Abstract
Purpose
To evaluate whether visual performance could be improved in pseudophakic subjects by correcting low levels of postoperative astigmatism.
Methods
An exploratory, noninterventional study was conducted using subjects who had been implanted with an aspheric intraocular lens and had 0.5–0.75 diopter postoperative astigmatism. Monocular visual performance using full correction was compared with visual performance using spherical equivalent correction. Testing consisted of high- and low-contrast visual acuity, contrast sensitivity, and reading acuity and speed using the Radner Reading Charts.
Results
Thirty-eight of 40 subjects completed testing. Visual acuities at three contrast levels (100%, 25%, and 9%) were significantly better using full correction than when using spherical equivalent correction (all P < 0.001). For contrast sensitivity testing under photopic, mesopic, and mesopic with glare conditions, only one out of twelve outcomes demonstrated a significant improvement with full correction compared with spherical equivalent correction (at six cycles per degree under mesopic without glare conditions, P = 0.046). Mean reading speed was numerically faster with full correction across all print sizes, reaching statistical significance at logarithm of the reading acuity determination (logRAD) 0.2, 0.7, and 1.1 (P < 0.05). Statistically significant differences also favored full correction in logRAD score (P = 0.0376), corrected maximum reading speed (P < 0.001), and logarithm of the minimum angle of resolution/logRAD ratio (P < 0.001).
Conclusions
In this study of pseudophakic subjects with low levels of postoperative astigmatism, full correction yielded significantly better reading performance and high- and low-contrast visual acuity than spherical equivalent correction, suggesting that cataractous patients may benefit from surgical correction of low levels of preoperative corneal astigmatism.
Keywords: aspheric intraocular lens, astigmatism, cataract surgery, contrast sensitivity, reading acuity, visual acuity
Introduction
The majority of patients who undergo cataract surgery have some degree of pre-existing corneal astigmatism, with a recent study reporting that 58% of over 4500 eyes exhibited between 0.25 diopter (D) and 1.0 D of corneal astigmatism.1 Low levels of corneal astigmatism (eg, ≤1.0 D) are often left uncorrected. For example, many peripheral corneal relaxing incision (PCRI) nomograms do not even include specifications for eyes with less than 1 D of preoperative astigmatism,2 and studies of toric intraocular lenses (IOLs) often have inclusion criteria specifying preoperative corneal astigmatism of at least 1 D or more.3 Even if patients with lower levels of corneal astigmatism have not been explicitly excluded from toric IOL studies, their visual outcomes have not been analyzed and presented separately.4,5 Thus, the clinical benefits of correcting low levels of corneal astigmatism are not well established.
Clinical studies have demonstrated that subjects with low levels of astigmatism who were being fitted for contact lenses experienced greater improvements in visual acuity with the use of toric contact lenses compared with either spherical6 or aspheric lenses,7 suggesting that correction of even low levels of astigmatism can positively influence visual outcomes. This study investigated whether improvements in visual performance could be demonstrated by correcting low levels of postoperative astigmatism using full correction, instead of spherical equivalent correction, in pseudophakic subjects.
Materials and methods
This was an exploratory, single-site, noninterventional study of subjects who had been implanted with the aspheric SN60WF IOL (Alcon Laboratories, Inc, Fort Worth, TX) more than 3 months prior to enrollment. The visual performance of these subjects using full correction was compared with their visual performance using spherical equivalent correction. The protocol was approved by the Institutional Review Board, and the study was performed in compliance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. All participating subjects provided written informed consent.
Subjects
Eligible subjects were adults who had undergone surgery for uncomplicated, age-related cataracts (Nuclear Sclerosis Grade 1–4) more than 3 months prior to enrollment. They also had to have a best-corrected Snellen visual acuity of 20/20 or better, 0.5–0.75 D of astigmatism at the spectacle plane (as measured with manifest refraction), and proof of an eye examination in the last year. One operative eye per subject was included. If both eyes qualified for inclusion, the dominant eye was selected.
Subjects were excluded if they met any of the following criteria: history of intraoperative complications during cataract surgery, extremes of axial length (<22 mm or >25 mm), amblyopia, corneal dystrophy, previous corneal transplant or retinal detachment, previous ocular or refractive surgery/trauma (other than cataract surgery), recurrent severe anterior or posterior segment inflammation of unknown etiology, uncontrolled glaucoma, uveitis, optic nerve atrophy, pregnancy, or diagnosed degenerative visual disorders that were predicted to cause future visual acuity losses greater than 0.2 logarithm of the minimum angle of resolution (logMAR).
