Abstract
Mononuclear cells may be important regulators of fibroblast glycosaminoglycan (GAG) biosynthesis. However, the soluble factors mediating these effects, the importance of intercytokine interactions in this regulation and the mechanisms of these alterations remain poorly understood. We analyzed the effect of recombinant (r) tumor necrosis factor (TNF), lymphotoxin (LT), and gamma, alpha, and beta 1 interferons (INF-gamma, -alpha and -beta 1), alone and in combination, on GAG production by normal human lung fibroblasts. rTNF, rLT, and rINF-gamma each stimulated fibroblast GAG production. In addition, rIFN-gamma synergized with rTNF and rLT to further augment GAG biosynthesis. In contrast, IFN-alpha A, -alpha D, and -beta 1 neither stimulated fibroblast GAG production nor interacted with rTNF or rLT to regulate GAG biosynthesis. The effects of the stimulatory cytokines and cytokine combinations were dose dependent and were abrogated by the respective monoclonal antibodies. In addition, these cytokines did not cause an alteration in the distribution of GAG between the fibroblast cell layer and supernatant. However, the stimulation was at least partially specific for particular GAG moieties with hyaluronic acid biosynthesis being markedly augmented without a comparable increase in the production of sulfated GAGs. Fibroblast prostaglandin production did not mediate these alterations since indomethacin did not decrease the stimulatory effects of the cytokines. In contrast, protein and mRNA synthesis appeared to play a role since the stimulatory effects of the cytokines were abrogated by cyclohexamide and actinomycin D, respectively. In addition, the cytokines and cytokine combinations increased cellular hyaluronate synthetase activity in proportion to their effects on hyaluronic acid suggesting that induction of this enzyme(s) is important in this stimulatory process. These studies demonstrate that IFN-gamma, TNF, and LT are important stimulators of fibroblast GAG biosynthesis, that interactions between these cytokines may be important in this regulatory process, that these cytokines predominantly stimulate hyaluronic acid production and that this effect may be mediated by stimulation of fibroblast hyaluronate synthetase activity.
Full text
PDF


