or obesity in the same child and other members of the same household. Research has shown that stunted children are more likely to be overweight in countries undergoing a rapid nutrition transition.8 Stunting is the most common type of undernutrition prevalent among preschool children (0–5 years) in the EMR. It has been estimated that the prevalence of stunting among children under five years ranged from 8% in Qatar to 53% in Yemen.148 In a study of overweight and stunting from large national surveys conducted between 2001 and 2004 in five countries in the EMR (Djibouti, Libya, Morocco, Syria, and Yemen), El-Taguri et al149 showed that the risk ratio for overweight in stunted children ranged from 2.14 in Djibouti to 3.85 in Libya.
Lack of nutrition and health awareness
The lack of knowledge and the negative attitudes of the public regarding risk factors for N-NCDs may be important barriers to the prevention and control of these diseases. In a study conducted to assess knowledge about stroke among the general public in the Arab Gulf countries, it was found that the majority (71%) of subjects had not even heard the term stroke. About 29% considered the age group 30–50 years to be at highest risk for stroke. The commonest risk factors identified were hypertension (23%) and smoking (27%). People who did not know the term stroke had a higher incidence of diabetes, hypertension, and had more than one risk factor (P < 0.05). In a univariate comparison, younger age (P < 0.001), higher education (P < 0.001), and female gender (P < 0.008) were the best predictors of knowledge about stroke. However, when a multivariate logistic regression model was used, educational level, monthly income, and smoking independently predicted knowledge about stroke.150
In Bahrain, Musaiger et al151 found that there was a large deficit in knowledge among adults about risk factors for osteoporosis. For example, 45% of men and 42% of women did not know that exposure to sunlight can help to reduce the risk. About 38% of men and 32% of women believed that green leafy vegetables are the best source of calcium rather than milk and dairy products. Only 40% of men and 53% of women believed that only women are at risk of osteoporosis, while the rest either disagreed or did not know. Misconceptions and inadequate knowledge regarding obesity were studied among Arab women,152 and it was found that 43% of women believed that drinking a lot of water led to obesity. About 54% of the women believed that wrapping the abdomen with clothes after delivery helps to decrease abdominal fat. Also, 31% of the women believed that skipping breakfast helps in reducing weight. Similar findings were reported among adolescents in Bahrain153 and university students in the United Arab Emirates.154
Physicians and medical students in the EMR were also shown to be deficient regarding important nutritional information. 155,156 It was found that 52% and 77% of physicians and medical students, respectively, in Iran did not know the amount of energy intake needed per kilogram per day for the average person. About 42% and 81% of physicians and medical students, respectively, did not know the correct percentage of energy needed from fat, carbohydrates, and protein in a healthy diet.155 Al-Numair156 reported that primary care physicians in Saudi Arabia had poor knowledge about hydrogenated fat, sources of vitamin B12, substances raising blood high-density lipoprotein cholesterol levels, type of dietary fiber contributing to lowering blood cholesterol, and nutrients associated with prevention of hypertension.
Conclusion
N-NCDs have reached critical levels in most EMR countries, representing more than 50%–60% of total deaths, and creating a health and economic burden on government health services.157 Intervention programs to prevent and control N-NCDs in these countries are lacking. Most of the current programs are focusing on education through mass media, especially television, the press, and booklets.28 There is a need to establish a comprehensive strategy to include all the N-NCDs rather than separate strategies for each disease, which is happening in many EMR countries. It is well documented that the risk factors for these diseases are related to each other, and that each disease may be a risk factor for another N-NCD. In order to combat N-NCDs, we need to focus on practical solutions that can be applied to prevent and control these diseases and make better use of the most reliable research data. Programs used in such a strategy should be long-term and sustainable, and the proposed activities should include all segments of the community. Private sectors as well as governmental sectors should be involved in the preparation and implementation of such programs. Three main components should be promoted in this strategy, ie, healthy eating, physical activity, and a healthy lifestyle, and avoidance of tobacco smoking.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization, Regional Office of East Mediterranean. Regional data on noncommunicable diseases risk factors. [Accessed February 25, 2009]. Available from: http//www.emro.who.int.ncd.
- 2.Mirkin B. Arab Human Development Report. United Nations Developing Programme; 2010. [Accessed January 25, 2012]. Population levels, trends and policies in the Arab region: Challenges and opportunities. Available from: http://www.arab-hdr.org/publications/other/ahdrps/paper01-en.pdf. [Google Scholar]
- 3.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 4.Kosti RI, Panagiotakos DB. The epidemic of obesity in children and adolescents in the world. Cent Eur J Public Health. 2006;14:151–159. doi: 10.21101/cejph.a3398. [DOI] [PubMed] [Google Scholar]
- 5.Mirmiran P, Sherafat-Kazemzadeh R, Jalali-Farahani S, Azizi F. Childhood obesity in the Middle East: a review. East Mediterr Health J. 2010;16:1009–1117. [PubMed] [Google Scholar]
