SUMMARY
Behavior cannot be predicted from a “connectome,” because the brain contains a chemical “map” of neuromodulation superimposed upon its synaptic connectivity map. Neuromodulation changes how neural circuits process information in different states, such as hunger or arousal. Here we describe a novel, genetically based method to map, in an unbiased and brain-wide manner, sites of neuromodulation under different conditions in the Drosophila brain. This method, and genetic perturbations, reveal that the well-known effect of hunger to enhance behavioral sensitivity to sugar is mediated, at least in part, by the release of dopamine onto primary gustatory sensory neurons, which enhances sugar-evoked calcium influx. These data reinforce the concept that sensory neurons constitute an important locus for state-dependent gain-control of behavior, and introduce a new methodology that can be extended to other neuromodulators and model organisms.
INTRODUCTION
The physiological responses of an animal’s nervous system to sensory stimuli can differ, depending on internal states such as hunger or arousal (Chiappe et al., 2010; Dubner, 1988; Maimon et al., 2010; Niell and Stryker, 2010; Shea and Margoliash, 2010; Tsuno and Mori, 2009). Such state-dependent influences enable animals to adjust their behavioral response to metabolic, emotional, attentional or other demands. Neuromodulators, such as biogenic amines and acetylcholine, as well as neuropeptides, play a major role in encoding or mediating internal states (Harris-Warrick and Marder, 1991; Pfaff et al., 2008), by altering the input-output properties of specific neural circuits (Birmingham and Tauck, 2003; Marder and Bucher, 2007).
Hunger and satiety represent a prototypic model for an internal state(s) that influences behavior. In the vinegar fly Drosophila melanogaster, for example, food deprivation is known to affect olfactory sensitivity (Root et al., 2011), formation and expression of food-associated memory (Krashes et al., 2009), the extent of feeding (Riemensperger et al., 2011) and locomotor activity (Lee and Park, 2004; Meunier et al., 2007). In addition, in Drosophila (Scheiner et al., 2004) as well as in other species (Berridge, 1991; Dethier, 1976; Gillette et al., 2000; Moskowitz et al., 1976; Moss and Dethier, 1983; Page et al., 1998) starvation changes the consummatory response to tastants, typically by enhancing the acceptance of energy resources such as sugar, with an associated increased tolerance for bitter-tasting contaminants. This dramatic starvation-dependent shift in sensitivity to sweet vs. unpalatable and potentially toxic energy resources illustrates how state-dependent control of behavior is critical for survival.
Despite the importance of hunger for regulating animal behavior, we know relatively little about the circuit-level mechanisms underlying such regulation. Studies in blowflies and honeybees have demonstrated that biogenic amines can modulate feeding-related behaviors (Brookhart et al., 1987; Long et al., 1986; Scheiner et al., 2002). Whether such modulators actually mediate the effect of hunger on these behaviors, however, has been more difficult to establish in these systems due to the lack of genetic tools. It has also been challenging to identify the circuitry through which such modulators mediate behavioral responses to starvation. Modulatory neurons often exhibit widespread projections throughout the brain (Mao and Davis, 2009; Monastirioti, 1999) and act via multiple receptors. Identifying the behaviorally relevant circuitry on which a given modulator acts, and demonstrating that such modulation is required for a specific state-dependent influence on a specific behavior in vivo, has been achieved in only a few cases (Crocker et al., 2010; Kong et al., 2010; Krashes et al., 2009; Lebestky et al., 2009; Root et al., 2011).
Drosophila provides an attractive system to address the circuit-level mechanisms underlying neuromodulation of feeding behavior, because of the availability of powerful genetic tools and our growing understanding of the gustatory receptors and neural circuitry that control feeding in this species (Dahanukar et al., 2007; Gordon and Scott, 2009; Marella et al., 2006; Montell, 2009; Scott et al., 2001; Thorne et al., 2004; Wang et al., 2004; Weiss et al., 2011). Although several neuropeptides, as well as biogenic amines, have been implicated in mediating the influence of food-deprivation on feeding behavior in Drosophila (Nassel and Winther, 2010), with few exceptions (Root et al., 2011; Wu et al., 2005) the circuit-level mechanisms underlying their influences remains poorly understood.
Here we have developed and applied a method, called TANGO-map, to detect the release of endogenous neuromodulators in vivo, and identify the circuits on which they act. We have used this method to examine the mechanisms that underlie a starvation-induced change in a feeding behavior in Drosophila. Our results identify a hunger-dependent, dopamine-mediated gain-control of behavior at the level of primary gustatory sensory neurons. They also provide proof-of-principle for a methodology that may have general applicability in the genetic dissection of circuit-level neuromodulatory mechanisms.
RESULTS
Design and validation of a Drosophila Dopamine Receptor-Tango system in vitro
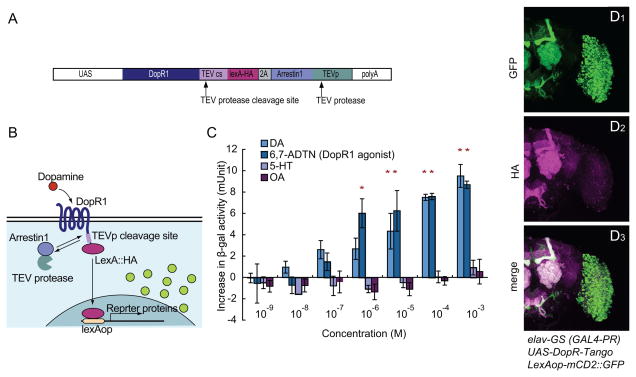
We sought to develop a genetically based tool that reports endogenous neuromodulator release and sites of action in vivo, with anatomic specificity. To do this, we adapted to Drosophila the Tango system (Barnea et al., 2008), which transforms a transient ligand/receptor interaction into a stable, anatomical readout of reporter gene expression. The reporter gene is activated by a “private,” synthetic signal transduction pathway, using a bacterial transcription factor (lexA) that is covalently coupled (via a specific tobacco etch virus (TEV) protease-sensitive cleavage site) to the exogenous dopamine receptor expressed in the cells of interest (Fig. 1B). The transcription factor is cleaved from the dopamine receptor following ligand binding, by recruitment of an arrestin-TEV protease fusion protein, and translocates to the nucleus where it activates a lexAop-driven reporter. This system was originally developed to detect receptor activation in cultured mammalian cell lines (Barnea et al., 2008), but whether it could also be used to detect receptor activation in vivo was not clear.
Figure 1. Characterization of DopR-Tango in vitro and in Drosophila.
(A) Design of the DopR-Tango transgene; note HA epitope tag on LexA.
(B) Schematic illustrating DopR-Tango mechanism.
(C) DopR-Tango reporter (β-gal) activity in response to indicated ligands in HEK293 cells co-transfected with CMV-GAL4, UAS-DopR-Tango and LexAop-β-gal. Increases in β-gal activity relative to background are shown. Error bars represent the standard error of mean (SEM). Asterisks represent statistically significant increases (p<0.05, t-test with Bonferroni correction, n=3).