Testing methods
All subjects received monocular visual testing in the study eye using both full correction and spherical equivalent correction; this design allowed each subject to serve as his or her own control. Testing consisted of high-contrast and low-contrast visual acuity, contrast sensitivity, and reading acuity and speed.
High-contrast visual acuity was tested using a 100% contrast Early Treatment Diabetic Retinopathy Study (ETDRS) chart under photopic (160 cd/m2) conditions at a distance of 4 meters. Low-contrast visual acuity was tested using 25% and 9% contrast ETDRS charts under photopic conditions at a distance of 4 meters.
Contrast sensitivity was tested at three, six, twelve, and 18 cycles per degree (cpd) using the CSV-1000E contrast sensitivity chart (VectorVision Inc, Greenville, OH) at a distance of 8 feet. Contrast sensitivity testing was conducted under photopic (chart luminance of 85 cd/m2) and mesopic (chart luminance of 3 cd/m2) conditions without glare and mesopic conditions with glare source.
Reading acuity and speed were measured with the Radner Reading Charts, a series of sentence optotypes that vary in size by 0.1 log unit steps.8 The visual acuity correction used for these assessments was best near power for reading at 40 centimeters. Logarithm of the reading acuity determination (logRAD) score was recorded for the sentence with the smallest print size that was read completely. Corrected logRAD score was determined by accounting for reading errors (total number of incorrectly read syllables × 0.005 + uncorrected logRAD score). Reading speed in words/minute was calculated by measuring the time taken to read a sentence from the Radner Reading Chart, each of which has 14 words (14 words ÷ X seconds × 60 seconds/minute). Corrected reading speed was calculated by subtracting words missed from the words/minute calculation. Both maximum and mean reading speeds were calculated. Critical print size was measured as the print size at which maximum reading speed was achieved. Corrected critical print size was determined by accounting for reading errors (total number of incorrectly read syllables × 0.005 + uncorrected critical print size). The logMAR/logRAD ratio was defined as (1 − logRAD) × 100 ÷ (1 − logMAR).
Statistics
Paired t-tests were used to estimate the difference in outcomes for full correction versus spherical equivalent correction. Analysis of variance was used to examine the least-squares mean differences between full correction and spherical equivalent correction for reading speed at each print size. With a sample size of 40 eyes, a two-sided 95% confidence interval on the mean was estimated to within ±0.45 standard deviations of the measurement. Results are presented as mean ± standard error of the mean unless otherwise noted.
Results
Forty subjects were enrolled in this study. Twenty-four (60%) were female and 16 (40%) were male. The mean age of these subjects was 70 ± 5 years. The mean prescription had −0.58 ± 0.06 D of spherical correction and 0.59 ± 0.02 D of cylindrical correction. Thirty-eight subjects completed testing.
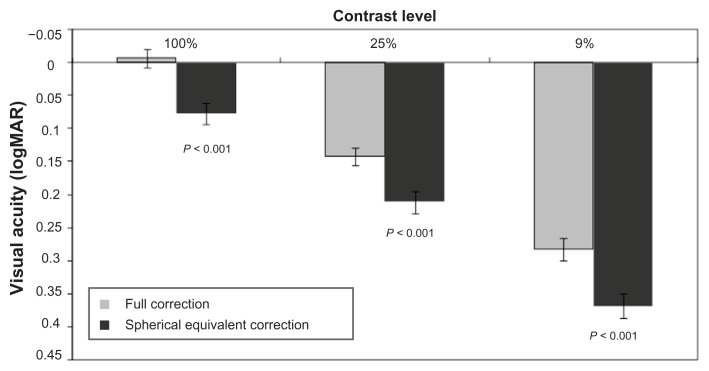
High-contrast visual acuity (100% contrast) using full correction was −0.01 ± 0.01 logMAR compared with 0.08 ± 0.02 logMAR using only spherical equivalent correction, which was a statistically significant difference favoring full correction (P < 0.001; Figure 1). Low-contrast visual acuity using 25% contrast was significantly better with full correction than with spherical equivalent correction (0.14 ± 0.01 logMAR vs 0.21 ± 0.02 logMAR, P < 0.001), as was low-contrast visual acuity using 9% contrast (0.28 ± 0.02 logMAR vs 0.37 ± 0.02 logMAR, P < 0.001).