- 6.Papandreou C, AbuMou’ad T, Jildeh C, Abdeen Z, Philalithis A, Tzanakis N. Obesity in Mediterranean region (1997–2007): a systematic review. Obes Rev. 2008;9:389–399. doi: 10.1111/j.1467-789X.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- 7.Sibai AM, Nasreddine L, Mokdad AH, Adra N, Tabet M, Hwalla N. Nutrition transition and cardiovascular disease risk factors in Middle East and North Africa Countries: reviewing the evidence. Ann Nutr Metab. 2010;57:193–203. doi: 10.1159/000321527. [DOI] [PubMed] [Google Scholar]
- 8.Musaiger AO. Overweight and obesity in the Eastern Mediterranean: prevalence and possible causes. J Obes. 2011;2011:407237. doi: 10.1155/2011/407237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Hazzaa HM. Physical activity, fitness and fatness among Saudi children and adolescents: implications for cardiovascular health. Saudi Med J. 2002;23:144–150. [PubMed] [Google Scholar]
- 10.Al-Sendi AM, Shetty P, Musaiger AO, Myatt M. Relationship between body composition and blood pressure in Bahraini adolescents. Br J Nutr. 2003;90:837–844. doi: 10.1079/bjn2003963. [DOI] [PubMed] [Google Scholar]
- 11.Bin-Zaal AA, Musaiger AO, D’Souza R. The association between obesity and blood pressure among adolescents in Dubai, UAE. Pak J Med Sci. 2010;26:271–276. [Google Scholar]
- 12.Al-Sabbah H, Vereecken C, Kolsteren P, Abdeen Z, Maes L. Food habits and physical activity patterns among Palestinian adolescents: findings from national study of Palestinian school children (HBSC-WBG 2004) Public Health Nutr. 2007;10:739–746. doi: 10.1017/S1368980007665501. [DOI] [PubMed] [Google Scholar]
- 13.Musharrafieh U, Tamim HM, Rahi AC, et al. Determinants of university students physical execises: a study from Lebanon. Int J Public Health. 2008;53:208–213. doi: 10.1007/s00038-008-7037-x. [DOI] [PubMed] [Google Scholar]
- 14.Henry CJK, Lightowler HJ, Al-Hourani HM. Physical activity and levels of inactivity in adolescent females aged 11–16 years in the United Arab Emirates. Am J Hum Biol. 2004;16:346–353. doi: 10.1002/ajhb.20022. [DOI] [PubMed] [Google Scholar]
- 15.Fazah A, Jacob C, Moussa E, El-Hage R, Youssef H, Delamarche P. Activity, inactivity and quality of life among Lebanese adolescents. Pediatr Int. 2010;52:573–578. doi: 10.1111/j.1442-200X.2009.03021.x. [DOI] [PubMed] [Google Scholar]
- 16.Bin Zaal AA, Musaiger AO, D’Souza R. Dietary habits associated with obesity among adolescents in Dubai, United Arab Emirates. Nutr Hosp. 2009;24:437–444. [PubMed] [Google Scholar]
- 17.Sayegh A, Dini EL, Holt RD, Bedi R. Food and drink consumption, sociodemographic factors and dental caries in 4–5-year-old children in Amman, Jordan. Br Dent J. 2002;193:37–42. doi: 10.1038/sj.bdj.4801478. [DOI] [PubMed] [Google Scholar]
- 18.Musaiger AO, Bader Z, Al-Roomi K, D’Souza R. Dietary and lifestyle habits among adolescents in Bahrain. Food Nutr Res. 2011 September 9; doi: 10.3402/fnr.v55i0.7122. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Shehri AN, Saleh ZA, Salama MM, Hassan YM. Prevalence of hyperlipidemia among Saudi school children in Riyadh. Ann Saudi Med. 2004;24:6–8. doi: 10.5144/0256-4947.2004.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azizi A, Rahmani M, Madjid M, Allahverdian A, Ghanbarian J, Hajipour R. Serum lipid levels in an Iranian population of children and adolescents: Tehran Lipid and Glucose Study. Eur J Epidemiol. 2001;17:281–288. doi: 10.1023/a:1017932212350. [DOI] [PubMed] [Google Scholar]
- 21.Benamer HT, Grosset D. Stroke in Arab countries: systematic literature review. J Neurol Sci. 2009;15:18–23. doi: 10.1016/j.jns.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Hosseini AA, Sobhania-Rad D, Ghandehari K, Benamer HTS. Frequency and clinical patterns of stroke in Iran – systematic and critical review. BMC Neurol. 2010;10:72. doi: 10.1186/1471-2377-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Global Status Report on Non-Communicable Diseases, 2010. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 24.El-Hazmi MAI, Warsy AS. Evaluation of serum cholesterol and triglyceride levels in 1–6-year-old Saudi children. J Trop Pediatr. 2001;47:181–185. doi: 10.1093/tropej/47.3.181. [DOI] [PubMed] [Google Scholar]
- 25.Harrabi I, Ghannem H, Gaha R, Hochlaf M, Limam K, Essoussi AS. Epidemiology of dyslipidemia among schoolchildren in Sousse, Tunisia. Diabetes Metab. 2005;31:285–289. doi: 10.1016/s1262-3636(07)70195-7. [DOI] [PubMed] [Google Scholar]
- 26.National Nutrition Institute. Diet, Nutrition and Prevention of Chronic Non-Communicable Diseases among Egyptian Adolescents. Cairo, Egypt: Ministry of Health; 2008. [Google Scholar]
- 27.Al Majed HT, AlAttar AT, Sadek AA, et al. Prevalence of dyslipidemia and obesity among college students in Kuwait. Alexandria J Med. 2011;47:67–71. [Google Scholar]
- 28.Musaiger AO, Hassan AS, Obeid O. The paradox of nutrition-related diseases in the Arab countries: the need for action. Int J Environ Res Public Health. 2011;8:3637–3671. doi: 10.3390/ijerph8093637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouguerra R, Alberti H, Salem LB, et al. The global diabetes pandemic: the Tunisian experiences. Eur J Clin Nutr. 2007;61:160–165. doi: 10.1038/sj.ejcn.1602478. [DOI] [PubMed] [Google Scholar]
- 30.Ajlouni K, Khader YS, Batieha A, Ajlouni H, El-Khateeb M. An increase in prevalence of diabetes mellitus in Jordan over 10 years. J Diabetes Complications. 2008;22:317–324. doi: 10.1016/j.jdiacomp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Khatib O. Non-communicable diseases. Risk factors and regional strategies for prevention and care. East Mediterr Health J. 2004;10:778–788. [PubMed] [Google Scholar]