(D) Representative confocal projections of whole-mount brains from DopR-Tango flies visualized with GFP native fluorescence (green) and anti-HA immunostaining (magenta).
To adapt this system to identify circuit-level sites of endogenous neuromodulator action in Drosophila in vivo, we generated a Tango system for dopamine (DA) (DopR-Tango), using the Drosophila DA receptor DopR1 (Gotzes et al., 1994; Sugamori et al., 1995) and Drosophila Arrestin1 (Figure 1A). Here, LexA is used as the tethered transcription factor. Stoichiometric co-expression of the Arrestin-TEV protease fusion was achieved using a 2A peptide (Szymczak and Vignali, 2005), which we have shown to permit bi-cistronic expression in Drosophila (Figure S1A–C).
To test whether DopR-Tango specifically reports cellular activation by DA, we co-expressed DopR-Tango in human embryonic kidney (HEK) 293 cells with a lexAop-β-galactosidase (β-gal) reporter. Treatment of these cells with DA or a DopR1 agonist (6,7-ADTN) resulted in a dose-dependent increase in reporter gene expression (Figure 1C). The EC50 of DopR1-Tango to DA and the D1 agonist are c.a. 1 μM in this experiment, similar to values previously reported in insect cell lines (Sugamori et al., 1995). In contrast, neuromodulators that are not ligands for DopR1, such as 5-HT or Octopamine (OA), did not induce reporter gene expression (Figure 1C). Together, these results indicate that (1) a Drosophila DA receptor and arrestin can be successfully used to generate a functional Tango system; (2) Drosophila DopR-Tango can activate reporter expression in response to DA receptor ligands, in a dose-dependent manner and (3) DopR-Tango maintains the ligand specificity of the original DA receptor. Analogous results in HEK293 cells were obtained with a Tango system constructed using a Drosophila OA receptor (OctR-Tango) (data not shown).
DopR-Tango induces reporter expression in a ligand-specific manner in Drosophila in vivo
We chose Drosophila as a model to test whether the Tango system can report ligand activity in vivo. To do this, we generated transgenic flies that express DopR-Tango components under the control of elav-GeneSwitch (elav-GS), a pan-neuronally expressed, hormone- (RU486) inducible form of GAL4 (GAL4-PR) (Osterwalder et al., 2001). This transgenic line (referred to subsequently as “DopR-Tango flies”), also contains a lexAop-mCD2::GFP transgene that encodes a membrane-tethered form of GFP, as the Tango reporter. The use of an inducible GAL4 was based on the assumption that background signal would be minimized by restricting expression of the DopR-Tango system to a 24 hr period just prior to the experimental manipulation, thereby avoiding developmental accumulation of the reporter.
After feeding with RU486 for 12–24 hrs, widespread expression of DopR-Tango was detected throughout the brain by immunostaining with an antibody to an HA epitope-tag present on LexA (Figure 1D2). Importantly, widespread brain expression of the GFP Tango reporter was also observed (Figure 1D1), beginning at 12 hr and peaking at 36 hrs after the onset of Tango expression (Figure S1E and Supplemental footnote 1). The pattern of reporter expression was not identical to that of the HA-tag, due to the different subcellular localization of the two markers (membrane vs. nuclear; Figure 1D3). Expression of the GFP reporter was not detected in control flies that expressed DopR fused to LexA without the Arrestin-TEV protease fusion protein (Figure S1D). These data indicate that GFP expression in DopR-Tango flies is Arrestin-TEV protease-dependent, and not due to basal transcription of the lexAop-mCD2::GFP reporter transgene, or TEV-protease independent cleavage of TEVcs-LexA.
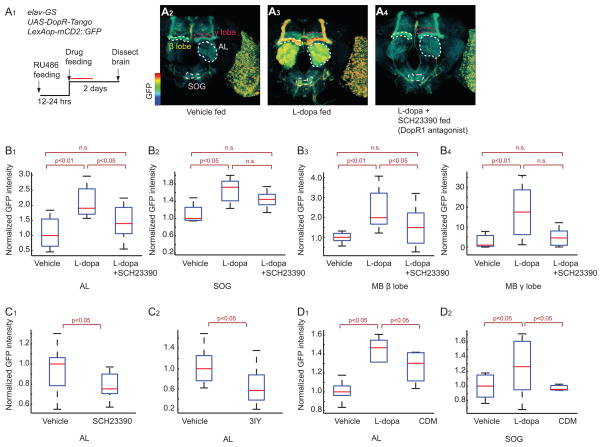
To investigate if Tango reporter expression in flies can report changes in levels of endogenous DA signaling, we examined expression of the reporter after drug treatments. Feeding DopR-Tango flies with L-dopa, a precursor of DA that is known to increase DA levels in the fly brain (Bainton et al., 2000), for 2 days after RU486 treatment, caused a statistically significant increase in reporter expression in various neural structures including the antennal lobe (AL), the sub-oesophageal ganglion (SOG), and β and γ lobes of the mushroom body (MB) ((Figure 2A2–3, 2B1–4, S2E; see Figure S2A–C for details of GFP reporter quantification). This increase, moreover, was reduced by SCH23390 (Sugamori et al., 1995), a D1 receptor antagonist, to a statistically significant extent in the AL (Figure 2B1) and MB β lobe (Figure 2B3), and exhibited a trend to reduction that did not reach significance in the SOG (Figure 2B2) and MB γ lobe (Figure 2B4). The dynamic range of this reporter (2–15 fold; Figure 2B1–4) is similar to that of the best currently available genetically encoded calcium indicators (GECIs) (Tian et al., 2009), although the signal-to-noise ratio (SNR; c.a. 4) is lower (see Supplemental footnote 2). These data confirm that DopR-Tango can read out a statistically significant increase in reporter gene expression in response to an experimentally induced increase in DA levels in vivo.
Figure 2. Characterization of DopR-Tango in transgenic flies.
(A). Specific activation of DopR-Tango by L-dopa in vivo. (A1) experimental design. Red line represents 24 hr detection window for Tango reporter (see Figure S1E and supplemental footnote 1). (A2–4) pseudocolor images of DopR-Tango reporter (GFP) expression; color scale to left. See Figure S2 for image processing details. Neuropils indicated by dashed outlines are: AL, Antennal Lobe (white); SOG, suboesophageal ganglion (pink); mushroom body (MB) β and γ lobes (yellow and red, respectively). (B–D). Quantification of reporter expression in the indicated neuropils. SCH23390, D1 receptor antagonist; 3IY (3-iodotyrosine, DA synthesis inhibitor). Unless otherwise indicated, p values in this and subsequent figures represent Kruskal-Wallis one-way ANOVA followed by Mann-Whitney U-tests with Bonferroni correction. n>5 for each experimental group. Boxplots: lower and upper whiskers represent 1.5 interquartile-range (IQR) of the lower and upper quartiles, respectively; boxes indicate lower quartile, median and upper quartile, from bottom to top.