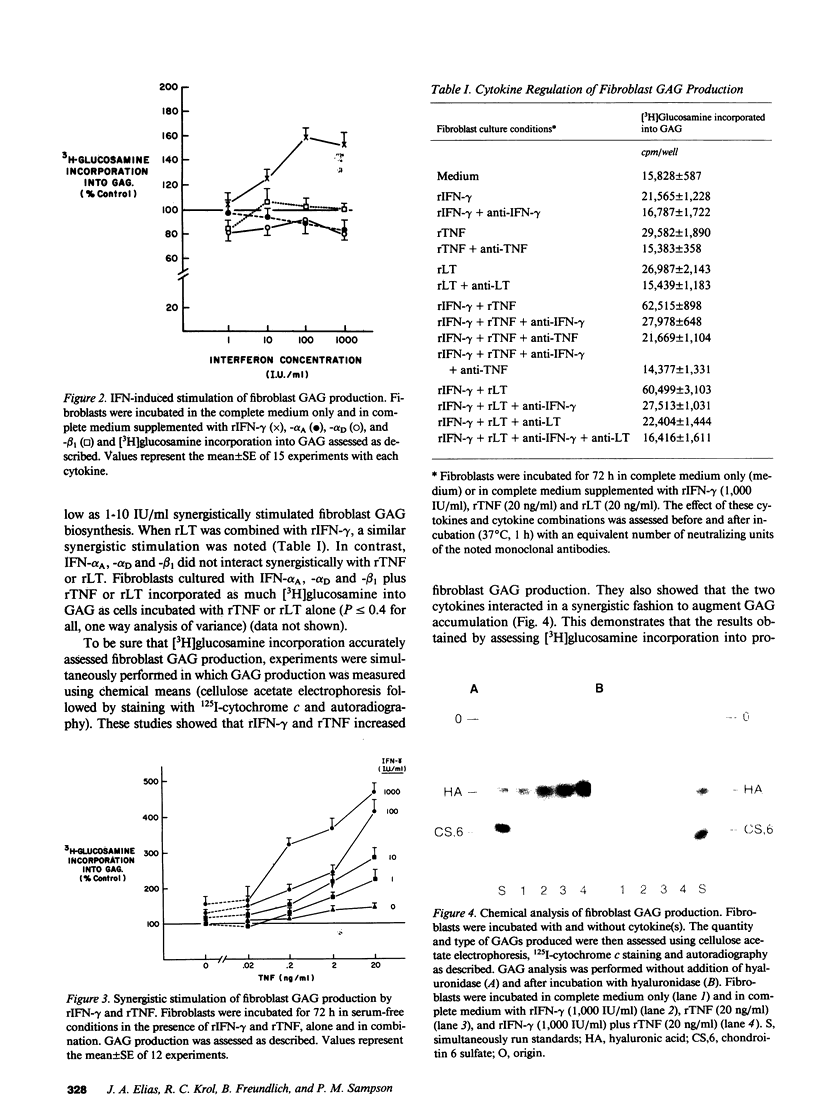
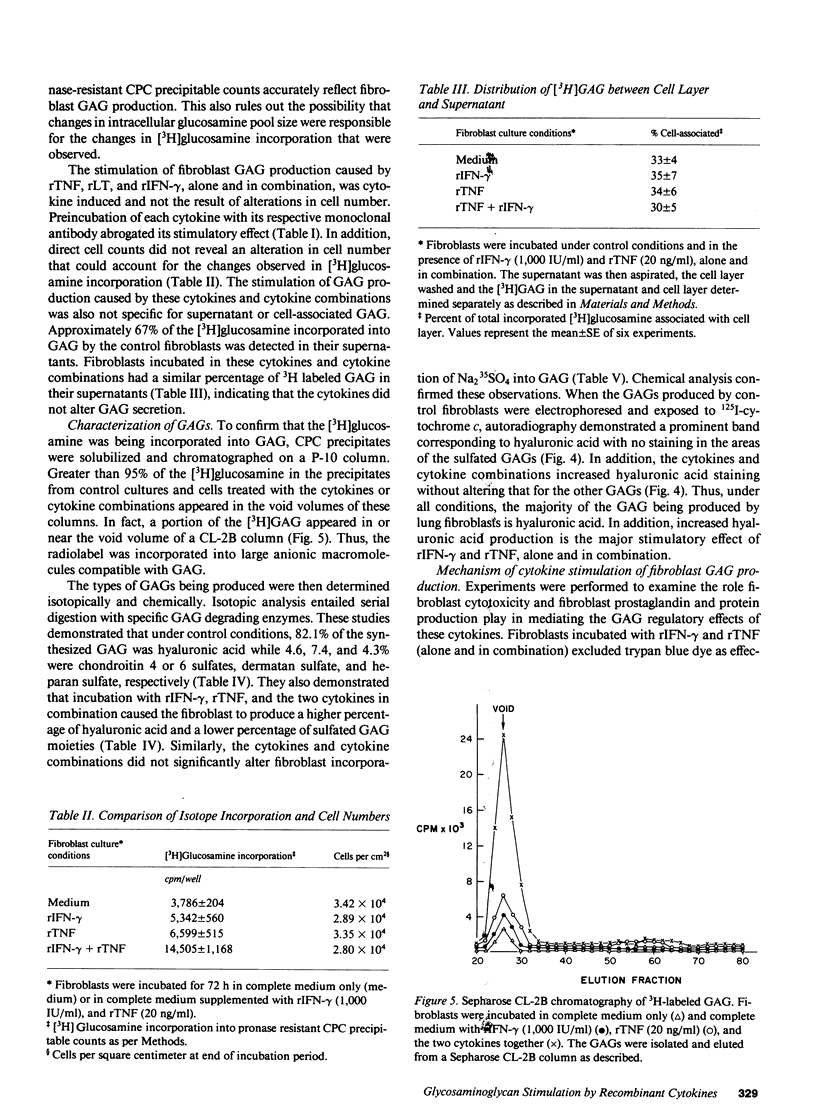
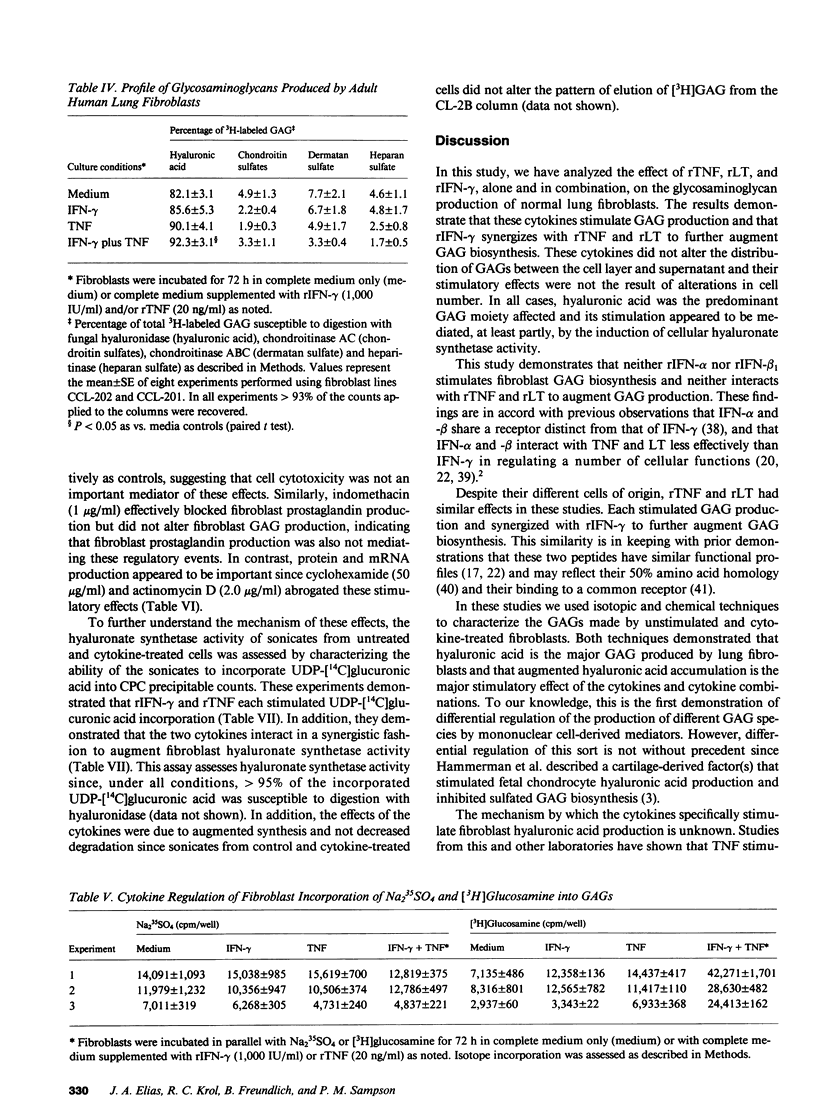



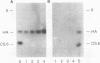
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Alcocer-Varela J., Martinez-Cordero E., Alarcon-Segovia D. Spontaneous production of, and defective response to, interleukin-1 by peripheral blood mononuclear cells from patients with scleroderma. Clin Exp Immunol. 1985 Mar;59(3):666–672. [PMC free article] [PubMed] [Google Scholar]
- Anastassiades T. P., Wood A. Effect of soluble products from lectin-stimulated lymphocytes on the growth, adhesiveness, and glycosaminoglycan synthesis of cultured synovial fibroblastic cells. J Clin Invest. 1981 Sep;68(3):792–802. doi: 10.1172/JCI110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiades T., Robertson W. Modulation of mitogen-dependent lymphocyte stimulation by hyaluronic acid. J Rheumatol. 1984 Dec;11(6):729–734. [PubMed] [Google Scholar]
- Appel A., Horwitz A. L., Dorfman A. Cell-free synthesis of hyaluronic acid in Marfan syndrome. J Biol Chem. 1979 Dec 10;254(23):12199–12203. [PubMed] [Google Scholar]
- Bashey R. I., Millan A., Jimenez S. A. Increased biosynthesis of glycosaminoglycans by scleroderma fibroblasts in culture. Arthritis Rheum. 1984 Sep;27(9):1040–1045. doi: 10.1002/art.1780270911. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Blackwood R. A., Cantor J. O., Moret J., Mandl I., Turino G. M. Glycosaminoglycan synthesis in endotoxin-induced lung injury. Proc Soc Exp Biol Med. 1983 Dec;174(3):343–349. doi: 10.3181/00379727-174-41746. [DOI] [PubMed] [Google Scholar]
- Bocquet J., Langris M., Daireaux M., Jouis V., Pujol J. P., Beliard R., Loyau G. Mononuclear cell-mediated modulation of synovial cell metabolism. II. Increased hyaluronic acid synthesis by a monocyte cell factor (MCF). Exp Cell Res. 1985 Sep;160(1):9–18. doi: 10.1016/0014-4827(85)90231-9. [DOI] [PubMed] [Google Scholar]
- Cantor J. O., Osman M., Cerreta J. M., Mandl I., Turino G. M. Glycosaminoglycan synthesis in explants derived from bleomycin-treated fibrotic hamster lungs. Proc Soc Exp Biol Med. 1983 Jul;173(3):362–366. doi: 10.3181/00379727-173-41657. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor C. W. Connective tissue activation. VII. Evidence supporting a role for prostaglandins and cyclic nucleotides. J Lab Clin Med. 1975 Mar;85(3):392–404. [PubMed] [Google Scholar]