- 33.Esteghamati A, Meysamie A, Khaizadeh O, et al. Third national surveillance of risk factors of non-communicable diseases (SuRFNCD-2007) in Iran: methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9:167–176. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization, Regional Office for the Eastern Mediterranean. Towards a strategy for cancer control in the Eastern Mediterranean region. Cairo: 2009. [Accessed January 25, 2012]. Available from: www.emro.who.int/dsaf/dsa1002.pdf. [Google Scholar]
- 35.El Salim, Moore MA, Al-Lawati JA, et al. Cancer epidemiology and control in the Arab World – past, present and future. Asian Pac J Cancer Prev. 2009;10:3–16. [PubMed] [Google Scholar]
- 36.Ravichandran K, Al-Zahrani AS. Association of reproductive factors with the incidence of breast cancer in Gulf Corporation Council countries. East Mediterr Health J. 2009;15:612–621. [PubMed] [Google Scholar]
- 37.Bener A. Colon cancer in rapidly developing countries: a review of the lifestyle, dietary, consanguinity and hereditary risk factors. Oncol Rev. 2011;5:5–11. [Google Scholar]
- 38.Al-Hamdan N, Ravichandran K, Al-Sayyed J, et al. Incidence of cancer in Gulf Corporation Council countries, 1998–2001. East Mediterr Health J. 2009;15:600–611. [PubMed] [Google Scholar]
- 39.World Health Organization, Regional Office for the Eastern Mediterranean. Report on the consultation on establishing regional guidelines on osteoporosis. Cairo: 2005. [Accessed January 25, 2012]. Available from: www.emro.who.int/ncd/pdf/who_em_ncd_044_e_en.pdf. [Google Scholar]
- 40.Hashemipour S, Larijani B, Adibi H, et al. Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health. 2004;4:38. doi: 10.1186/1471-2458-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Hajj Fuleihan G, Baddoura R, Awada H, Salam N, Salamoun M, Rizk P. Low peak bone mineral density in healthy Lebanese subjects. Bone. 2002;31:520–528. doi: 10.1016/s8756-3282(02)00845-1. [DOI] [PubMed] [Google Scholar]
- 42.Bener A, Rizk DE, Shaheen H, Micallef R, Osman N, Dunn EV. Measurement-specific quality of life satisfaction during menopause in an Arabian Gulf country. Climacteric. 2000;3:43–49. doi: 10.3109/13697130009167598. [DOI] [PubMed] [Google Scholar]
- 43.Ardawi MS, Maimany AA, Bahksh TM, Nasrat HA, Milaat WA, Al-Raddadi RM. Bone mineral density of the spine and femur in healthy Saudis. Osteoporos Int. 2005;16:43–55. doi: 10.1007/s00198-004-1639-9. [DOI] [PubMed] [Google Scholar]
- 44.El-Dessouki MI. Osteoporosis in post menopausal Saudi women using x-ray bone densitometry. Saudi Med J. 2003;24:953–956. [PubMed] [Google Scholar]
- 45.Meddeb N, Sahli H, Chahed M, et al. Vitamin D deficiency in Tunisia. Osteoporos Int. 2005;16:180–183. doi: 10.1007/s00198-004-1658-6. [DOI] [PubMed] [Google Scholar]
- 46.Allali F, El Aichaoui S, Khazani H, et al. High prevalence of hypovitaminosis D in Morocco: relation to lifestyle, physical performance, bone makers, and bone mineral density. Semin Arthritis Rheum. 2009;38:441–451. doi: 10.1016/j.semarthrit.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Dougherty G, Al-Mazrouk N. Bone density measured by dual-energy x-ray absorptiometry in healthy Kuwaiti women. Calcif Tissue Int. 2001;68:225–229. doi: 10.1007/s002230020015. [DOI] [PubMed] [Google Scholar]
- 48.Gannage-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res. 2000;15:1856–1862. doi: 10.1359/jbmr.2000.15.9.1856. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqui AM, Kamfar HZ. Prevalence of vitamin D deficiency rickets in adolescent school girls in Western region, Saudi Arabia. Saudi Med J. 2007;28:441–444. [PubMed] [Google Scholar]
- 50.Maalouf G, Gannage-Yared MH, Ezzedine J, et al. Middle East and North Africa consensus on osteoporosis. J Musculoskelet Neuronal Interact. 2007;7:131–143. [PubMed] [Google Scholar]
- 51.Al-Hazzaa HM. Prevalence and trends in obesity among school boys in Central Saudi Arabia between 1988 and 2005. Saudi Med J. 2007;28:1569–1574. [PubMed] [Google Scholar]
- 52.Esteghamati A, Khalilzadeh O, Mohammad K, et al. Secular trends of obesity in Iran between 1999–2007: national surveys of risk factors of non-communicable diseases. Metab Syndr Relat Disord. 2010;8:209–213. doi: 10.1089/met.2009.0064. [DOI] [PubMed] [Google Scholar]
- 53.Yusuf S, Hawken S, Ounpuu S, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 54.Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. 2004;5:4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 55.Thanopoulou A, Karamanos B, Angelico F, et al. Epidemiological evidence for the non-random clustering of the components of the metabolic syndrome: multicenter study of the Mediterranean Group for the Study of Diabetes. Eur J Clin Nutr. 2006;60:1376–1383. doi: 10.1038/sj.ejcn.1602467. [DOI] [PubMed] [Google Scholar]
- 56.AL-Lawati JA, Mohammed AJ, Al-Hinai HQ, Jousilahti P. Prevalence of the metabolic syndrome among Omani adults. Diabetes Care. 2003;26:1781–1785. doi: 10.2337/diacare.26.6.1781. [DOI] [PubMed] [Google Scholar]
- 57.Al-Rashan I, Al-Nesef Y. Prevalence of overweight, obesity, and metabolic syndrome among Kuwaitis: result from community-based national survey. Angiology. 2010;61:42–49. doi: 10.1177/0003319709333226. [DOI] [PubMed] [Google Scholar]
- 58.Bener A, Zirie M, Musallam M, Khader YS, Al-Hamaq AO. Prevalence of metabolic syndrome according to Adult International Panell III and International Diabetes Federation Criteria: a population-based study. Metab Syndr Relat Disord. 2009;7:221–230. doi: 10.1089/met.2008.0077. [DOI] [PubMed] [Google Scholar]
- 59.Sibai AM, Obeid O, Batal M, Afra N, Elkhoury D, Hwalla N. Prevalence and correlates of metabolic syndrome in an adult Lebanese population. CVD Prev Control. 2008;3:83–90. [Google Scholar]
- 60.Harzallah F, Alberti H, Ben-Khalifa F. The metabolic syndrome in an Arab population. a first look at the next International Diabetes Federation criteria. Diabet Med. 2006;23:441–444. doi: 10.1111/j.1464-5491.2006.01866.x. [DOI] [PubMed] [Google Scholar]