We also investigated the source of the baseline expression of the Tango reporter observed in un-manipulated flies (Figure 2A2). Genetic elimination of DA in DopR-Tango flies was not feasible, as null mutations in Tyrosine hydroxylase (Th) are embryonic lethal (Riemensperger et al., 2011). Instead, we fed flies with SCH23390, or the DA synthesis inhibitor, 3-iodotyrosine (3IY) (Bainton et al., 2000). SCH23390 feeding significantly decreased, but did not abolish, Tango reporter expression in both the AL and SOG (Figure 2C1 and 3C1). 3IY feeding also decreased reporter expression in the AL (Figure 2C2) in statistically significant manner, but the decrease in the SOG did not reach significance (Figure 3C2). The incomplete effects of the antagonist to inhibit basal (as well as L-dopa-induced; Figure 2B1–4) expression of the reporter may reflect limits on the effective levels of the drug that can be achieved in vivo, due to instability, non-specific absorption or toxicity. Alternatively, it may reflect some level of DA-independent expression of the Tango reporter, for example due to ligand-independent binding of Arrestin-TEVp to DopR-Tango. Whatever the explanation, these results indicate that the level of baseline GFP reporter expression in DopR-Tango flies is, at least in part, a reflection of endogenous DA signaling in the brain.
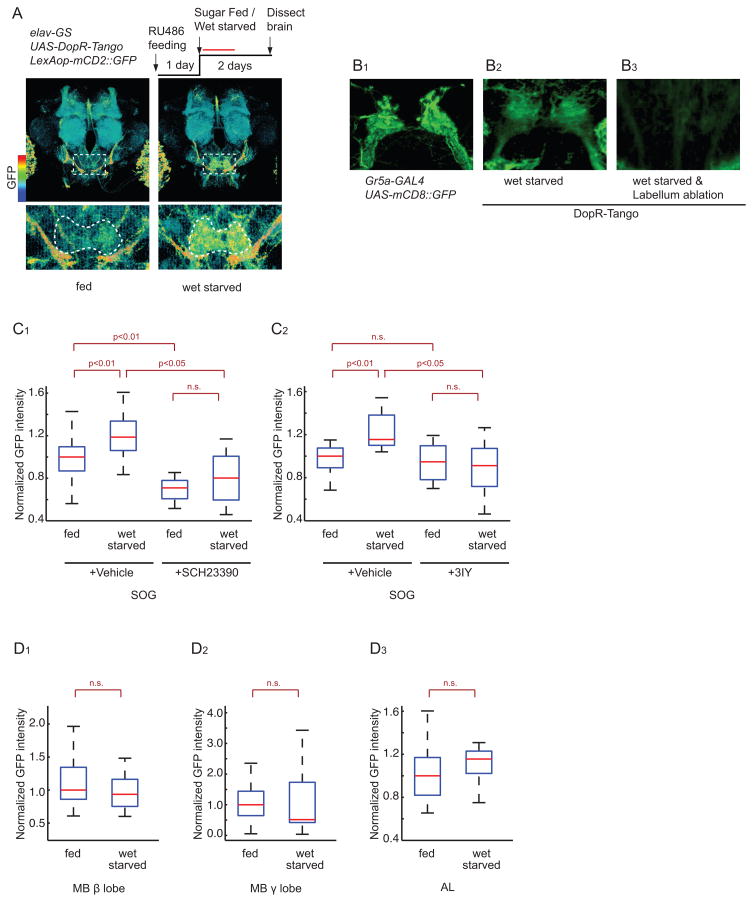
Figure 3. DA release onto GRNs increases during starvation.
(A) Experimental design and normalized Tango reporter (GFP) expression in brains of fed vs. 48-hr wet-starved flies; color scale to left. Laser scanning was performed at a higher gain setting to increase sensitivity. Dashed boxes delineate SOG (enlarged in lower panels). White dashed line in lower panels show ROIs used for quantification, based on UAS-DsRed expression in SOG neuropil.
(B) Representative confocal projections of sugar-sensing GRNs (B1), and Tango reporter expression (B2, B3) in the SOG of normal (B1, B2) or labellum-ablated (B3) flies.
(C, D) Normalized GFP expression in DopR-Tango flies quantified in the SOG (C1–2), MB β lobe (D1), MB γ lobe (D2) and AL (D3). n >6 for each experimental group.
DopR-Tango reporter expression also exhibited ligand specificity in vivo. When DopR-Tango flies were fed with either L-dopa or chlordimeform (CDM), an OA receptor agonist, only L-dopa feeding increased expression of the reporter in the SOG (Figure 2D2). L-dopa feeding also yielded an increase in DopR-Tango reporter signal in the AL (Figure 2D1), but in this case a smaller but still significant induction was observed using CDM. This difference may reflect an indirect effect of CDM to increase dopaminergic signaling in the AL, given that OA did not activate DopR-Tango in vitro (Figure 1C). In OctR-Tango flies fed with L-dopa or CDM, only CDM increased expression of the GFP reporter in the AL (Figure S2D). These data suggest that in vivo, as well as in HEK293 cells, DopR-Tango can specifically report an artificially induced increase in DA signaling
DopR-Tango reveals increased dopamine release onto primary gustatory neurons during starvation
To investigate whether DopR-Tango can identify neural circuits that are targets of modulation by endogenous DA, we exposed DopR-Tango flies to various treatments and looked for increases in reporter expression. Wet starvation of DopR-Tango flies for 2 days produced a statistically significant increase in GFP expression in the SOG, the primary gustatory center (Figure 3A and 3C1–2), but not in the MB β and γ lobes or the AL (Figure 3D1–3). Inclusion of the DopR antagonist SCH23390, or the DA synthesis inhibitor 3IY, abolished the starvation-induced increase in GFP expression in the SOG (Figure 3C1–2). Based on the time-course of Tango reporter expression, we estimate that the enhanced GFP expression likely reflects cumulative DopR-Tango activation integrated over the first 24 hrs of food deprivation (Figure S1E and Supplemental footnote 1).
Two lines of evidence suggest that the starvation-induced increase in GFP expression in the SOG occurs, at least in part, in the terminals of primary gustatory receptor neurons (GRNs). First, the pattern of Tango reporter expression in the SOG resembled that of the projections of sugar-sensing GRNs, as visualized using a Gr5-GAL4 transgene specifically expressed in these neurons (Wang et al., 2004) to drive mCD8::GFP expression (Figure 3B1). Second, surgical removal of the labellum (tip of proboscis, a mouth part of a fly; Figure S3A), which contains the cell bodies of GRNs, strongly reduced Tango reporter expression (Figure 3B2–3).