- Cesario T. C., Andrews B. S., Martin D. A., Jason M., Treadwell T., Friou G., Tilles J. G. Interferon in synovial fluid and serum of patients with rheumatic disease. J Rheumatol. 1983 Aug;10(4):647–650. [PubMed] [Google Scholar]
- Dahl I. M., Husby G. Hyaluronic acid production in vitro by synovial lining cells from normal and rheumatoid joints. Ann Rheum Dis. 1985 Oct;44(10):647–657. doi: 10.1136/ard.44.10.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Demczuk S. Cytokines and other mediators in rheumatoid arthritis. Springer Semin Immunopathol. 1984;7(4):387–413. doi: 10.1007/BF00201968. [DOI] [PubMed] [Google Scholar]
- Duncan M. R., Berman B. Gamma interferon is the lymphokine and beta interferon the monokine responsible for inhibition of fibroblast collagen production and late but not early fibroblast proliferation. J Exp Med. 1985 Aug 1;162(2):516–527. doi: 10.1084/jem.162.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Gustilo K., Baeder W., Freundlich B. Synergistic stimulation of fibroblast prostaglandin production by recombinant interleukin 1 and tumor necrosis factor. J Immunol. 1987 Jun 1;138(11):3812–3816. [PubMed] [Google Scholar]
- Elias J. A., Jimenez S. A., Freundlich B. Recombinant gamma, alpha, and beta interferon regulation of human lung fibroblast proliferation. Am Rev Respir Dis. 1987 Jan;135(1):62–65. doi: 10.1164/arrd.1987.135.1.62. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Rossman M. D., Daniele R. P. Inhibition of human lung fibroblast growth by mononuclear cells. Am Rev Respir Dis. 1982 Jun;125(6):701–705. doi: 10.1164/arrd.1982.125.6.701. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Rossman M. D., Zurier R. B., Daniele R. P. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985 Jan;131(1):94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- Engström-Laurent A., Hällgren R. Circulating hyaluronate in rheumatoid arthritis: relationship to inflammatory activity and the effect of corticosteroid therapy. Ann Rheum Dis. 1985 Feb;44(2):83–88. doi: 10.1136/ard.44.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig J. J., Ward-Bailey P. F. Sulfated glycosaminoglycans inhibit hyaluronic acid synthesizing activity in mouse cumuli oophori. Exp Cell Res. 1984 Feb;150(2):459–465. doi: 10.1016/0014-4827(84)90590-1. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Reeves J. R. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977 May;20(4):975–984. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Sasse J., Klagsbrun M. A cartilage-derived growth factor enhances hyaluronate synthesis and diminishes sulfated glycosaminoglycan synthesis in chondrocytes. J Cell Physiol. 1986 May;127(2):317–322. doi: 10.1002/jcp.1041270220. [DOI] [PubMed] [Google Scholar]
- Hiro D., Ito A., Matsuta K., Mori Y. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun. 1986 Oct 30;140(2):715–722. doi: 10.1016/0006-291x(86)90790-4. [DOI] [PubMed] [Google Scholar]
- Hällgren R., Eklund A., Engström-Laurent A., Schmekel B. Hyaluronate in bronchoalveolar lavage fluid: a new marker in sarcoidosis reflecting pulmonary disease. Br Med J (Clin Res Ed) 1985 Jun 15;290(6484):1778–1781. doi: 10.1136/bmj.290.6484.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez S. A., Freundlich B., Rosenbloom J. Selective inhibition of human diploid fibroblast collagen synthesis by interferons. J Clin Invest. 1984 Sep;74(3):1112–1116. doi: 10.1172/JCI111480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlinsky J. B. Glycosaminoglycans in emphysematous and fibrotic hamster lungs. Am Rev Respir Dis. 1982 Jan;125(1):85–88. doi: 10.1164/arrd.1982.125.1.85. [DOI] [PubMed] [Google Scholar]
- Kramer S. L., Granger G. A. The role of lymphotoxin in target cell destruction by mitogen-activated human lymphocytes. I. The correlation of target cell sensitivity to lymphotoxin and the intact lymphocyte. Cell Immunol. 1975 Jan;15(1):57–68. doi: 10.1016/0008-8749(75)90164-1. [DOI] [PubMed] [Google Scholar]
- Lamberg S. I., Stoolmiller A. C. Glycosaminoglycans. A biochemical and clinical review. J Invest Dermatol. 1974 Dec;63(6):433–449. doi: 10.1111/1523-1747.ep12680346. [DOI] [PubMed] [Google Scholar]
- Mason R. M., Kimura J. H., Hascall V. C. Biosynthesis of hyaluronic acid in cultures of chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1982 Mar 10;257(5):2236–2245. [PubMed] [Google Scholar]