- 61.Abdul-Rahim HF, Husseini A, Bjertness E, Gicaman R, Gordon NH, Jervell J. The metabolic syndrome in the West Bank population, an urban-rural comparison. Diabetes Care. 2001;24:275–279. doi: 10.2337/diacare.24.2.275. [DOI] [PubMed] [Google Scholar]
- 62.Delavari A, Forouzanfar MH, Alikhani S, Sharifian A, Kelishadi R. First national study of the prevalence of the metabolic syndrome and optimal cutoff points of waist circumference in the Middle East: the national survey of risk factors for noncommunicable diseases of Iran. Diabetes Care. 2009;32:1092–1097. doi: 10.2337/dc08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malik M, Abdel-Razig S. The prevalence of metabolic syndrome among the multiethnic population of the United Arab Emirates: a report of a national survey. Metab Syndr Relat Disord. 2008;6:177–186. doi: 10.1089/met.2008.0006. [DOI] [PubMed] [Google Scholar]
- 64.Mabry RM, Reeves MM, Eakin EG, Owen N. Gender differences in the prevalence of the metabolic syndrome in Gulf Corporation Council countries: a systematic review. Diabet Med. 2010;27:593–597. doi: 10.1111/j.1464-5491.2010.02998.x. [DOI] [PubMed] [Google Scholar]
- 65.Esmaillzadeh A, Mirmiran P, Azedbakht L, Etemadi A, Azizi F. High prevalence of the metabolic syndrome in Iranian adolescents. Obesity. 2006;14:377–382. doi: 10.1038/oby.2006.50. [DOI] [PubMed] [Google Scholar]
- 66.Kelishadi R, Ardalan G, Gheiratmand R, Adeli K, Delavari A, Majdzadehet R. Pediatric metabolic syndrome and associated anthropometric indices: CASPIAN study. Acta Pediatr. 2006;95:1625–1634. doi: 10.1080/08035250600750072. [DOI] [PubMed] [Google Scholar]
- 67.Al-Daghri NM. Extremely high prevalence of metabolic syndrome manifestations among Arab youth: a call for early intervention. Eur J Clin Invest. 2010;40:1063–1066. doi: 10.1111/j.1365-2362.2010.02341.x. [DOI] [PubMed] [Google Scholar]
- 68.Kelishadi R. Childhood overweight, obesity and metabolic syndrome in developing countries. Epidemiol Rev. 2007;29:62–76. doi: 10.1093/epirev/mxm003. [DOI] [PubMed] [Google Scholar]
- 69.Kelishadi R, Gouya MM, Adeli K, et al. Factors associated with metabolic syndrome in a national sample of youths: CASPIAN study. Nutr Metab Cardiovasc Dis. 2008;18:461–470. doi: 10.1016/j.numecd.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Ghassemi H, Harrison G, Mohammad K. An accelerated nutrition transition in Iran. Public Health Nutr. 2002;5:149–155. doi: 10.1079/PHN2001287. [DOI] [PubMed] [Google Scholar]
- 71.Galal OM. The nutrition transition in Egypt: obesity, undernutrition and the food consumption context. Public Health Nutr. 2002;5:141–148. doi: 10.1079/PHN2001286. [DOI] [PubMed] [Google Scholar]
- 72.Benjelloun S. Nutrition transition in Morocco. Public Health Nutr. 2002;5:135–140. doi: 10.1079/PHN2001285. [DOI] [PubMed] [Google Scholar]
- 73.Madanat HN, Troutman KP, Al-Madi B. The nutrition transition in Jordan: the political, economic and food consumption context. Promot Educ. 2008;15:6–10. doi: 10.1177/1025382307088092. [DOI] [PubMed] [Google Scholar]
- 74.Sibai AM, Nasreddine L, Mokdad AH, Adra N, Tabet M, Hwalla N. Nutrition transition and cardiovascular disease risk factors in Middle East and North Africa Countries: reviewing the evidence. Ann Nutr Metab. 2010;57:193–203. doi: 10.1159/000321527. [DOI] [PubMed] [Google Scholar]
- 75.Food and Agriculture Organization of the United Nations. Food Balance Sheet. [Accessed January 25, 2012]. Available from: http://faostat.fao.org/
- 76.Esmaillzadeh A, Azadbakht L. Major dietary patterns in relation to general obesity and central adiposity among Iranian women. J Nutr. 2008;138:358–363. doi: 10.1093/jn/138.2.358. [DOI] [PubMed] [Google Scholar]
- 77.Galal O. Nutrition-related health patterns in the Middle East. Asia Pac J Clin Nutr. 2003;12:337–343. [PubMed] [Google Scholar]
- 78.Musaiger AO. Diet and prevention of coronary heart disease in the Arab Middle East countries. Med Princ Pract. 2002;11:9–16. doi: 10.1159/000066415. [DOI] [PubMed] [Google Scholar]
- 79.Kimigar SM, Ghaffarpour M, Houshiar-Red A, Homozdyari H, Zellipour L. Food consumption pattern in the Islamic Republic of Iran and its relation to coronary heart disease. East Mediterr Health J. 1998;4:539–547. [Google Scholar]
- 80.Nasreddine L, Hwalla N, Sibai A, Hamze M, Parent-Massin D. Food consumption patterns in an adult urban population in Beirut, Lebanon. Public Health Nutr. 2006;9:194–203. doi: 10.1079/phn2005855. [DOI] [PubMed] [Google Scholar]
- 81.Mahmood AH. Nutritional status and anthropometric measurements among women in Egypt, National Survey 2001–2002. Arab J Food Nutr. 2004;11:98–107. [Google Scholar]
- 82.Kelishadi R, Ardalan G, Gheiratmand R, et al. Association of physical activity and dietary behaviours in relation to the body mass index in a national sample of Iranian children and adolescents: CASPIAN study. Bull World Health Organ. 2007;85:19–26. doi: 10.2471/BLT.06.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mirmiran P, Azadbakht L, Azizi L. Dietary quality-adherence to the dietary guidelines in Tehranian adolescents: Tehran Lipid and Glucose Study. Int J Vitam Nutr Res. 2005;75:195–200. doi: 10.1024/0300-9831.75.3.195. [DOI] [PubMed] [Google Scholar]
- 84.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health. 2001;22:309–335. doi: 10.1146/annurev.publhealth.22.1.309. [DOI] [PubMed] [Google Scholar]
- 85.Bashour HN. Survey of dietary habits of in-school adolescents in Damascus, Syria Arab Republic. East Mediterr Health J. 2004;10:853–862. [PubMed] [Google Scholar]
- 86.Hejazi N, Mazloom Z. Socioeconomic status, youth’s eating patterns and meal consumed away from home. Pak J Biol Sci. 2009;12:730–733. doi: 10.3923/pjbs.2009.730.733. [DOI] [PubMed] [Google Scholar]