Figure 1.
High-contrast (100%) and low-contrast (25% and 9%) visual acuity with full correction versus spherical equivalent correction (N = 37).
Note: Error bars represent standard error of the mean.
Abbreviation: logMAR, logarithm of the minimum angle of resolution.
No statistically significant differences between full correction and spherical equivalent correction were observed for contrast sensitivity performed under photopic conditions (Table 1). Under mesopic conditions, the six cpd spatial frequency demonstrated a significant improvement with full correction (P = 0.046). When a glare source was added to the mesopic condition, none of the spatial frequencies exhibited a significant difference between correction conditions.
Table 1.
Mean differences in contrast sensitivity between full correction and spherical equivalent correction (N = 38)
| Contrast sensitivity condition | Mean differencea ± SEM (logMAR) | |||
|---|---|---|---|---|
|
|
||||
| 3 cpd | 6 cpd | 12 cpd | 18 cpd | |
| Photopic without glare | 0.008 ± 0.04 | 0.01 ± 0.04 | 0.01 ± 0.06 | 0.02 ± 0.05 |
| Mesopic without glare | −0.05 ± 0.05 | 0.12 ± 0.06b | 0.03 ± 0.06 | 0.02 ± 0.05 |
| Mesopic with glare | 0.02 ± 0.04 | 0.03 ± 0.05 | 0.03 ± 0.045 | 0.07 ± 0.04 |
Notes:
Mean difference = score with full correction – score with spherical equivalent correction;
P = 0.046.
Abbreviations: cpd, cycles per degree; logMAR, logarithm of the minimum angle of resolution; SEM, standard error of the mean.
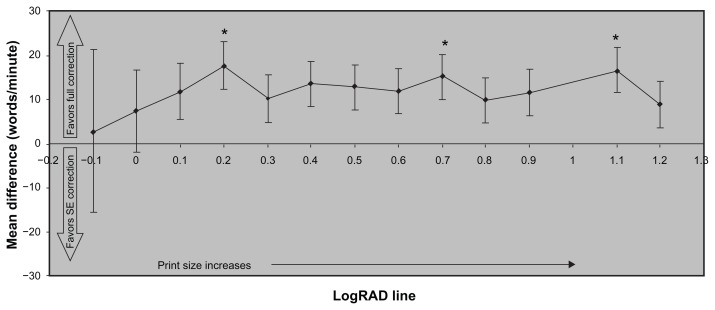
Across all print sizes on the Radner Reading Chart, the mean reading speed was numerically faster with full correction than with spherical equivalent correction, reaching a statistically significant difference at print sizes of logRAD 0.2, 0.7, and 1.1 (P < 0.05; Figure 2). Statistically significant differences also favored full correction over spherical equivalent correction in logRAD score (P = 0.0376), corrected maximum reading speed (P < 0.001), and logMAR/logRAD ratio (P < 0.001; Table 2). No significant differences between correction conditions were noted for critical print size.
Figure 2.
Mean differences in reading speed across print sizes between use of full correction and spherical equivalent correction (N = 37).
Notes: Error bars represent standard error of the mean; *Hommel’s adjusted P value, P < 0.05.
Abbreviation: logRAD, logarithm of the reading acuity determination; SE, spherical equivalent.
Table 2.
Mean differences in additional Radner reading test outcomes between full correction and spherical equivalent correction (N = 37)
| Outcome | Mean differencea 95% confidence limit (lower, upper) | P value |
|---|---|---|
| Corrected critical print size, logRAD | 0.02 (−0.09, 0.14) | 0.6803 |
| Reading acuity @ 40 cm, logRAD | −0.02 (−0.04, −0.001) | 0.0376b |
| Corrected maximum reading speed, words/minute | 19.2 (10.4, 28.0) | <0.001b |
| LogMAR/logRAD ratio | −6.54 (−9.95, −3.12) | <0.001b |
Notes:
Mean difference = score with full correction – score with spherical equivalent correction;
P values signify statistically significant differences, all of which favored outcomes using full correction.