- Mason R. M., d'Arville C., Kimura J. H., Hascall V. C. Absence of covalently linked core protein from newly synthesized hyaluronate. Biochem J. 1982 Dec 1;207(3):445–457. doi: 10.1042/bj2070445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuoka K., Namba M., Mitsui Y. Hyaluronate synthetase inhibition by normal and transformed human fibroblasts during growth reduction. J Cell Biol. 1987 Apr;104(4):1105–1115. doi: 10.1083/jcb.104.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian N. Analysis of cell-growth-phase-related variations in hyaluronate synthase activity of isolated plasma-membrane fractions of cultured human skin fibroblasts. Biochem J. 1986 Jul 15;237(2):333–342. doi: 10.1042/bj2370333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuni-Takagaki Y., Toole B. P. Hyaluronate-protein complex of rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1981 Aug 25;256(16):8463–8469. [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedwin G. E., Svedersky L. P., Bringman T. S., Palladino M. A., Jr, Goeddel D. V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985 Oct;135(4):2492–2497. [PubMed] [Google Scholar]
- Ohya T., Kaneko Y. Novel hyaluronidase from streptomyces. Biochim Biophys Acta. 1970 Mar 18;198(3):607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Toole B. P. Isolation and characterization of hyaluronidase from cultures of chick embryo skin- and muscle-derived fibroblasts. J Biol Chem. 1980 Feb 10;255(3):1036–1042. [PubMed] [Google Scholar]
- Orkin R. W., Underhill C. B., Toole B. P. Hyaluronate degradation in 3T3 and simian virus-transformed 3T3 cells. J Biol Chem. 1982 May 25;257(10):5821–5826. [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Mechanism of chain growth. Biochem J. 1983 Apr 1;211(1):191–198. doi: 10.1042/bj2110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Sullivan S. A., Williamson B. D., Carswell E. A., Old L. J. High affinity binding of 125I-labeled human tumor necrosis factor (LuKII) to specific cell surface receptors. J Exp Med. 1985 Sep 1;162(3):1099–1104. doi: 10.1084/jem.162.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero V., Tavernier J., Fiers W., Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-gamma. J Immunol. 1986 Apr 1;136(7):2445–2450. [PubMed] [Google Scholar]