Starvation and L-dopa both increase behavioral sensitivity to sucrose
The proboscis extension reflex (PER; Figure S3A) (Dethier, 1976), is a simple feeding behavior elicited by presentation of sugar to Gr5a-expressing GRNs located in the labella or legs (Gordon and Scott, 2009; Marella et al., 2006). In Drosophila, the sucrose-sensitivity of the PER (elicited from the legs) has been reported to increase with the duration of food-deprivation (Scheiner et al., 2004), although a direct comparison to unstarved flies was not performed. Surprisingly, an effect of starvation to enhance the PER in response to activation of labellar sugar receptors has not previously been reported in this species. Therefore, to identify a behavioral correlate of the starvation-induced Tango signal on labellar sugar-sensing GRNs, we first investigated whether starvation indeed increases the sensitivity of the PER to sucrose applied to labellar taste receptors.
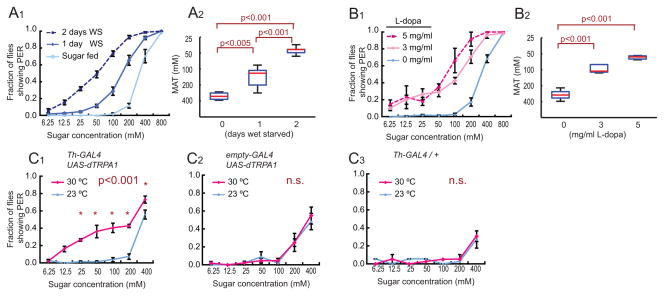
Wet starvation indeed increased the fraction of flies exhibiting a PER across a broad range of sugar concentrations (Figure 4A1), while decreasing sensitivity to bitter tastants (H.K.I. and D.J.A. unpublished result). In addition, the mean acceptance threshold (MAT; the sucrose concentration at which the probability of a PER response at the population level is 50%; see Figure S3B–D and Supplemental footnote 3) (Long et al., 1986) significantly decreased as the starvation time was increased from 1 to 2 days (Figure 4A2; note that the y-axis/ordinate is inverted: when sensitivity increases the threshold decreases). This increase in sugar sensitivity is gradual and reversible (Figure S3E; significant changes observed as early as 6 hours of wet starvation). Thus, Drosophila exhibits a starvation-induced enhancement of PER behavior induced by sucrose applied to labellar GRNs, whose magnitude depends on the duration of food-deprivation.
Figure 4. Hunger and DA increase the sugar sensitivity of the PER.
(A) Fraction of fed vs. wet-starved (WS) flies showing a PER at different concentrations of sucrose. (A1). Average responses. Error bars represent S.E.M. (A2) MAT (mean acceptance threshold; the sugar concentration where 50% of the flies show PER), plotted as a function of starvation time. One-way ANOVA followed by t-test with Bonferroni correction (n>4 for each experimental group).
(B) PER responses in non-starved flies fed with the indicated concentrations of L-dopa (n>4 for each experimental group).
(C) Genetic activation of DA neurons increases sugar sensitivity. PER vs. sugar concentration curves are shown for experimental Th-GAL4;UAS-dTRPA1 (C1) and genetic control flies (C2 and C3) at the permissive (red) and non-permissive (blue) temperatures for dTRPA1. Within-genotype differences between temperatures were analyzed using a two-way ANOVA with replication followed by post-hoc t-tests with the Bonferroni correction at each sugar concentration. *; p<0.05, n.s.; not significant (n>4 for each experimental group).
Because our DopR-Tango results suggested that Gr5a+ GRNs may be a target of dopaminergic regulation, we next asked whether experimental elevation of DA levels in fed flies would mimic the effect of food-deprivation to enhance the PER. We performed such an elevation in two ways: pharmacologically and genetically. After two days of L-dopa feeding, we observed a dose-dependent increase in PER sugar sensitivity similar to that produced by starvation (Figure 4B1–2). The sugar sensitivity of the PER was also increased in fed flies by artificial activation of dopaminergic neurons using dTRPA1, a Drosophila thermosensitive cation channel (Hamada et al., 2008), expressed under the control of Th-GAL4 (Friggi-Grelin et al., 2003) (Figure 4C1–3). This behavioral phenotype was detectable within 10 min of the temperature shift to 27°C. Together, these data indicate that elevating endogenous levels of DA can increase behavioral sensitivity to sucrose in fed flies, mimicking the effect of starvation. Importantly, DopR-Tango flies also showed a starvation-induced increase in the sugar-sensitivity of the PER (Figure S3F1–2), indicating that expression of this detector system in GRNs does not impair the physiological function of these neurons in feeding behavior.
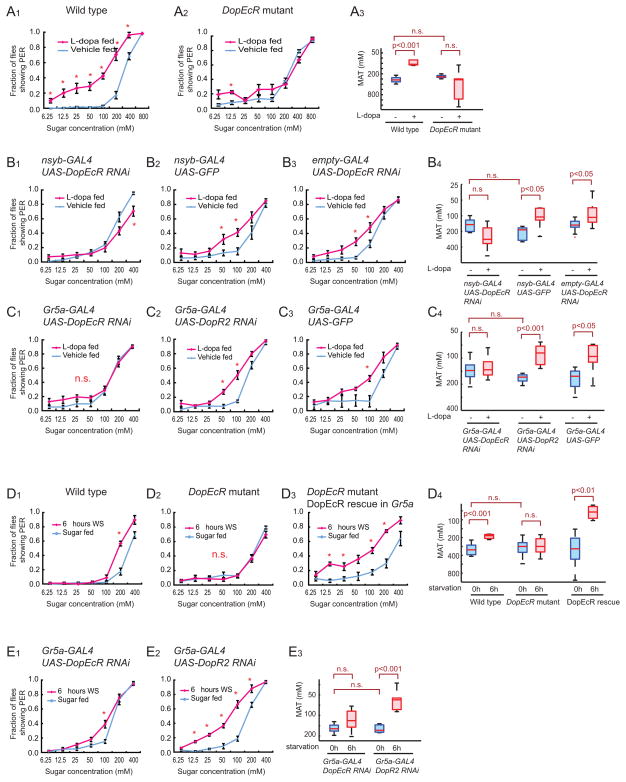
The DA receptor DopEcR expressed in sugar sensing GRNs mediates the effect of L-dopa feeding to enhance the PER
Given that both starvation and the experimental elevation of endogenous DA levels increase DopR-Tango reporter levels on sugar-sensing GRNs, and also enhance the PER, we next investigated whether DA receptors expressed in GRNs mediate this behavioral effect. We approached this objective by: 1) identifying the DA receptors expressed in sugar-sensing GRNs; 2) testing whether genetic inactivation of any of these receptors blocks the effect of L-dopa feeding to enhance the PER; 3) testing whether the same genetic manipulations block the effect of starvation to enhance the PER.