Abbreviations: logMAR, logarithm of the minimum angle of resolution; logRAD, logarithm of the reading acuity determination.
Discussion
Moderate to high levels of corneal astigmatism in patients with cataract have conventionally been corrected with spectacles after surgery or with toric IOLs or PCRIs (also called limbal relaxing incisions) at the time of cataract surgery. For lower levels of corneal astigmatism (eg, <1 D), few studies have been published examining the visual outcomes of cataract patients who have had low levels of astigmatism surgically corrected. Of these studies, most reported only astigmatic reduction and postoperative uncorrected and/or best-corrected visual acuities;9–11 another study reported on postoperative higher-order aberrations.12 The current study focused on visual performance parameters, including contrast visual acuity, contrast sensitivity, and reading performance. Specifically, it examined the postoperative visual performance of pseudophakic subjects having low levels of residual astigmatism after applying either full correction or spherical equivalent correction in order to isolate the impact of astigmatic correction on several performance parameters. Little difference in contrast sensitivity was observed between full correction and spherical equivalent correction, with a significant improvement observed with full correction for only one of twelve outcomes (perception of four spatial densities tested under three lighting conditions). This is consistent with the results from a previous study, which reported that contrast sensitivity was not affected by low levels of astigmatism (0.25–1.0 D).13 The current study did demonstrate that subjects achieved significant improvements in visual acuity under high- and low-contrast conditions and in reading performance when astigmatism was corrected compared with spherical equivalent correction alone. The fact that these subjects could benefit from postoperative correction of low-level astigmatism suggests that they may also have benefited from surgical correction of their pre-existing astigmatism at the time of cataract surgery.
Astigmatism can be corrected during cataract surgery in a number of ways, including placement of the phacoemulsification incision on the steepest meridian of the cornea (commonly referred to as the steep axis), use of opposite clear corneal incisions (OCCIs), use of PCRIs, and implantation of a toric IOL.14 Placement of the incision on the steep axis is attractive due to its simplicity; however, outcomes can be highly variable.15 Variability in surgically induced astigmatism has been correlated with age, preoperative astigmatism, and postoperative IOP.16 This type of on-axis incision is performed primarily in patients with low levels of astigmatism, because it can correct only approximately 0.5–0.6 D of astigmatism.14,17 Paired on-axis OCCIs can provide additional astigmatic correction and thus are typically used when higher levels of astigmatism are present.17,18 Although on-axis incisions are meant to reduce corneal astigmatism, in one recent study these incisions produced similar proportions of eyes having an increase in residual astigmatism compared with conventional temporal incisions.15 In another study they produced significantly more axis shifts greater than 30° than did temporal incisions (P < 0.05).19
Compared with on-axis incisions, PCRIs have been reported to be less variable and more stable over time.20 In a consecutive case series studying the predictability of PCRIs, 54% of eyes with low-level astigmatism (0.5 D or 1.0 D) exhibited a postoperative increase, rather than decrease, in cylinder magnitude with PCRIs.2 Most PCRI nomograms do not currently include specifications for eyes with less than 1 D of pre-existing astigmatism.2 In addition to the challenges of variability, lack of precision, and limited range, both on-axis incisions and PCRIs also have the potential to induce complications associated with an additional surgical step, including delayed wound healing, corneal epithelial defects, and exacerbation of dry-eye symptoms.21 These surgical approaches require additional surgical skill and operating time to achieve desired results.
Toric IOL implantation has the advantage of bypassing an additional surgical interventional step and has been shown to produce superior astigmatic correction compared with both OCCIs22 and PCRIs.23 Toric IOLs have also been shown to provide lower refractive cylinder/postoperative astigmatism24 and superior uncorrected visual acuity than spherical IOLs in patients with low levels of astigmatism.11,25 The main disadvantage of toric IOLs is the potential for IOL misalignment to reduce the astigmatic correction or even, in some cases, to worsen preoperative astigmatism. However, rotational stability has improved over time as better haptics have been introduced. For instance, two recent studies of toric IOLs each reported a mean axis rotation of less than 4° and IOL rotation between 10° and 20° in a small proportion of eyes (1% [N = 100] and 6.7% [N = 243]).4,5
The current study does have some limitations. First, the population consisted of pseudophakic subjects having low levels of postoperative astigmatism, whereas other studies of low astigmatism focused on pre-existing astigmatism. In the current study, preoperative astigmatic levels were not reported and were almost certainly different from postoperative levels. Furthermore, the astigmatism was corrected only with phoropter lenses, rather than surgically. Thus, although these results do not allow us to draw firm conclusions regarding the ability of surgical correction to improve visual performance in patients with preoperative low levels of corneal astigmatism, they do strongly suggest that surgical correction of astigmatism less than 1 D should be explored with comparative clinical studies.