- 87.Bray GA. Soft drinks and obesity: the evidence. CMR J. 2009;2:10–14. [Google Scholar]
- 88.Honkala S, Honkala E, Al-Sahli N. Consumption of sugar products and associated-life and school-satisfaction and self-esteem factors among schoolchildren in Kuwait. Acta Odontol Scand. 2006;64:79–88. doi: 10.1080/00016350500420048. [DOI] [PubMed] [Google Scholar]
- 89.Collison KS, Zaidi MZ, Subhani SM, Al-Rubeaan K, Shoukri M, Al-Mohanna FA. Sugar sweetened carbonated beverage consumption correlates with waist circumference and poor dietary choices in school children. BMC Public Health. 2010;10:234. doi: 10.1186/1471-2458-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng SW, Zaghloal S, Ali H, Harrison G, El-Sadig M, Popkin BM. Nutrition transition in the United Arab Emirates. Eur J Clin Nutr. 2011;65:1328–1337. doi: 10.1038/ejcn.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ismail AI, Tanzer JM, Dingle JL. Current trends of sugar consumption in developing countries. Community Dent Oral Epidemiol. 1997;25:438–443. doi: 10.1111/j.1600-0528.1997.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 92.Higgins J. Whole gain, and the subsequence meal effect: Implications for glucose control and the role of fermentation. J Nutr Metab. 2011 October 30; doi: 10.1155/2012/829238. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Esmaillzadeh A, Mirmiran P, Azizi F. Whole-grain intake and prevalence of hypertriglyceridemic waist phenotype in Tehranian adults. Am J Clin Nutr. 2005;81:55–63. doi: 10.1093/ajcn/81.1.55. [DOI] [PubMed] [Google Scholar]
- 94.Nasreddine L, Mehio-Sibai A, Mrayati M, Adra N, Hwalla N. Adolescent obesity in Syria: prevalence and associated factors. Child Care Health Dev. 2010;36:404–413. doi: 10.1111/j.1365-2214.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 95.Kelishadi R, Ardalan G, Gheiratmand R, et al. Association of physical activity and dietary behaviours in relation to the bodymass index in a national sample of Iranian children and adolescents: CASPIAN Study. Bull World Health Organ. 2007;85:19–26. doi: 10.2471/BLT.06.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.El-Ghazali S, Ibrahim JM, Kanari BM, Ismail NA. The relationship between lifestyle and body mass index among university students in Kuwait. Egyptian J Com Med. 2010;28:69–76. [Google Scholar]
- 97.Dastgiri S, Mahdavi R, TuTunchi H, Faramarzi E. Prevalence of obesity, food choices and socio-economic status: a cross-sectional study in the north-west of Iran. Public Health Nutr. 2006;9:996–1000. doi: 10.1017/s1368980006009827. [DOI] [PubMed] [Google Scholar]
- 98.Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bull World Health Organ. 2005;83:100–108. [PMC free article] [PubMed] [Google Scholar]
- 99.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 100.Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- 101.Al-Roomi K, Musiger AO, Al-Awadi H. Lifestyle and risk of acute myocardial infarction in a Gulf Arab population. Int J Epidemiol. 1994;23:931–939. doi: 10.1093/ije/23.5.931. [DOI] [PubMed] [Google Scholar]
- 102.Mirmiran P, Noori N, Zavareh MB, Azizi F. Fruit and vegetable consumption and risk factors for cardiovascular disease. Metabolism. 2009;58:460–468. doi: 10.1016/j.metabol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 103.Pourfarzi F, Whelan A, Kaldor J, Malikzadeh R. The role of diet and other environmental factors in the causation of gastric cancer in Iran – a population based study. Int J Cancer. 2009;125:1953–1960. doi: 10.1002/ijc.24499. [DOI] [PubMed] [Google Scholar]
- 104.Kelishadi R, Pour MH, Sarraf-Zadegan N, Sadry, et al. Obesity and associated modifiable environmental factors in Iranian adolescents: Isfahan Healthy Heart Programme-Heart Health Promotion from Childhood. Pediatr Int. 2003;45:435–442. doi: 10.1046/j.1442-200x.2003.01738.x. [DOI] [PubMed] [Google Scholar]
- 105.Rashad H. Demographic transition in Arab countries: a new perspective. J Popul Res. 2000;17:83–101. [Google Scholar]
- 106.World Health Organization/Regional Off ice for the Eastern Mediterranean. Active Aging and Promotion of the Health of the Older People. Cairo, Egypt: Regional Off ice of the Eastern Mediterranean; 2006. [Google Scholar]
- 107.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 108.Monteiro CA, Moura EC, Conde WL, Popkin BM. Socioeconomic status and obesity in adult populations of developing countries: a review. Bull World Health Organ. 2004;82:940–946. [PMC free article] [PubMed] [Google Scholar]
- 109.Aounallah-Skhiri H, Traissal P, El-Ali J, et al. Nutrition transition among adolescents of a South-Mediterranean country: dietary patterns association with socio-economic factors, overweight and blood pressure; a cross-sectional study in Tunisia. Nutr J. 2011;10:38. doi: 10.1186/1475-2891-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soliman AS, Khorshid A, Ibrahim N, Dorgham L, McPherson RS. Diet and cooking practices in Egypt: exploration of potential relationship to early-chest colorectal cancer. Nutr Res. 1998;18:785–797. [Google Scholar]
- 111.Jamal HA, Sheiham A, Cowell CR, Walt RG. Taste preference for sweetness in urban and rural population in Iraq. J Dent Res. 1996;76:1879–1889. doi: 10.1177/00220345960750111001. [DOI] [PubMed] [Google Scholar]
- 112.Hakeem R, Thomas J, Badruddin SH. Food habits and nutrient density of diet of Pakistani children living in different urban and rural settings. J Health Popul Nutr. 2002;20:255–263. [PubMed] [Google Scholar]
- 113.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kopiszewsti A. Arab versus Asian migrant workers in the GCC countries Population Division, United Nations Secretariat. 2006. [Accessed January 26, 2012]. Available from: http://www.un.org/esa/population/meetings/EGM_Ittmig_Arab/P02_Kapiszewski.pdf.