In the absence of immune reagents specific for each of the DA receptor subtypes, we investigated whether GRNs normally express any of the 4 known Drosophila DA receptors (Gotzes et al., 1994; Han et al., 1996; Hearn et al., 2002; Srivastava et al., 2005), by carrying out Q-RTPCR experiments using RNA isolated from sugar-sensing GRNs via the “TU-tagging” method (See Supplemental Experimental Procedures; (Miller et al., 2009)). qPCR of cDNA synthesized from this RNA showed a 10-fold enrichment for Gr5a mRNA itself, relative to mRNA encoding the bitter sensing receptor, Gr66, which is not expressed in sugar sensing neurons (Figure S4A). This result implied successful synthesis of cDNAs enriched in sugar sensing neurons. qPCR analysis of this cDNA revealed that 3 of the 4 Drosophila DA receptors, namely DopR1, D2R and DopEcR, are expressed in Gr5a GRNs to varying levels, while DopR2 mRNA was not detectable (Figure S4A).
We next asked whether any of the 3 DA receptors expressed in Gr5a GRNs is required for the effects of L-dopa feeding or starvation to enhance sugar sensitivity. In flies bearing a hypomorphic mutation in DopEcR, DopEcRc02142 (Fig. S4B; Ishimoto et al., in prep) (Thibault et al., 2004), L-dopa feeding failed to produce an increase in sugar sensitivity (Figure 5A2–3). Moreover, expression of a DopEcR RNAi using pan-neuronal Gal4 driver, neuronal synaptobrevin (nsyb)-GAL4 (Pauli et al., 2008) (Fig. S4C), similarly blocked the effect of L-dopa to enhance the PER (Figure 5B1–4). By contrast, flies bearing a hypomorphic mutation in DopR1 (Lebestky et al., 2009) showed a normal L-dopa-dependent increase in sugar sensitivity (Figure S4D), as did flies with a pan-neuronal RNAi-mediated knock down of D2R (Figure S4E1–3).
Figure 5. DopEcR expression in Gr5a GRNs is necessary and sufficient for L-dopa feeding- and starvation-induced increases in PER sugar sensitivity.
(A) Sugar sensitivity of wild type, and DopEcR mutant flies after L-dopa (3 mg/ml) feeding. The wild-type data are identical to Figure 4B1 and are reproduced here for ease of comparison.
(B–E) Sugar sensitivity of RNAi flies or mutant flies after L-dopa feeding (B, C) or 6 hrs wet-starvation (WS; D, E). UAS-DopEcR RNAi and UAS-DopR2 RNAi are in the same genetic background. Note that DopR2 is not expressed in a detectable level in sugar-sensing GRNs (Figure S4A).
The statistical significance of within-genotype differences between PER curves, or MAT values, for L-dopa vs. vehicle treatment or feeding vs. wet-starvation was analyzed using two-way ANOVA with replication followed by post hoc t-tests with Bonferroni correction. *; p<0.05, n.s.; not significant (n>4 for each experimental group). A significant interaction between genotype and feeding manipulation was revealed by a 2-way ANOVA in (A3) p<0.0001; (B4) p<0.005; (C4) p<0.01; (D4) p<0.05; and (E3) p<0.005, indicating that the genetic manipulations interfered with the effect of wet-starvation or L-dopa feeding.
Importantly, cell-specific knock-down of DopEcR in Gr5a GRNs also prevented the L-dopa feeding-induced enhancement of the sugar sensitivity of the PER, while control flies expressing either UAS-GFP or UAS-DopR2 RNAi showed a statistically significant enhancement of PER behavior by L-dopa (Figure 5C1–4). The MAT of vehicle-fed flies of both the DopEcR RNAi and DopEcR mutant genotypes was not significantly different from that of the genetic control flies (Figure 5A3, 5B4 and 5C4), indicating that DopEcR is not necessary for baseline PER behavior per se, but rather for its enhancement by L-dopa feeding. Taken together, these data indicate that DopEcR expressed in Gr5a GRNs is necessary for the effect of L-dopa feeding to increase sugar sensitivity.
DopEcR expressed in sugar-sensing GRNs is required for the effect of starvation to enhance PER behavior
Having demonstrated that DopEcR in Gr5a neurons is necessary for the effect of L-dopa feeding to enhance the sugar sensitivity of the PER, we next tested whether DopEcR in Gr5a GRNs is also necessary for starvation to exert the same behavioral effect. Indeed, in flies wet-starved for 6 hours, DopEcR mutant flies failed to exhibit an increase in sugar sensitivity, in contrast to wild-type controls (Figure 5D1–2). Importantly, this phenotype could be rescued by specific expression in DopEcR mutant flies of a UAS-DopEcR transgene in Gr5a neurons (Figure 5D3). Over-expression of DopEcR, (but not of DopR1) in Gr5a neurons of DopEcR+ flies also enhanced the sucrose sensitivity of the PER in starved, but not in fed, animals (Figure S4H1–4 and S4I1–3).
Finally, specific knock-down of DopEcR in sugar sensing neurons using RNAi also strongly attenuated the increase in sugar sensitivity caused by 6 hours of starvation (Figure 5E1–3). Thus, both selective rescue of the DopEcR mutant phenotype, and selective expression of RNAi, implicate Gr5a neurons as a site of DopEcR action. Interestingly, although the DopEcR mutation and RNAi both impaired PER enhancement by 48 hrs of L-dopa feeding, they did not do so in flies wet-starved for 24 hrs or more (Figure S4F1–3 and S4G1–3). This observation suggests either a time-dependent recruitment of redundant DA receptors, or of DA-independent mechanisms, mediating enhanced sugar sensitivity at later stages of starvation. Flies lacking both DopEcR and DopR1 did not show an impaired PER response after 24 hrs of starvation, suggesting the involvement of additional neuromodulators (data not shown). Whatever the explanation, at early times of starvation DA, acting through DopEcR expressed in Gr5a GRNs, is required for enhancement of PER behavior.
Cellular mechanism of the starvation-induced increase in behavioral sensitivity to sucrose
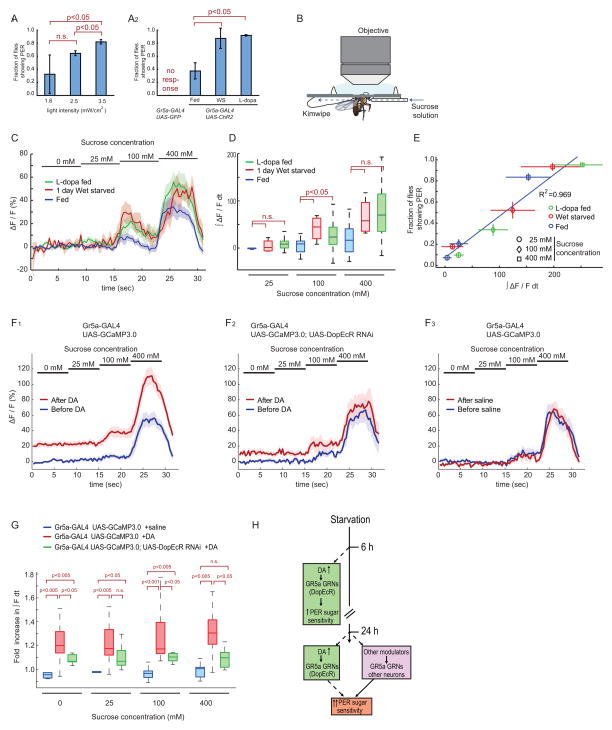
Lastly, we approached the cellular mechanism through which starvation and DA enhance the sugar-sensitivity of the PER. As a first step, we asked whether starvation and DA act to modify the activity of gustatory receptors (GRs) themselves, or rather on a downstream physiological process. To do this, we bypassed the requirement for GR activation in the PER response using Channelrhodopsin 2 (ChR2), a light sensitive cation channel (Zhang et al., 2006), to artificially activate sugar-sensing GRNs (Gr5a-GAL4; UAS-ChR2) (Zhang et al., 2007).