Conclusion
In this study of pseudophakic subjects with low levels of postoperative astigmatism, full correction of astigmatism was significantly better than spherical equivalent correction for outcomes of reading performance, high-contrast visual acuity, and low-contrast visual acuity, though most contrast sensitivity outcomes indicated no difference between full correction and spherical equivalent correction. These results indicate that correcting low levels of preoperative astigmatism at the time of cataract surgery may be efficacious in providing optimal visual outcomes; however, the balance of safety with this expected efficacy should be investigated in a randomized, controlled manner.
Acknowledgments
This study was supported by Alcon Laboratories, Inc. Medical writing assistance was provided by Jennifer Klem PhD and was funded by Alcon Laboratories, Inc.
Footnotes
Disclosures
This study has not been previously presented. The authors have no proprietary interest in this study. Diane Houtman is an employee of Alcon Laboratories, Inc. Robert Lehmann provides consulting services to Alcon Laboratories, Inc.
References
- 1.Ferrer-Blasco T, Montés-Micó R, Peixoto-de-Matos SC, González-Méijome JM, Cerviño A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–75. doi: 10.1016/j.jcrs.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 2.Gills JP, Wallace RB, Miller K, et al. Reducing pre-existing astigmatism with limbal relaxing incisions. In: Gills JP, editor. A complete surgical guide for correcting astigmatism: an ophthalmic manifesto. Thorofare, NJ: Slack, Inc; 2003. pp. 99–119. [Google Scholar]
- 3.Bauer NJ, de Vries NE, Webers CA, Hendrikse F, Nuijts RM. Astigmatism management in cataract surgery with the AcrySof toric intraocular lens. J Cataract Refract Surg. 2008;34(9):1483–1488. doi: 10.1016/j.jcrs.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM. The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism: a randomized, subject-masked, parallel-group, 1-year study. Ophthalmology. 2010;117(11):2104–2111. doi: 10.1016/j.ophtha.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 5.Chang DF. Comparative rotational stability of single-piece open-loop acrylic and plate-haptic silicone toric intraocular lenses. J Cataract Refract Surg. 2008;34(11):1842–1847. doi: 10.1016/j.jcrs.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Richdale K, Berntsen DA, Mack CJ, Merchea MM, Barr JT. Visual acuity with spherical and toric soft contact lenses in low- to moderate-astigmatic eyes. Optom Vis Sci. 2007;84(10):969–975. doi: 10.1097/OPX.0b013e318157c6dc. [DOI] [PubMed] [Google Scholar]
- 7.Morgan PB, Efron SE, Efron N, Hill EA. Inefficacy of aspheric soft contact lenses for the correction of low levels of astigmatism. Optom Vis Sci. 2005;82(9):823–828. doi: 10.1097/01.opx.0000177792.62460.58. [DOI] [PubMed] [Google Scholar]
- 8.Stifter E, König F, Lang T, et al. Reliability of a standardized reading chart system: variance component analysis, test-retest and inter-chart reliability. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):31–39. doi: 10.1007/s00417-003-0776-8. [DOI] [PubMed] [Google Scholar]
- 9.Kremer I, Gabbay U, Blumenthal M. One-year follow-up results of photorefractive keratectomy for low, moderate, and high primary astigmatism. Ophthalmology. 1996;103(5):741–748. doi: 10.1016/s0161-6420(96)30621-0. [DOI] [PubMed] [Google Scholar]
- 10.Schnitzler EM, Kohnen T, Steinkamp GW, Ohrloff C. Photoastigmatic refractive keratectomy for low, moderate, and high astigmatism using a broad beam excimer laser: evaluation according to new international criteria. Klin Monbl Augenheilkd. 1999;215(5):267–274. doi: 10.1055/s-2008-1034713. [DOI] [PubMed] [Google Scholar]