- 115.Al-Hazzaa HM. The public health burden of physical inactivity in Saudi Arabia. J Fam Community Med. 2004;11:45–51. [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Nozha M, Al-Hazzaa H, Arafah MR, et al. Physical activity and inactivity among Saudi aged 30–70 years: A population-based cross-sectional study. Saudi Med J. 2007;28:559–568. [PubMed] [Google Scholar]
- 117.Al-Refaee SA, Al-Hazzaa HM. Physical activity profile of Saudi males: implications for health. Saudi Med J. 2001;22:784–789. [PubMed] [Google Scholar]
- 118.Al-Hazzaa HM. Health-enhancing physical activity among Saudi adults using the International Physical Activity Questionnaire (IPAQ) Public Health Nutr. 2007;10:59–64. doi: 10.1017/S1368980007184299. [DOI] [PubMed] [Google Scholar]
- 119.World Health Organization. STEPwise approach to surveillance (STEPS) [Accessed September 17, 2011]. Available from: http://www.who.int/chp/steps/en/
- 120.Guthold R, Cowan MJ, Autenrieth CS, Kann L, Riley LM. Physical activity and sedentary behavior among schoolchildren: a 34-country comparison. J Pediatr. 2010;157:43–49. e1. doi: 10.1016/j.jpeds.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 121.Al-Hazzaa HM, Musaiger AO. Physical activity patterns and eating habits of adolescents living in major Arab cities: The Arab Teens Lifestyle Study. Saudi Med J. 2010;31:210–211. [PubMed] [Google Scholar]
- 122.Farahat TM, Mechael AA, Abu-Salem M. Prevalence of obesity among preschool children in Menofia, Egypt. Arab J Food Nutr. 2003;8:107–118. [Google Scholar]
- 123.Al-Hazzaa HM, Al-Rasheedi AA. Adiposity and physical activity levels among preschool children in Jeddah, Saudi Arabia. Saudi Med J. 2007;28:766–773. [PubMed] [Google Scholar]
- 124.Lafta RK, Kadhim MJ. Childhood obesity in Iraq: prevalence and possible risk factors. Ann Saudi Med. 2005;25:389–393. doi: 10.5144/0256-4947.2005.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.World Health Organization/Regional Off ice for the Eastern Mediterranean. Report on the Regional Consultation on Hypertension, Prevention and Control. Cairo, Egypt: World Health Organization/Regional Office for the Eastern Mediterranean; 2004. [Google Scholar]
- 126.Abdulla AM, Nagelkerke NJ, Abouchacra S, Pathan JY, Adem A, Obineche EN. Under-treatment and under diagnosis of hypertension: a serious problem in the United Arab Emirates. BMC Cardiovasc Disord. 2006;6:24. doi: 10.1186/1471-2261-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bener A, Al-Suwaidi J, Al-Jaber K, Al-Marri S, Elbagi IE. Epidemiology of hypertension and its associated risk factors in the Qatari population. J Hum Hypertens. 2004;18:529–530. doi: 10.1038/sj.jhh.1001691. [DOI] [PubMed] [Google Scholar]
- 128.Al-Salloum AA, El-Mouzan MI, Al-Herbish AS, Al-Omar AA, Qurashi MM. Blood pressure standards for Saudi children and adolescents. Ann Saudi Med. 2009;29:173–178. doi: 10.4103/0256-4947.51787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Subahi MD. Blood pressure profile and hypertension in Iraqi primary school children. Saudi Med J. 2006;27:482–486. [PubMed] [Google Scholar]
- 130.Villablanca AC, McDonald JM, Rutledge JC. Smoking and cardiovascular disease. Clin Chest Med. 2000;21:159–172. doi: 10.1016/s0272-5231(05)70015-0. [DOI] [PubMed] [Google Scholar]
- 131.National Cancer Institute. Smoking and Tobacco Control Monograph No. 10. Bethesda, MD: US Department of Health and Human Service, National Institutes of Health, National Cancer Institute; 1999. Health effects of exposure to environmental tobacco smoke: a report of the California Environmental Protection Agency. NIH Pub No 99-4645. [Google Scholar]
- 132.Siziya S, Muula AS, Rudatsikira E. Correlates of current smoking in-school adolescents in the Kurdistan region of Iraq. Confl Health. 2007;1:13–19. doi: 10.1186/1752-1505-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harrabi I, Chahed H, Maatoug J, Gaha J, Essoussi S, Ghannem H. Predictors of smoking initiation among schoolchildren in Tunisia: a 4 years cohort study. Afr Health Sci. 2009;9:147–152. [PMC free article] [PubMed] [Google Scholar]
- 134.El-Roueiheb Z, Tamim H, Kanj M, Jabbour S, Alayan I, Musharrafieh U. Cigarette and waterpipe smoking among Lebanese adolescents, a cross-sectional study, 2003–2004. Nicotine Tob Res. 2008;10:309–314. doi: 10.1080/14622200701825775. [DOI] [PubMed] [Google Scholar]
- 135.Amin TT, Amar MA, Zaza BO, Suleman W. Harm perception, attitudes and predicators of waterpipe (shisha) smoking among secondary school adolescent in Al-Hassa, Saudi Arabia. Asian Pac J Cancer Prev. 2010;11:293–301. [PubMed] [Google Scholar]
- 136.Kelishadi R, Mokhtari MR, Tavasoli AA, et al. Determinants of tobacco smoking among youths in Isfahan, Iran. Int J Public Health. 2007;52:173–179. doi: 10.1007/s00038-007-6017-x. [DOI] [PubMed] [Google Scholar]