Increasing the strength of blue light illumination (from 1.6 to 2.9 mW/cm2) increased the fraction of flies exhibiting a PER (Figure 6A1), similar to the effect of stimulating the labellum with increasing sugar concentrations. Strikingly, both wet-starved and L-dopa fed Gr5a-GAL4;UAS-ChR2 flies showed an increased light sensitivity of the PER, compared to control non-starved flies (Figure 6A2). These data suggest that both starvation and DA enhance sugar sensitivity by acting downstream of the sugar sensing receptors themselves. Consistent with this idea, extracellular recordings from GRN somata in the labella indicated no change in the frequency of sucrose-evoked spiking in wet-starved vs. control fed flies (Figure S5A–B).
Figure 6. Starvation or L-dopa feeding enhance calcium transients in sugar sensing GRNs.
(A) Channelrhodopsin-2-evoked PER. Gr5a-GAL4;UAS-ChR2 or Gr5a-GAL4;UAS-GFP control flies were stimulated with blue (470/40 nm: centerwavelength/bandwidth) light at the indicated intensities (A1) or at 1.6 mW/cm2 under the indicated conditions (A2).
(B) The setup for calcium imaging of sugar-sensing GRNs. Blue dashed arrow indicates direction of flow of sugar solution.
(C) Responses (ΔF/F) to different concentrations of sucrose in the central projections of sugar-sensing GRNs in Gr5a-GAL4;UAS-GCaMP3.0 flies. The solid lines represent average trace, and envelopes indicate SEM (n>7 for each condition).
(D) Quantification of fluorescent changes. ∫ ΔF/F dt, integrated ΔF/F during stimulus period. Data analyzed from (C). Mann-Whitney U-tests with Bonferroni correction.
(E) Correlation between GCaMP signals (analyzed in B, C) and behavioral responses (PER) of Gr5a-GAL4;UAS-GCaMP3.0 flies (n>4). Error bars represent SEM.
(F) Responses (ΔF/F) to different concentrations of sucrose in the central projections of sugar-sensing GRNs before and after 5 min exposure of the brain to saline with or without 1mM DA. The solid lines represent average trace, and envelopes indicate SEM (n>7 for each condition).
(G) Fold increase in ∫ F dt (∫ F dt[After DA]/∫ F dt[Before DA]) during each stimulus period, calculated from the data in (F). Mann-Whitney U-tests with Bonferroni correction.
(H) Schematic illustrating mechanisms controlling starvation-induced increases in the sugar sensitivity of PER behavior.
To pin down the physiological mechanism underlying starvation-dependent enhancement of PER behavior, we tested whether starvation and DA augment pre-synaptic Ca2+ influx in sugar-sensing GRNs. For this purpose, we performed calcium imaging, using two-photon microscopy, of sugar-sensing GRNs in flies expressing a genetically encoded calcium sensor (GCaMP3.0 (Tian et al., 2009)) under the control of Gr5a-GAL4. Delivery of increasing concentrations of sucrose (from 0 mM to 400 mM) to the labellum yielded increasing GCaMP 3.0 fluorescence signal in Gr5a-expressing nerve fibers in the SOG (Figure 6B–D), consistent with a previous report (Marella et al., 2006). Strikingly, both wet-starved and L-dopa fed flies showed a statistically significant enhancement of sucrose-evoked GCaMP fluorescence, compared to non-starved control flies, at 100 mM sucrose, and a non-significant trend to enhancement at 400 mM sucrose (Nusbaum and Beenhakker, 2002) (Figure 6D).
A scatter-plot of integrated GCaMP fluorescence signal intensity vs. the fraction of flies showing a PER response at each sucrose concentration revealed a strong positive correlation between the two measures (R2=0.969) (Figure 6E). The simplest interpretation of this correlation is that the starvation-induced enhancement of calcium influx in sugar-sensing GRNs underlies the parallel enhancement of PER behavior.
Finally, to examine more directly whether DA acts on Gr5a+ GRNs to modulate Ca2+ influx, we compared the sugar responses of these GRNs before vs. after exposure to 1mM DA in the bath. Following 5 min of such exposure, there was a ~1.2 fold increase in basal Ca2+ influx, and a ~1.3–1.4 fold influx in Ca2+ influx caused by 400mM sucrose; the fold increase at 400mM sucrose was significantly higher than at 0mM sucrose (p<0.05, Wilcoxon matched pairs test) (Figure 6F1, 6G). Importantly, RNAi-mediated knockdown of DopEcR expression in sugar-sensing GRNs attenuated this increase in Ca2+ influx (Figure 6F2, and 6G). These data indicate that DA acts directly on Gr5a GRNs via DopEcR to enhance both baseline and sucrose-induced increases in intracellular free Ca2+.
DISCUSSION
Drosophila is a potentially powerful model system for understanding how neuromodulators control state-dependent changes in behavior. However establishing the behaviorally relevant, circuit-level mechanisms of action of neuromodulators remains challenging. This is partially because standard methods used to measure the release of endogenous neuromodulators in vertebrates, such as fast-scan cyclic voltammetry (Phillips et al., 2003) or micro-dialysis (Benveniste and Huttemeier, 1990), are of limited applicability in Drosophila. Moreover, such methods cannot identify the neurons on which released neuromodulators act. The data presented here provide proof-of-principle for the utility of a new method, called TANGO-map, to identify, in a brain-wide and relatively unbiased manner, circuit-level substrates of neuromodulation relevant to a particular state-dependent influence on behavior.
Starvation regulates gustatory sensitivity in Drosophila and causes DA release onto sugar-sensing GRNs
We show here that sweet taste-sensitivity in the labellum is enhanced with increasing duration of food-deprivation in Drosophila. This observation confirms and extends previous reports in Drosophila (Meunier et al., 2007; Scheiner et al., 2004), and is consistent with observations in many other animal species (Dethier, 1976; Moskowitz et al., 1976; Page et al., 1998). We have used this phenomenon as a prototypic case of a state-dependent change in behavior, to investigate the ability of TANGO-map to identify underlying neuromodulatory mechanisms.
Our results indicate that starvation enhances endogenous DA release onto primary GRNs, as detected by increased expression of the DopR-Tango reporter in vivo. In contrast, starvation did not increase the DopR-Tango reporter in the MB or AL, although L-dopa feeding did so. These data indicate that DopR-Tango is capable of revealing selective sites of endogenous DA release in a brain-wide manner, under specific behavioral conditions.
