Abstract
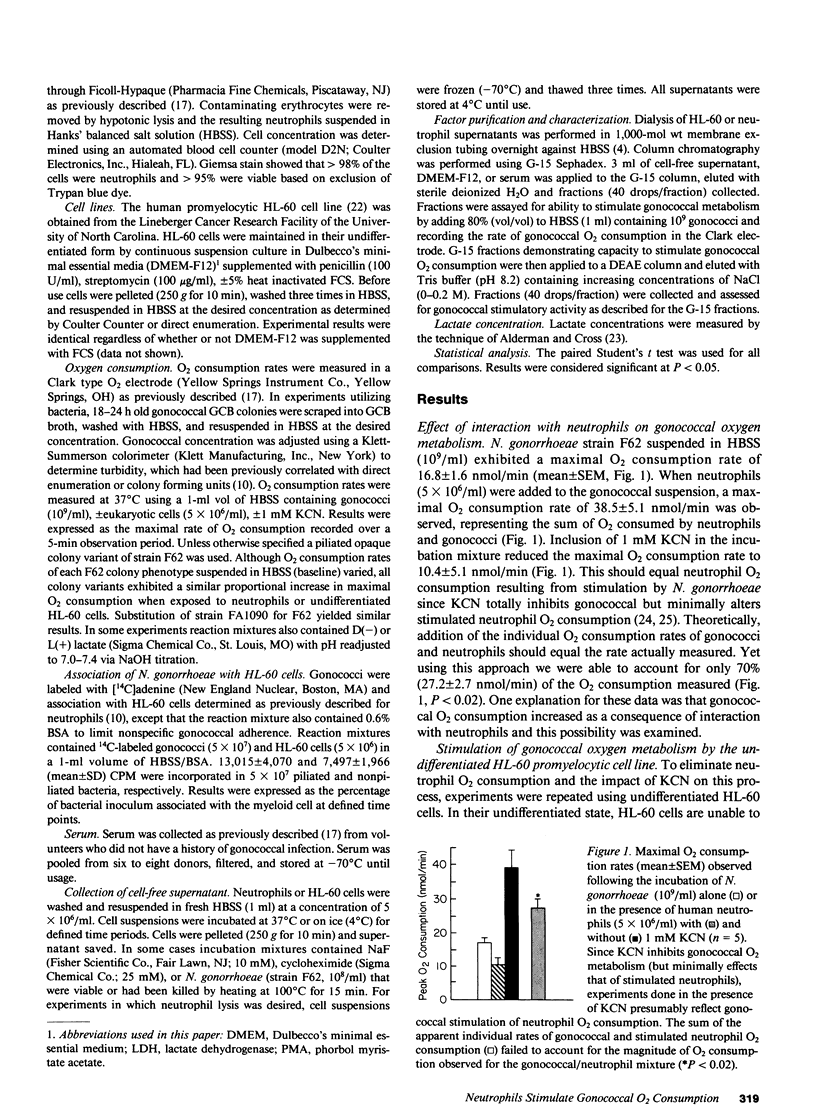
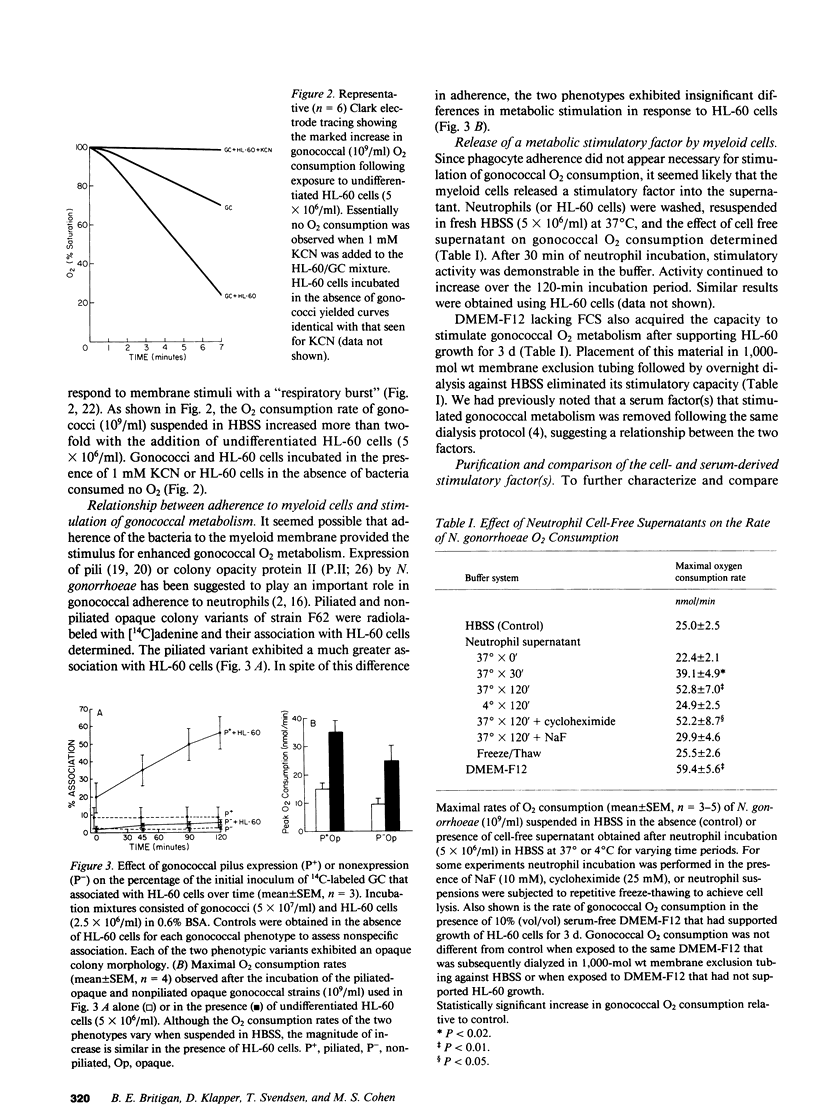
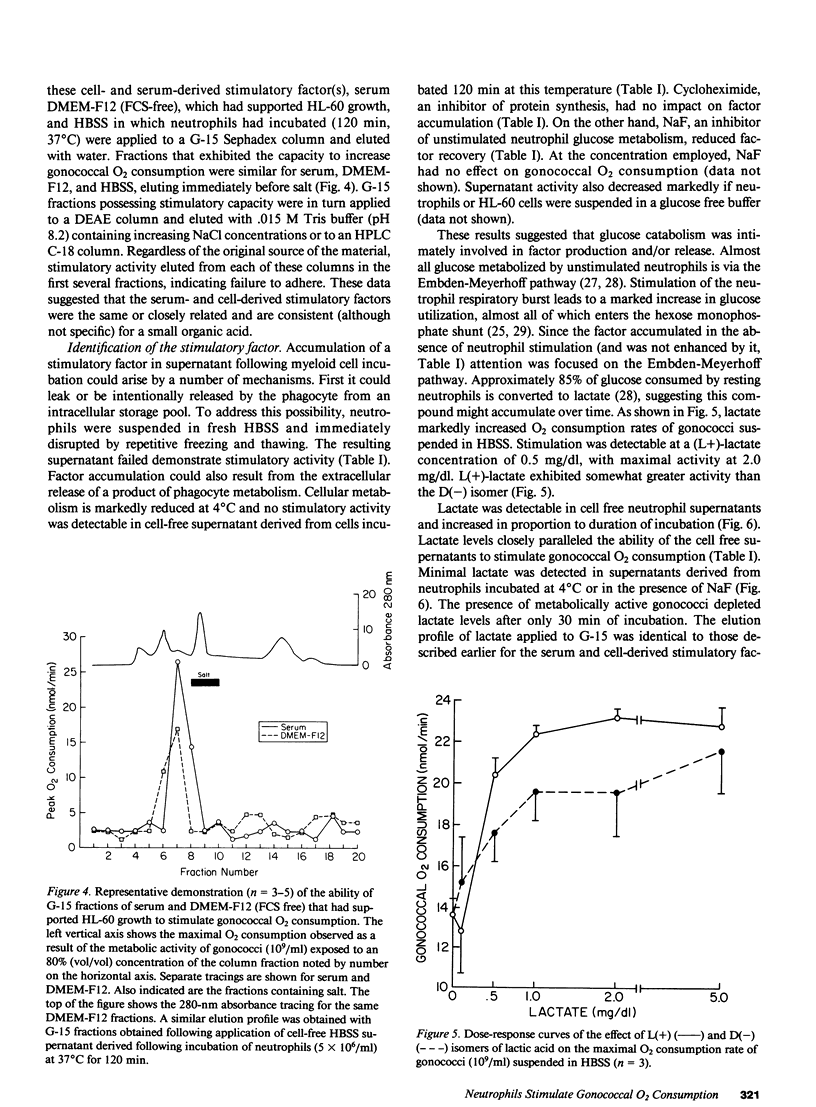
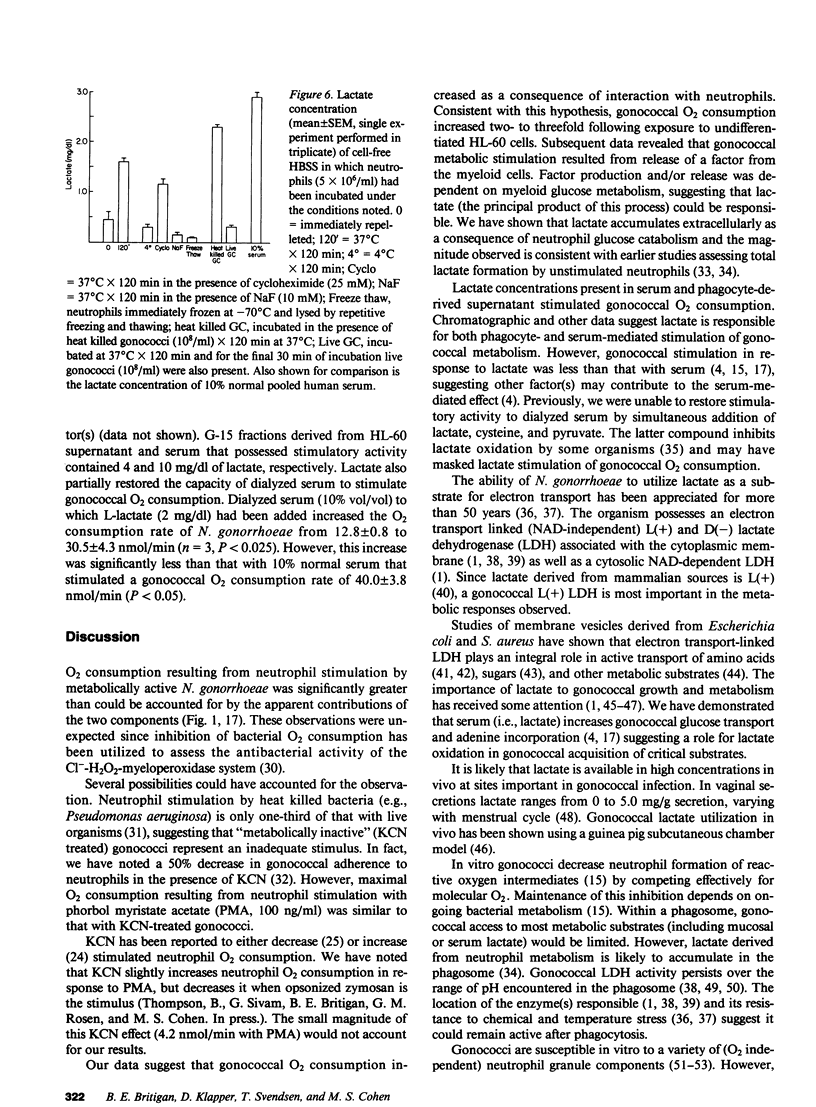
O2 consumption resulting from interaction of Neisseria gonorrhoeae and human neutrophils represents a composite of O2 consumed by the two cell systems. Experiments studying the relative contribution of each system suggested the possibility that gonococci increased their metabolic activity in response to interaction with neutrophils. This hypothesis was confirmed by demonstrating that undifferentiated HL-60 cells, which are unable to undergo a respiratory burst, induce a two- to three-fold increase in gonococcal O2 consumption. Gonococcal capacity to adhere to HL-60 cells did not correlate with extent of metabolic stimulation. Stimulatory activity was demonstrable in cell-free supernatant from neutrophils or HL-60 cells, and increased with duration of incubation. Supernatant applied to a G-15 Sephadex column yielded fractions that stimulated gonococcal O2 consumption. Elution profiles were similar for HL-60 cells, neutrophils, and a stimulatory factor previously isolated from pooled human serum. This stimulatory factor(s) failed to adhere to DEAE or C-18 HPLC columns. Stimulatory activity release from myeloid cells was inhibited by incubation at 4 degrees C or in the presence of NaF, indicating a critical role for glucose metabolism. Lactate, the principal product of resting neutrophil glucose catabolism, was demonstrable in cell-free supernatants after incubation at 37 degrees C. Lactate accumulation was inhibited by NaF and decreased temperature of incubation. Lactate at levels present in cell-free supernatant increased gonococcal O2 consumption twofold and restored stimulatory activity to dialyzed serum. Live, but not heat-killed