- 11.Statham M, Apel A, Stephensen D. Comparison of the AcrySof SA60 spherical intraocular lens and the AcrySof Toric SN60T3 intraocular lens outcomes in patients with low amounts of corneal astigmatism. Clin Experiment Ophthalmol. 2009;37(8):775–779. doi: 10.1111/j.1442-9071.2009.02154.x. [DOI] [PubMed] [Google Scholar]
- 12.Wigledowska-Promienska D, Zawojska I. Changes in higher order aberrations after wavefront-guided PRK for correction of low to moderate myopia and myopic astigmatism: two-year follow-up. Eur J Ophthalmol. 2007;17(4):507–514. doi: 10.1177/112067210701700405. [DOI] [PubMed] [Google Scholar]
- 13.Zheng GY, Du J, Zhang JS, et al. Contrast sensitivity and higher-order aberrations in patients with astigmatism. Chin Med J (Engl) 2007;120(10):882–885. [PubMed] [Google Scholar]
- 14.Amesbury EC, Miller KM. Correction of astigmatism at the time of cataract surgery. Curr Opin Ophthalmol. 2009;20(1):19–24. doi: 10.1097/ICU.0b013e328319c27a. [DOI] [PubMed] [Google Scholar]
- 15.Borasio E, Mehta JS, Maurino V. Torque and flattening effects of clear corneal temporal and on-axis incisions for phacoemulsification. J Cataract Refract Surg. 2006;32(12):2030–2038. doi: 10.1016/j.jcrs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Storr-Paulsen A, Madsen H, Perriard A. Possible factors modifying the surgically induced astigmatism in cataract surgery. Acta Ophthalmol Scand. 1999;77(5):548–551. doi: 10.1034/j.1600-0420.1999.770513.x. [DOI] [PubMed] [Google Scholar]
- 17.Khokhar S, Lohiya P, Murugiesan V, Panda A. Corneal astigmatism correction with opposite clear corneal incisions or single clear corneal incision: comparative analysis. J Cataract Refract Surg. 2006;32(9):1432–1437. doi: 10.1016/j.jcrs.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Lever J, Dahan E. Opposite clear corneal incisions to correct pre-existing astigmatism in cataract surgery. J Cataract Refract Surg. 2000;26(6):803–805. doi: 10.1016/s0886-3350(00)00378-3. [DOI] [PubMed] [Google Scholar]
- 19.Borasio E, Mehta JS, Tacker M, Maurino V. Astigmatism axis shift after sutureless phacoemulsification: Comparison between clear-corneal temporal and clear-corneal on-axis incision. Invest Ophthalmol Vis Sci. 2005;46 E-Abstract 780. [Google Scholar]
- 20.Kaufmann C, Peter J, Ooi K, Phipps S, Cooper P, Goggin M. Limbal relaxing incisions versus on-axis incisions to reduce corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2005;31(12):2261–2265. doi: 10.1016/j.jcrs.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 21.Mamalis N. Correction of astigmatism during cataract surgery. J Cataract Refract Surg. 2009;35(3):403–404. doi: 10.1016/j.jcrs.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Mendicute J, Irigoyen C, Ruiz M, Illarramendi I, Ferrer-Blasco T, Montés-Micó R. Toric intraocular lens versus opposite clear corneal incisions to correct astigmatism in eyes having cataract surgery. J Cataract Refract Surg. 2009;35(3):451–458. doi: 10.1016/j.jcrs.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 23.Poll JT, Wang L, Koch DD, Weikert MP. Correction of astigmatism during cataract surgery: Toric intraocular lens compared to peripheral corneal relaxing incisions. J Refract Surg. 2011;27(3):165–171. doi: 10.3928/1081597X-20100526-01. [DOI] [PubMed] [Google Scholar]
- 24.Ernest P, Potvin R. The effects of preoperative corneal astigmatism orientation on results with a low cylinder power toric IOL. J Cataract Refract Surg. 2011;37(4):727–732. doi: 10.1016/j.jcrs.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 25.Lane SS, Ernest P, Miller KM, Hileman KS, Harris B, Waycaster CR. Comparison of clinical and patient-reported outcomes with bilateral AcrySof toric or spherical control intraocular lenses. J Refract Surg. 2009;25(10):899–901. doi: 10.3928/1081597X-20090617-05. [DOI] [PubMed] [Google Scholar]