DA release onto sugar-sensing GRNs is required for the behavioral effect of starvation to enhance PER sensitivity
Our results indicate that a mutation in the DA receptor DopEcR, as well as specific knock-down of this receptor in sugar-sensing GRNs, eliminates the effect of starvation to enhance the sucrose-sensitivity of the PER. However, this phenotype was only observed at 6 hr of starvation; after 24 hr of food deprivation these genetic manipulations no longer had an effect. This is not because these manipulations themselves became ineffective at later times, since the same manipulations did attenuate the increased PER sensitivity caused by L-dopa feeding for 24 hr. This suggests that at an early stage of starvation, DA is necessary to enhance the sugar sensitivity of the PER, while at later stages additional factors come into play (Figure 6H).
The slow kinetics of Tango reporter accumulation (Supplementary Figure S1E) preclude the detection of statistically significant increases in signal as early as 6 hr following an experimental manipulation. However, the level of reporter expression detected in animals examined after 48 hrs of treatment likely reflects the integration of increases in dopaminergic signaling occurring throughout the first 12–24 hr of the treatment period (see Supplemental Footnote 1). Thus, although we detected an increase in DopR-Tango signal at a starvation timepoint when genetic reduction of DopEcR levels no longer impaired the behavioral effect of starvation, and observed a behavioral phenotype at a time point too early to be evaluated directly by the TANGO-map method, this should not be taken to imply that no DA release occurred after 6 hrs of starvation. Importantly, given the kinetics of the system, the DopR-Tango signals we detect in vivo are likely to reflect primarily changes in tonic levels of DA signaling, rather than brief episodes of phasic DA release. Further improvements of the TANGO-map method are required to increase its temporal resolution. Nevertheless, the present methodology provides a powerful method to identify sites where dopaminergic modulation of a given behavior may occur, even if it cannot reveal precisely how quickly such regulation is exerted.
Mechanism of dopaminergic regulation of GRN sensitivity
Several lines of evidence suggest that the dopaminergic modulation of sugar-sensing GRNs revealed here may involve an enhancement of Ca2+ influx at the nerve terminal. Both starvation and L-dopa feeding increased sucrose-evoked Ca2+ influx, without changing the frequency of action potentials measured extracellularly at GRN somata (Figure S5), despite a previous report to the contrary (Meunier et al., 2007). Furthermore, we found that direct exposure of the brain to DA increased Ca2+ influx at the presynaptic terminals of sugar-sensing GRNs, in a DopEcR-dependent manner. A model consistent with these data is that starvation leads to increased DA release, which increases calcium influx into sugar-sensing GRNs via DopEcR, leading to increased neurotransmitter release. The fact that DopEcR signals via the cAMP/PKA pathway (Srivastava et al., 2005), and that this pathway has been reported to increase Ca2+ channel currents in Drosophila (Bhattacharya et al., 1999), is also consistent with this scenario. Nevertheless, our genetic data suggest that there are additional pathways through which starvation modulates feeding behavior in this system.
Our finding that DA modulates primary GRNs to control starvation-dependent changes in behavioral sensitivity to sugar echoes the observation of a similar influence of food-deprivation on odorant sensitivity in Drosophila (Root et al., 2011). Such neuromodulatory gain control at the level of primary sensory neurons has also been reported in variety of other invertebrate, as well as vertebrate, species (Bicker and Menzel, 1989; Hurley et al., 2004). While we cannot exclude the possibility that hunger also influences PER behavior at higher-order synapses in the circuit (Gordon and Scott, 2009), our data add to a growing body of information indicating that modulation of primary sensory neurons is a general mechanism for implementing state-dependent changes in behavioral responses to the stimuli detected by these neurons.
TANGO-map as a new tool to monitor neuromodulation at the circuit level
TANGO-map affords a number of unique advantages to study neuronal modulation in the brain (see Table S1 for comparison to other methods). Firstly, and most importantly, it permits the detection of increases in endogenous neuromodulator release in vivo, in an organism in which the application of conventional methods is not feasible. Secondly, it provides an anatomical readout of neuromodulation at the neural circuit level. The use of a pan-neuronal GAL4 driver to express the sensor permits, in principle, an unbiased survey of potential sites of neuromodulatory activity throughout the brain. Thirdly, the sensor has ligand-specificity. The modular design of the Tango system (Barnea et al., 2008) affords the ability to develop in vivo Tango reporters for other biogenic amines and neuropeptides that work via GPCRs. Importantly, because the method employs a synthetic, “private” signal transduction pathway (Barnea et al., 2008), the readout of the reporter should be relatively insensitive to interference from conventional signal transduction pathways activated by other endogenous receptors. Systematic and comprehensive application of this approach could, in principle, provide an overview of anatomic patterns of neuromodulation in the brain in a given behavioral setting. Finally, since the Tango system is transcriptionally based, in principle it permits the expression not only of neutral reporters, but also of effectors such as RNAi’s or ion channels, in the neurons receiving neuromodulatory input.
While the TANGO-map system can certainly benefit from improvements in its kinetics and signal-to-noise ratio (Supplemental Footnotes 1 and 2), it affords a means of identifying points-of-entry for studying circuit-level mechanisms of behaviorally relevant neuromodulation, that are currently difficult to access in any other way. The extension of this methodology to other neuromodulators and model organisms should further our understanding of state-dependent control of neural activity and behavior.
EXPERIMENTAL PROCEDURES
Fly strains
Adult female Drosophila melanogaster were used for all experiments. All control genotypes were tested in the same genetic background as the experimental genotype. Construction of recombinant DNA, descriptions of transgenic fly strains, and procedures for TANGO-map are described in the Supplemental Experimental Procedures.
PER assays
For standard PER assays, 3–7 day-old female flies were wet-starved or fed in vials and tested as described previously (Shiraiwa and Carlson, 2007). For details, see Supplemental Experimental Procedures.
Calcium imaging
Two-photon imaging was performed on an Ultima two-photon laser scanning microscope (Prairie Technology) with an imaging wavelength at 925nm Details of the preparation are described in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Dr. K. J. Lee for sharing Tango DNA constructs prior to publication. We also thank Dr. T. Lee, K. Deisseroth, A. Stathopoulos, and B. Pfeiffer for plasmids. Fly stocks were generously provided by the Bloomington Stock Center, the VDRC stock center, Drosophila RNAi Screening Center, Drs. G. M. Rubin, J. Simpson, L. L Looger, H. Keshishian, K. Scott, T. Lee, S. Birman, P. A. Garrity, C. Q. Doe, and K. Ito. We also thank Dr. H. Otsuna and Y. Wan for Fluorender, and members of the Anderson lab for helpful discussion and sharing of flies. H.K.I. is supported by the Nakajima Foundation. D.J.A. is an investigator of the Howard Hughes Medical Institute. This work was supported in part by NIH grant 1RO1 DA031389 to D.J. Anderson.