gonococci eliminated lactate released from neutrophils during phagocytosis. Gonococci are able to utilize host-derived lactate to enhance their rate of O2 metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman J. A., Cross R. E. Adaptation to the centrifugal analyzer of an enzymatic method for the measurement of lactate in plasma and cerebrospinal fluid. Clin Chem. 1977 Oct;23(10):1917–1920. [PubMed] [Google Scholar]
- BECK W. S. The control of leukocyte glycolysis. J Biol Chem. 1958 May;232(1):251–270. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Chai Y., Cohen M. S. Effects of human serum on the growth and metabolism of Neisseria gonorrhoeae: an alternative view of serum. Infect Immun. 1985 Dec;50(3):738–744. doi: 10.1128/iai.50.3.738-744.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S. Effects of human serum on bacterial competition with neutrophils for molecular oxygen. Infect Immun. 1986 Jun;52(3):657–663. doi: 10.1128/iai.52.3.657-663.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Britigan B., Cohen M. S. Effect of growth in serum on uptake of Neisseria gonorrhoeae by human neutrophils. J Infect Dis. 1985 Aug;152(2):330–338. doi: 10.1093/infdis/152.2.330. [DOI] [PubMed] [Google Scholar]
- COHN Z. A., MORSE S. I. Functional and metabolic properties of polymorphonuclear leucocytes. II. The influence of a lipopolysaccharide endotoxin. J Exp Med. 1960 May 1;111:689–704. doi: 10.1084/jem.111.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey S. G., Shafer W. M., Spitznagel J. K. Anaerobiosis increases resistance of Neisseria gonorrhoeae to O2-independent antimicrobial proteins from human polymorphonuclear granulocytes. Infect Immun. 1985 Feb;47(2):401–407. doi: 10.1128/iai.47.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Campbell L. A., Palermo D. A., Evans T. M., Klimpel K. W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect Immun. 