- 137.Almerie MQ, Matar HE, Salam M, et al. Cigarettes of waterpipe smoking among medical students in Syria: a cross-sectional study. Int J Tuberc Lung Dis. 2008;12:1085–1091. [PMC free article] [PubMed] [Google Scholar]
- 138.Wali SO. Smoking habits among medical students in Western Saudi Arabia. Saudi Med J. 2011;32:843–448. [PubMed] [Google Scholar]
- 139.Behbehani NN, Hamadeh RR, Macklai NS. Knowledge of and attitudes towards tobacco control among smoking and non-smoking physicians in 2 Gulf Arab states. Saudi Med J. 2004;25:585–591. [PubMed] [Google Scholar]
- 140.Akl EA, Gunukula SK, Aleen S, Obeid R, Abou-Jaoude P, Honeine R. The prevalence of waterpipe tobacco smoking among the general and specific population: a systematic review. BMC Public Health. 2011;11:244–255. doi: 10.1186/1471-2458-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Moh’d Al-Mulla A, Abdou Helmy S, Al-Lawati J, et al. Prevalence of tobacco use among students aged 13–15 years in Health Ministers’ Council/Gulf Corporation Council Member Status, 2001–2004. J Sch Health. 2008;78:337–343. doi: 10.1111/j.1746-1561.2008.00311.x. [DOI] [PubMed] [Google Scholar]
- 142.Chaaya M, El-Roueiheb Z, Chemaitelly H, Azar G, Nasr J, Al-Sahab B. Argileh smoking among university students: a new tobacco epidemic. Nicotine Tob Res. 2004;6:457–463. doi: 10.1080/14622200410001696628. [DOI] [PubMed] [Google Scholar]
- 143.Al-Turki YA. Smoking habits among medical students in Central Saudi Arabia. Saudi Med J. 2006;27:700–703. [PubMed] [Google Scholar]
- 144.Mandil A, Hussein A, Omer H, et al. Characteristics and risk factors of tobacco consumption among University of Sharjah students, 2005. East Mediterr Health J. 2007;13:1449–1458. doi: 10.26719/2007.13.6.1449. [DOI] [PubMed] [Google Scholar]
- 145.Maziak W, Eissenberg T, Rastam S, et al. Beliefs and attitudes related to narghile (waterpipe) smoking among university students in Syria. Ann Epidemiol. 2004;14:646–654. doi: 10.1016/j.annepidem.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 146.Maziak W, Fouad FM, Asfar T, et al. Prevalence and characteristics of narghile smoking among university students in Syria. Int J Tuber Lung Dis. 2004;8:882–889. [PubMed] [Google Scholar]
- 147.Ali VVM, Zabaid M, Al-Motarreb A, et al. Association of khat chewing with increased risk of stroke and death in patients presenting with acute coronary syndrome. Mayo Clin Proc. 2010;85:974–980. doi: 10.4065/mcp.2010.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.United Nations Children’s Fund. Child Survival. New York, NY: United Nations Children’s Fund; 2008. 2007. The State of World’s Children. [Google Scholar]
- 149.El Taguri A, Besmar F, Abdel Monem A, Betilmal I, Ricour C, Rolland-Cachera MF. Stunting is a major risk factor for overweight: results from national surveys in 5 Arab countries. East Mediterr Health J. 2009;15:549–562. [PubMed] [Google Scholar]
- 150.Kamran S, Bener AB, Deleu D, et al. The level of awareness of stroke risk factors and symptoms in the Gulf Cooperation Council Countries: Gulf Cooperation Council Stroke Awareness Study. Neuroepidemiology. 2009;29:235–242. doi: 10.1159/000112856. [DOI] [PubMed] [Google Scholar]
- 151.Musaiger AO, Al-Maskari A, Alawati H, et al. Factors associated with the risk of osteoporosis among adults in Bahrain. Arab J Food Nutr. 2008;19:49–62. [Google Scholar]
- 152.Musaiger AO, Shahbeek NE. The effect of education and obesity on attitudes toward fads related to weight reduction among Arab women in Qatar. J Nutr Food Sci. 2001;31:201–204. [Google Scholar]
- 153.Musaiger AO, Matter AM, Alekri SA, Mahdi AE. Knowledge and attitudes of Bahraini adolescents toward obesity. J Consumer Studies Home Economics. 1991;15:321–325. [Google Scholar]
- 154.Musaiger AO. Knowledge and attitudes of university female students towards obesity. Int Q Community Health Educ. 1993;14:337–344. doi: 10.2190/2M8D-M26R-8U6X-PWNJ. [DOI] [PubMed] [Google Scholar]
- 155.Hosseini S, Nayebi N, Amirkalali B, Seyedkhoei N, Heshmat R. Are physicians good candidates for recommending diet. Iran J Public Health. 2008;37:48–53. [Google Scholar]
- 156.Al-Numair K. Nutrition knowledge of primary health care physicians in Saudi Arabia. Pak J Nutr. 2004;3:344–347. [Google Scholar]
- 157.Habib S, Saha S. Burden of non-communicable disease: global overview. Diabetes and Metabolic Synd. Clinical Research and Reviews. 2008;4:41–47. [Google Scholar]
- 158.de Onis M, Blossner M. Prevalence and trends of overweight among preschool children in developing countries. Am J Clin Nutr. 2000;72:1032–1039. doi: 10.1093/ajcn/72.4.1032. [DOI] [PubMed] [Google Scholar]