Footnotes
Supplemental Information, including Supplemental Figures, a Supplemental Table, Supplemental Experimental Procedures and Supplemental Footnotes can be found with this article online at.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bainton RJ, Tsai LT, Singh CM, Moore MS, Neckameyer WS, Heberlein U. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci U S A. 2008;105:64–69. doi: 10.1073/pnas.0710487105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Huttemeier PC. Microdialysis--theory and application. Prog Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16:103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Gu GG, Singh S. Modulation of dihydropyridine-sensitive calcium channels in Drosophila by a cAMP-mediated pathway. J Neurobiol. 1999;39:491–500. doi: 10.1002/(sici)1097-4695(19990615)39:4<491::aid-neu3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bicker G, Menzel R. Chemical codes for the control of behaviour in arthropods. Nature. 1989;337:33–39. doi: 10.1038/337033a0. [DOI] [PubMed] [Google Scholar]
- Birmingham JT, Tauck DL. Neuromodulation in invertebrate sensory systems: from biophysics to behavior. J Exp Biol. 2003;206:3541–3546. doi: 10.1242/jeb.00601. [DOI] [PubMed] [Google Scholar]
- Brookhart GL, Edgecomb RS, Murdock LL. Amphetamine and reserpine deplete brain biogenic amines and alter blow fly feeding behavior. J Neurochem. 1987;48:1307–1315. doi: 10.1111/j.1471-4159.1987.tb05662.x. [DOI] [PubMed] [Google Scholar]
- Chiappe ME, Seelig JD, Reiser MB, Jayaraman V. Walking modulates speed sensitivity in Drosophila motion vision. Current biology: CB. 2010;20:1470–1475. doi: 10.1016/j.cub.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Shahidullah M, Levitan IB, Sehgal A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron. 2010;65:670–681. doi: 10.1016/j.neuron.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. The hungry fly: a physiological study of the behavior associated with feeding. Cambridge, Mass: Harvard University Press; 1976. [Google Scholar]
- Dubner R. The effect of behavioral state on the sensory processing of nociceptive and non-nociceptive information. Prog Brain Res. 1988;77:213–228. doi: 10.1016/s0079-6123(08)62788-0. [DOI] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Gillette R, Huang RC, Hatcher N, Moroz LL. Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc Natl Acad Sci U S A. 2000;97:3585–3590. doi: 10.1073/pnas.97.7.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzes F, Balfanz S, Baumann A. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors Channels. 1994;2:131–141. [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci. 1991;14:39–57. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- Hearn MG, Ren Y, McBride EW, Reveillaud I, Beinborn M, Kopin AS. A Drosophila dopamine 2-like receptor: Molecular characterization and identification of multiple alternatively spliced variants. Proc Natl Acad Sci U S A. 2002;99:14554–14559. doi: 10.1073/pnas.202498299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol. 2004;14:488–495. doi: 10.1016/j.conb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebestky T, Chang JS, Dankert H, Zelnik L, Kim YC, Han KA, Wolf FW, Perona P, Anderson DJ. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long TF, Edgecomb RS, Murdock LL. Effects of substituted phenylethylamines on blowfly feeding behavior. Comp Biochem Physiol C. 1986;83:201–209. doi: 10.1016/0742-8413(86)90037-x. [DOI] [PubMed] [Google Scholar]
- Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nature neuroscience. 2010;13:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Meunier N, Belgacem YH, Martin JR. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J Exp Biol. 2007;210:1424–1434. doi: 10.1242/jeb.02755. [DOI] [PubMed] [Google Scholar]
- Miller MR, Robinson KJ, Cleary MD, Doe CQ. TU-tagging: cell type-specific RNA isolation from intact complex tissues. Nat Methods. 2009;6:439–441. doi: 10.1038/nmeth.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–121. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Montell C. A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz HR, Kumraiah V, Sharma KN, Jacobs HL, Sharma SD. Effects of hunger, satiety and glucose load upon taste intensity and taste hedonics. Physiol Behav. 1976;16:471–475. doi: 10.1016/0031-9384(76)90326-7. [DOI] [PubMed] [Google Scholar]
- Moss CF, Dethier VG. Central nervous system regulation of finicky feeding by the blowfly. Behav Neurosci. 1983;97:541–548. doi: 10.1037//0735-7044.97.4.541. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Winther AM. Drosophila neuropeptides in regulation of physiology and behavior. Prog Neurobiol. 2010;92:42–104. doi: 10.1016/j.pneurobio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Jr, Erber J, Fondrk MK. The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.) J Comp Physiol A. 1998;182:489–500. doi: 10.1007/s003590050196. [DOI] [PubMed] [Google Scholar]
- Pauli A, Althoff F, Oliveira RA, Heidmann S, Schuldiner O, Lehner CF, Dickson BJ, Nasmyth K. Cell-type-specific TEV protease cleavage reveals cohesin functions in Drosophila neurons. Dev Cell. 2008;14:239–251. doi: 10.1016/j.devcel.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Kieffer BL, Swanson LW. Mechanisms for the regulation of state changes in the central nervous system: an introduction. Ann N Y Acad Sci. 2008;1129:1–7. doi: 10.1196/annals.1417.031. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods Mol Med. 2003;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, Iche-Torres M, Cassar M, Strauss R, Preat T, et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci U S A. 2011;108:834–839. doi: 10.1073/pnas.1010930108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiner R, Pluckhahn S, Oney B, Blenau W, Erber J. Behavioural pharmacology of octopamine, tyramine and dopamine in honey bees. Behav Brain Res. 2002;136:545–553. doi: 10.1016/s0166-4328(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Scheiner R, Sokolowski MB, Erber J. Activity of cGMP-dependent protein kinase (PKG) affects sucrose responsiveness and habituation in Drosophila melanogaster. Learn Mem. 2004;11:303–311. doi: 10.1101/lm.71604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Behavioral state-dependent reconfiguration of song-related network activity and cholinergic systems. J Chem Neuroanat. 2010;39:132–140. doi: 10.1016/j.jchemneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraiwa T, Carlson JR. Proboscis extension response (PER) assay in Drosophila. J Vis Exp. 2007:193. doi: 10.3791/193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- Szymczak AL, Vignali DA. Development of 2A peptide-based strategies in the design of multicistronic vectors. Expert Opin Biol Ther. 2005;5:627–638. doi: 10.1517/14712598.5.5.627. [DOI] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno Y, Mori K. Behavioral state-dependent changes in the information processing mode in the olfactory system. Commun Integr Biol. 2009;2:362–364. doi: 10.4161/cib.2.4.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The molecular and cellular basis of bitter taste in Drosophila. Neuron. 2011;69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ge W, Wang Z. A toolbox for light control of Drosophila behaviors through Channelrhodopsin 2-mediated photoactivation of targeted neurons. Eur J Neurosci. 2007;26:2405–2416. doi: 10.1111/j.1460-9568.2007.05862.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.