1987 Jun;55(6):1359–1364. doi: 10.1128/iai.55.6.1359-1364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Black J. R., Proctor R. A., Sparling P. F. Host defences and the vaginal mucosa. A re-evaluation. Scand J Urol Nephrol Suppl. 1984;86:13–22. [PubMed] [Google Scholar]
- Cohen M. S., Cooney M. H. A bacterial respiratory burst: stimulation of the metabolism of Neisseria gonorrhoeae by human serum. J Infect Dis. 1984 Jul;150(1):49–56. doi: 10.1093/infdis/150.1.49. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Mullikin D., Shirley P. S., McCall C. E. Phagocytosis of live versus heat-killed bacteria by human polymorphonuclear leukocytes. Infect Immun. 1974 Jul;10(1):25–29. doi: 10.1128/iai.10.1.25-29.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner M., Penn C. W., Sanyal S. C., Veale D. R., Smith H. Phenotypically determined resistance of Neisseria gonorrhoeae to normal human serum: environmental factors in subcutaneous chambers in guinea pigs. J Gen Microbiol. 1979 Sep;114(1):169–177. doi: 10.1099/00221287-114-1-169. [DOI] [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma K. Metabolic control and intracellular pH during phagocytosis by polymorphonuclear leucocytes. J Biochem. 1970 Aug;68(2):177–185. doi: 10.1093/oxfordjournals.jbchem.a129344. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Major N. C., Davies D. B., Robinson A. Physiology and virulence determinants of Neisseria gonorrhoeae grown in glucose-, oxygen- or cystine-limited continuous culture. J Gen Microbiol. 1986 Dec;132(12):3289–3302. doi: 10.1099/00221287-132-12-3289. [DOI] [PubMed] [Google Scholar]