- 159.Al-Raees GY, Al-Amer M, Shehab M. Nutritional Status of Bahraini Children Aged 2–6 Years old. Juffair, Kingdom of Bahrain: Ministry of Health; 2003. [Google Scholar]
- 160.Administration of Food and Nutrition. Kuwait Nutritional Surveillance System. Murgab, Kuwait: Ministry of Health; 2005. [Google Scholar]
- 161.Alasfoor D, Mohammed AJ. Implications of the use of the new WHO growth charts on the interpretation of malnutrition and obesity in infants and young children in Oman. East Mediterr Health J. 2009;15:890–898. [PubMed] [Google Scholar]
- 162.Kamal AA, Bener A, Kareem Al-Mulla AM. Growth pattern of Qatari preschool children. Croat Med J. 2004;45:461–465. [PubMed] [Google Scholar]
- 163.Food and Agriculture Organization of the United Nations. Nutrition Country Profile – Sudan. Rome, Italy: Food and Nutrition Division, Food and Agriculture Organization of the United Nations; 2005. [Google Scholar]
- 164.United Nations Environment Programme, United Nations Children’s Fund. MICS 111 Survey in 2006. Tunisia: National Office of Population and Family, Ministry of Public Health; 2007. [Google Scholar]
- 165.Bader Z, Musaiger AO, Al-Roomi K, D’Souza R. Overweight and obesity among adolescents in Bahrain. Anthropol Anz. 2008;66:401–408. [PubMed] [Google Scholar]
- 166.National Nutrition Institute. Diet, Nutrition and Prevention of Chronic Non-Communicable Diseases among Egyptian Adolescents. Cairo, Egypt: Ministry of Health; 2008. [Google Scholar]
- 167.Kelishadi R, Ardalan G, Gheiratmand R, et al. Thinness, overweight and obesity in a national sample of Iranian children and adolescents: CASPIAN Study. Child Care Health Dev. 2008;34:44–54. doi: 10.1111/j.1365-2214.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 168.El-Bayoumy I, Shady I, Lotfy H. Prevalence of obesity among adolescents (10 to 14 years) in Kuwait. Asia Pac J Public Health. 2009;21:153–159. doi: 10.1177/1010539509331786. [DOI] [PubMed] [Google Scholar]
- 169.Sibai AM, Hwalla N, Adra N, Rahal B. Prevalence and covariates of obesity in Lebanon: findings from the first epidemiological study. Obes Res. 2003;11:1353–1361. doi: 10.1038/oby.2003.183. [DOI] [PubMed] [Google Scholar]
- 170.Bener A, Kamal AA. Growth patterns of Qatari school children and adolescent aged 6–18 years. J Health Popul Nutr. 2005;23:250–258. [PubMed] [Google Scholar]
- 171.El Mouzan MI, Foster PJ, Al Herbish AS, et al. Prevalence of overweight and obesity in Saudi children and adolescents. Ann Saudi Med. 2010;30:203–208. doi: 10.4103/0256-4947.62833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aounallah-Skhiri H, Romdhane HB, Traissac P, et al. Nutritional status of Tunisian adolescents: associated gender, environmental and socio-economic factors. Public Health Nutr. 2008;11:1306–1317. doi: 10.1017/S1368980008002693. [DOI] [PubMed] [Google Scholar]
- 173.United Arab Emirates Global School-based Student Survey. Abu Dhabi, United Arab Emirates. 2005. [Accessed January 26, 2012]. Available from: http://www.who.int/chp/gshs/2005_United_Arab_Emirates_GSHS_Country_Report.pdf.
- 174.Ministry of Health. National Non-Communicable Diseases Risk Factor Survey, 2007. Juffair, Kingdom of Bahrain: Ministry of Health; 2010. [Google Scholar]
- 175.Janghorbani M, Amini M, Willett WC, et al. First nationwide survey of prevalence of overweight, underweight, and abdominal obesity in Iranian adults. Obesity. 2007;15:2797–2808. doi: 10.1038/oby.2007.332. [DOI] [PubMed] [Google Scholar]
- 176.Ministry of Health. Risk Factors for Non-Communicable Diseases in Kuwait. Murgab, Kuwait: Ministry of Health; 2007. [Google Scholar]
- 177.Food and Agriculture Organization of the United Nations. Nutrition Country Profile – Libyan Arab Jamahiriya. Food and Nutrition Division; 2005. [Accessed January 26, 2012]. Available from: ftp://ftp.fao.org/es/esn/nutrition/ncp/lby.pdf. [Google Scholar]
- 178.Mokhtar N, Elati J, Chabir R, et al. Diet culture and obesity in northern Africa. J Nutr. 2001;131:887S–892S. doi: 10.1093/jn/131.3.887S. [DOI] [PubMed] [Google Scholar]
- 179.Ministry of Health. National Health Survey 2000, Study of Lifestyle Risk Factors. Sultanate of Oman: Ministry of Health; 2000. [Google Scholar]
- 180.Ministry of Health. Country-Specific Standard Report. Riyadh, Saudi Arabia: Ministry of Health; 2010. [Google Scholar]
- 181.El-Ati J, Traissac P, Chiraz B, et al. Change in nutritional status of adult women in Tunisia from 1996 to 2005: effect of living environment and socio-economic factors. Int J Obes. 2008;32(Suppl 1):S215. [Google Scholar]
- 182.Food and Agriculture Organization of the United Nations. Nutrition Country Profile – Palestine. Rome, Italy: Food and Nutrition Division, Food and Agriculture Organization of the United Nations; 2005. [Google Scholar]