- Sampson P. M., Boyd R. B., Pietra G. G., Fishman A. P. Glycosaminoglycan biosynthesis in the isolated perfused rat lung. J Appl Physiol Respir Environ Exerc Physiol. 1984 Dec;57(6):1648–1654. doi: 10.1152/jappl.1984.57.6.1648. [DOI] [PubMed] [Google Scholar]
- Sampson P. M., Heimer R., Fishman A. P. Detection of glycosaminoglycans at the one-nanogram level by 125I-cytochrome c. Anal Biochem. 1985 Dec;151(2):304–308. doi: 10.1016/0003-2697(85)90180-0. [DOI] [PubMed] [Google Scholar]
- Sampson P. M., Heimer R., Fishman A. P. Detection of glycosaminoglycans with 125I-labeled cytochrome c. Methods Enzymol. 1987;138:260–267. doi: 10.1016/0076-6879(87)38021-8. [DOI] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Sisson J. C., Castor C. W., Klavons J. A. Connective tissue activation. XVIII. Stimulation of hyaluronic acid synthetase activity. J Lab Clin Med. 1980 Aug;96(2):189–197. [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Turino G. M. The lung parenchyma--a dynamic matrix. J. Burns Amberson lecture. Am Rev Respir Dis. 1985 Dec;132(6):1324–1334. doi: 10.1164/arrd.1985.132.6.1324. [DOI] [PubMed] [Google Scholar]
- Uitto J., Helin G., Helin P., Lorenzen I. Connective tissue in scleroderma. A biochemical study on the correlation of fractionated glycosaminoglycans and collagen in human skin. Acta Derm Venereol. 1971;51(6):401–406. [PubMed] [Google Scholar]
- Vasan N. S., La Manna O., Lamb K. M. Differential accumulation of matrix material during limb development. Prog Clin Biol Res. 1984;151:387–398. [PubMed] [Google Scholar]
- Whiteside T. L., Worrall J. G., Prince R. K., Buckingham R. B., Rodnan G. P. Soluble mediators from mononuclear cells increase the synthesis of glycosaminoglycan by dermal fibroblast cultures derived from normal subjects and progressive systemic sclerosis patients. Arthritis Rheum. 1985 Feb;28(2):188–197. doi: 10.1002/art.1780280214. [DOI] [PubMed] [Google Scholar]
- Wiebkin O. W., Muir H. Synthesis of proteoglycans by suspension and monolayer cultures of adult chondrocytes and de novo cartilage nodules-the effect of hyaluronic acid. J Cell Sci. 1977;27:199–211. doi: 10.1242/jcs.27.1.199. [DOI] [PubMed] [Google Scholar]
- Yarden A., Kimchi A. Tumor necrosis factor reduces c-myc expression and cooperates with interferon-gamma in HeLa cells. Science. 1986 Dec 12;234(4782):1419–1421. doi: 10.1126/science.3097823. [DOI] [PubMed] [Google Scholar]