- Kerwar G. K., Gordon A. S., Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. IV. Galactose transport by isolated membrane vesicles from Escherichia coli. J Biol Chem. 1972 Jan 10;247(1):291–297. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Komatsu Y., Tanaka K. Deoxycytidine uptake by isolated membrane vesicles from Escherichia coli K 12. Biochim Biophys Acta. 1973 Jul 18;311(4):496–506. doi: 10.1016/0005-2736(73)90125-9. [DOI] [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- Lysko P. G., Morse S. A. Effects of steroid hormones on Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Aug;18(2):281–288. doi: 10.1128/aac.18.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak P. A. The normal microbial flora. N Engl J Med. 1982 Jul 8;307(2):83–93. doi: 10.1056/NEJM198207083070203. [DOI] [PubMed] [Google Scholar]
- Mandell G. L. Intraphagosomal pH of human polymorphonuclear neutrophils. Proc Soc Exp Biol Med. 1970 Jun;134(2):447–449. doi: 10.3181/00379727-134-34810. [DOI] [PubMed] [Google Scholar]
- Mietzner T. A., Luginbuhl G. H., Sandstrom E., Morse S. A. Identification of an iron-regulated 37,000-dalton protein in the cell envelope of Neisseria gonorrhoeae. Infect Immun. 1984 Aug;45(2):410–416. doi: 10.1128/iai.45.2.410-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Hebeler B. H. Effect of pH on the growth and glucose metabolism of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):87–95. doi: 10.1128/iai.21.1.87-95.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe J. F., Shipp J. C. Glucose metabolism in leucocytes from patients with diabetes mellitus, with and without hypercholesteremia. Diabetes. 1965 Sep;14(9):584–590. doi: 10.2337/diab.14.9.584. [DOI] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F., Fischer S. H., Ingham Z. Z., Jones J. F. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982 May;36(2):737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rest R. F. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect Immun. 1979 Aug;25(2):574–579. doi: 10.1128/iai.25.2.574-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Shafer W. M., Onunka V., Hitchcock P. J. A spontaneous mutant of Neisseria gonorrhoeae with decreased resistance to neutrophil granule proteins. J Infect Dis. 1986 May;153(5):910–917. doi: 10.1093/infdis/153.5.910. [DOI] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. Further studies on amino acid transport in Staphylococcus aureus membrane vesicles. J Biol Chem. 1974 Jul 10;249(13):4275–4281. [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978 Jul;21(1):292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: effect of exogenous amines on antibacterial action against Escherichia coli. Infect Immun. 1979 Jul;25(1):110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley F. P., Blackwell C. C., Tan E. L., Patel P. V., Parsons N. J., Martin P. M., Smith H. Alteration of pyocin-sensitivity pattern of Neisseria gonorrhoeae is associated with induced resistance to killing by human serum. J Gen Microbiol. 1984 May;130(5):1303–1306. doi: 10.1099/00221287-130-5-1303. [DOI] [PubMed] [Google Scholar]
- Winter D. B., Morse S. A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975 Aug;123(2):631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Elmros T., Normark S., Bloom G. D. Cell envelope of Neisseria gonorrhoeae: outer membrane and peptidoglycan composition of penicillin-sensitive and-resistant strains. Infect Immun. 1975 Jun;11(6):1332–1341. doi: 10.1128/iai.11.6.1332-1341.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zwieten R., Wever R., Hamers M. N., Weening R. S., Roos D. Extracellular proton release by stimulated neutrophils. J Clin Invest. 1981 Jul;68(1):310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]



